Cover Crop Selection by Jointly Optimizing Biomass Productivity, Biological Nitrogen Fixation, and Transpiration Efficiency: Application to Two Crotalaria Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Biomass Productivity and Characteristics of Nodules
2.3. Determination of Transpiration Efficiency
2.4. Gas Exchange Measurements
2.5. Determination of Nitrogen Concentration and Stable Isotopic Composition of Plant Parts
- BNF is the percentage of N in the plant, derived from BNF.
- δ15Nref is the δ15N value of the non-fixing reference plant.
- δ15Nfix is the δ15N value of the fixing plant.
- B is the δ15N value of a fixing plant growing in N-free growth medium.
2.6. Experimental Setup
2.7. Statistical Analysis
3. Results and Discussion
3.1. Biomass and Nitrogen Productivity from Fixation
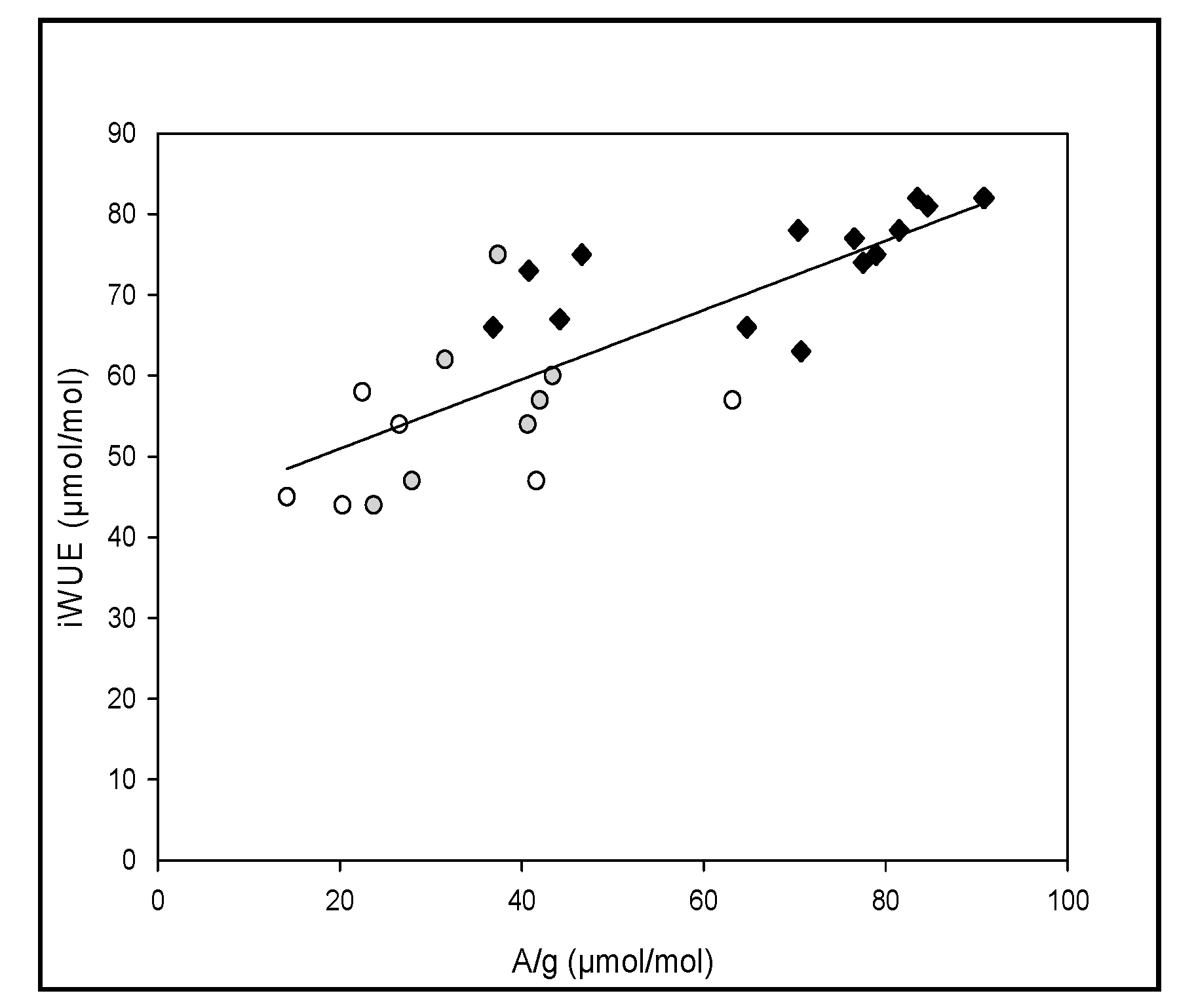
3.2. Transpiration Efficiency and Water Use Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cherr, C.M.; Scholberg, J.M.S.; McSorley, R. Green manure approaches to crop production: A synthesis. Agron. J. 2006, 98, 302–319. [Google Scholar] [CrossRef]
- Martins, F.; Hungria, M.; de Carvalho, L.; Bueno, F.; Souza, D. Fixação biológica de nitrogeno em adubos verdes In Adubação Verde e Plantas de Cobertura no Brasil: Fundamentos e Práticas; Lima Filho, O.F., Ambrosano, E.J., Rossi, F., Carlos, J.A.D., Eds.; Embrapa. Brasília—DF: Brasilia, Brazil, 2014; pp. 309–334. [Google Scholar]
- Folorunso, O.A.; Rolston, D.E.; Lovi, D.T. Soil surface strength and infiltration rate as affected by winter cover crops. Soil Technol. 1992, 5, 189–197. [Google Scholar] [CrossRef]
- Pacheco, J.S.; Silva-López, R.E.S. Genus Crotalaria L. (Leguminoseae). Rev. Fitos 2010, 5, 43–52. [Google Scholar]
- Meena, R.S.; Lal, R. Legumes and Sustainable Use of Soils. In Legumes for Soil Health and Sustainable Management; Meena, R., Das, A., Yadav, G., Lal, R., Eds.; Springer: Singapore, 2018; pp. 1–31. [Google Scholar]
- Soratto, R.P.; Crusciol, C.A.C.; Costa, C.H.M.; Ferrani Neto, J.; Castrp, G.S.A. Produção, decomposição e ciclagem de nutrientes em resíduos de crotalária e milheto, cultivados solteiros e consorciados. Pesqui. Agropecu. Bras. 2012, 47, 1462–1470. [Google Scholar] [CrossRef]
- Menezes, L.A.S.; Leandro, W.M.; de Oliveira Junior, J.P.; Ferreira, A.C.B.; das Santana, J.G.; Barros, R.G. Produção de fitomassa de diferentes espécies, isoladas e consorciadas, com potencial de utilização para cobertura do solo. Biosci. J. 2009, 25, 7–12. [Google Scholar]
- Perin, A.; Santos, R.H.S.; Urquiaga, S.C.; Guerra, J.G.M.; Cecon, P.R. Produção de fitomassa, acúmulo de nutrientes e fixação biológica de nitrogênio por adubos verdes em cultivo isolado e consorciado. Pesqui. Agropecu. Bras. 2004, 39, 35–40. [Google Scholar] [CrossRef][Green Version]
- Balkcom, K.S.; Reeves, D.W. Sunn-hemp utilized as a legume cover crop for corn production. Agron. J. 2005, 97, 26–31. [Google Scholar] [CrossRef]
- Wutke, E.B.; Calegari, A.; Wildner, L.P. Espécies de adubos verdes e plantas de cobertura e recomendações para seu uso. In Adubação Verde e Plantas de Cobertura no Brasil: Fundamentos e Práticas; Lima Filho, O.F., Ambrosano, E.J., Rossi, F., Carlos, J.A.D., Eds.; Embrapa, Brasília—DF: Brasilia, Brazil, 2014; pp. 59–167. [Google Scholar]
- Mendonça, E.; Lima, P.C.; Guimarães, G.P.; Moura, W.; Andrade, F.V. Biological Nitrogen Fixation by Legumes and N Uptake by Coffee Plants. Rev. Bras. Ciênc. Solo Viçosa 2017, 41, e0160178. [Google Scholar] [CrossRef]
- Lombardi, M.L.; Moreira, M.; Ambrosio, L.A.; Cardoso, E.J. Occurence and host specificity of indigenous rhizobia from soils of São Paulo State, Brazil. Sci. Agric. 2009, 66, 543–548. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Arf, O.; Soratto, R.P.; Andreotti, M.; Rodrigues, R.A.F. Absorção, exportação e eficiência de utilização de nutrientes pela cultura do arroz de terras altas em função de lâminas de agua aplicadas por aspersão. Acta Sci. Agron. 2003, 25, 97–102. [Google Scholar] [CrossRef]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- de Alves, F.J.S.; Miranda, J.P.H.V.; Moura, D.A.; Reis, B.R.; Soares, J.P.G.; Fernandes, F.D.; Ramos, A.K.B.; Malaquias, J.V. Produção de biomassa e valor nutricional do Cajanus Cajan cv. Mandarin sob manejo orgânico e convencional. In Proceedings of the XXIV Congresso Brasileiro de Zootecnia, Vitória, Brazil, 12–14 May 2014. [Google Scholar]
- Macedo, I.; Otaño, C.; Barrios, E.; Beyhaut, E.; Rossi, C.; Sawchick, J.; Terra, J.A. Leguminosas anuales de verano como opciones de cobertura en sistemas agrícolas. Rev. INIA Urug. 2015, 43, 50–54. [Google Scholar]
- Matos, E.S.; Mendoca, E.S.; Lima, P.C.; Coelho, M.S.; Mateus, R.F.; Cardoso, I.M. Green manure in coffee systems in the region of Zona da Mata, Minas Gerais: Characteristics and kinetics of carbon and nitrogen mineralization. Rev. Bras. Cienc. Solo 2008, 32, 2027–2035. [Google Scholar] [CrossRef][Green Version]
- Fishman, R.; Devineni, N.; Raman, S. Can improved agricultural water use efficiency save India’s groundwater? Environ. Res. Lett. 2015, 10, 084022. [Google Scholar] [CrossRef]
- Ren, C.F.; Guo, P.; Yang, G.Q.; Li, R.H.; Liu, L. Spatial and temporal analyses of water resources use efficiency based on data envelope analysis and malmquist index: Case study in Gansu Province, China. J. Water Resour. Plan. Manag. 2016, 142, 04016066. [Google Scholar] [CrossRef]
- Wunsch, E.M.; Bell, L.W.; Bell, M.J. Can legumes provide greater benefits than millet as a spring cover crop in southern Queensland farming systems? Crop Pasture Sci. 2017, 68, 746. [Google Scholar] [CrossRef]
- Gregory, P.J. Concepts of water use efficiency. In Soil and Crop Management for Improved Water Use Efficiency in Rainfed Areas; Harris, H.C., Cooper, P.J.M., Pala, M., Eds.; Proceedings of International Workshop; ICARDA: Ankara, Turkey; Aleppo, Syria, 1991; pp. 9–20. [Google Scholar]
- Franks, P.J.; Doheny-Adams, T.W.; Britton-Harper, Z.J.; Gray, J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef]
- Bhattacharya, A. Water-use efficiency under changing climatic conditions. In Changing Climate and Resource Use Efficiency in Plants; Bhattacharya, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 111–180. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Pronger, J.; Campbell, D.I.; Clearwater, M.J.; Mudge, P.L.; Rutledge, S.; Wall, A.M.; Schipper, L.A. Toward optimisation of water use efficiency in dryland pastures using carbon isotope discrimination as a tool to select plant species mixtures. Sci. Total Environ. 2019, 665, 698–708. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [PubMed]
- Berriel, V.; Perdomo, C.; Monza, J. Carbon Isotope Discrimination and Water-Use Efficiency in Crotalaria Cover Crops under Moderate Water Deficit. J. Soil Sci. Plant Nutr. 2020, 20, 537–545. [Google Scholar] [CrossRef]
- Sulzman, E.W. Stable isotope chemistry and measurement: A primer. In Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Michener, R., Lajtha, K., Eds.; Blackwell Publishing: Boston, NJ, USA, 2007; pp. 1–21. [Google Scholar]
- Unkovich, M.; Herridge, D.; Peoples, M.; Boddey, R.; Cadisch, G.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; Australian Center of International Agricultural Research (ACIAR): Canberra, Australia, 2008; p. 258.
- Okito, A.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Isotopic fractionation during N2 fixation by four tropical legumes. Soil Biol. Biochem. 2004, 36, 1179–1190. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Version 2011; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2018. [Google Scholar]
- Addinsoft. XLSTAT; Statistical Software: Paris, France, 2020. [Google Scholar]
- Irisarri, P.; Cardozo, G.; Tartaglia, C.; Reyno, R.; Gutiérrez, P.; Lattanzi, F.A.; Rebuffo, M.; Monza, J. Selection of Competitive and Efficient Rhizobia Strains for White Clover. Front. Microbiol. 2019, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Buchmann, N.; Sprent, J.; Buckley, T.N.; Turnbull, T.L. Crops, Nitrogen, Water: Are Legumes Friend, Foe, or Misunderstood Ally? Trends Plant Sci. 2018, 23, 539–550. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Sant’Anna, S.A.C.; Martins, M.R.; Goulart, J.M.; Araújo, S.N.; Araújo, E.S.; Zaman, M.; Jantalia, C.P.; Alves, B.J.R.; Boddey, R.M.; Urquiaga, S. Biological nitrogen fixation and soil N2O emissions from legume residues in an Acrisol in SE Brazil. Geoderma Reg. 2018, 15, e00196. [Google Scholar] [CrossRef]
- Veettil, A.V.; Mishra, A.K. Water security assessment using blue and green water footprint concepts. J. Hydrol. 2016, 542, 589–602. [Google Scholar] [CrossRef]
- Sinclair, T.R. Is transpiration efficiency a viable plant trait in breeding for crop improvement? Funct. Plant Biol. 2012, 39, 359–365. [Google Scholar] [CrossRef]
- Fu, Q.A.; Button, T.W.; Ehleringer, J.R.; Flager, R.B. Environmental and Developmental effects on carbon isotope discrimination by two species of Phaseolus. In Stable Isotopes and Plant Carbon-Water Relations; Ehleringer, J.R., Hall, A.E., Farquhar, G.D., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 297–310. [Google Scholar]
- Johnson, R.C.; Basset, L.M. Carbon isotope discrimination and water use efficiency in four cool season grasses. Crop Sci. 1991, 31, 157–162. [Google Scholar] [CrossRef]
- Read, J.J.; Johnson, D.A.; Asay, K.H.; Tieszen, L.T. Carbon isotope discrimination, gas exchange, and water use efficiency in crested wheatgrass clones. Crop Sci. 1991, 31, 1203–1208. [Google Scholar] [CrossRef]
- Kumarasinghe, K.S.; Kirda, C.; Mohamed, A.R.A.G.; Zapata, F.; Danso, S.K.A. 13C isotope discrimination correlates with biological nitrogen fixation in soybean (Glycine max (L.) Merrill). Plant Soil 1992, 139, 145–147. [Google Scholar] [CrossRef]
- Knight, J.D.; Verhees, F.; Van Kessel, C.; Slinkard, A.E. Does carbon isotope discrimination correlate with biological nitrogen fixation? Plant Soil 1993, 153, 151–153. [Google Scholar] [CrossRef]


| Species | Nodules | Shoot DM | T | Nleaf | A | g | E | δ13C | δ15N |
|---|---|---|---|---|---|---|---|---|---|
| g | kg | gN/100gDM | µmol/m2 s | mol/m2 s | mmol/m2 s | ‰ | |||
| C. spectabilis | + | 6.29 ± 1.16 | 2.11 ± 0.56 | 2.26 ± 0.43 | 6.78 ± 2.15 | 0.11 ± 0.06 | 1.70 ± 0.80 | −27.75 ± 0.56 | −0.45 ± 0.72 |
| C. juncea | +/− | 3.60 ± 2.61 | 1.48 ± 0.76 | 1.85 ± 0.70 | 5.09 ± 3.72 | 0.15 ± 0.09 | 2.21 ± 1.14 | −29.46 ± 0.78 | 1.13 ± 2.27 |
| + | 5.05 ± 2.72 | 1.87 ± 0.81 | 2.23 ± 0.70 | 7.06 ± 3.96 | 0.19 ± 0.10 | 2.72 ± 1.15 | −29.23 ± 0.90 | −0.74 ± 0.95 | |
| − | 1.91 ± 1.05 | 1.03 ± 0.39 | 1.40 ± 0.37 | 2.80 ± 1.63 | 0.10 ± 0.07 | 1.61 ± 0.87 | −29.74 ± 0.55 | 3.30 ± 0.87 | |
| Water-Use Efficiency | N fixation | ||||||||
| TE | A/g | A/E | iWUE | BNF | |||||
| g/kg | µmol/mol | mmol/mol | µmol/mol | % | |||||
| C. spectabilis | + | 3.10 ± 0.58 | 68.95 ± 17.86 | 4.25 ± 0.93 | 74.06 ± 6.57 | 92.65 ± 7.50 | |||
| C. juncea | +/− | 2.21 ± 0.68 | 33.44 ± 13.01 | 2.17 ± 0.89 | 54.15 ± 8.91 | 67.08 ± 22.90 | |||
| + | 2.54 ± 0.74 | 35.22 ± 7.59 | 2.43 ± 0.70 | 57.00 ± 10.30 | 85.30 ± 9.27 | ||||
| − | 1.81 ± 0.34 | 31.37 ± 18.09 | 1.87 ± 1.06 | 50.83 ± 6.24 | 45.82 ± 8.50 | ||||
| Nodules | Shoot DM | δ15N | BNF | g | A/g | δ13C | iWUE | |
|---|---|---|---|---|---|---|---|---|
| p | ||||||||
| Model | 0.0026 | <0.0001 | <0.0001 | NS | 0.0003 | 0.0001 | 0.0001 | |
| Species | Ranks mean and Groups | |||||||
| C. juncea | - | 4.8A | 26.50A | 3.50A | - | 6.67A | 5.83A | 5.83A |
| + | 14.9B | 10.14B | 17.4B | - | 9.00A | 9.29A | 9.29A | |
| C. spectabilis | + | 18.9B | 12.81B | 19.2B | - | 20.75B | 20.94B | 20.94B |
| Nleaf | T | TE | A | E | A/E | |
|---|---|---|---|---|---|---|
| Model | p | |||||
| Species | 0.0352 | 0.0099 | 0.0004 | NS | NS | <0.0001 |
| Species > Fix | 0.0061 | 0.0173 | 0.0339 | 0.0067 | 0.0363 | NS |
| Contrasts | p | |||||
| C. juncea vs. C. spectabilis | 0.0245 | 0.0071 | 0.0003 | 0.0687 | NS | <0.0001 |
| C. juncea (+) vs. C. juncea (−) | 0.0061 | 0.0173 | 0.0339 | 0.0067 | 0.0363 | NS |
| C juncea (+) vs. C. spectabilis | NS | 0.4011 | 0.0467 | NS | 0.0421 | 0.0002 |
| Variable | TE | A/g | δ15N | BNF | N | iWUE |
|---|---|---|---|---|---|---|
| TE | 1 | |||||
| A/g | 0.49 ** | 1 | ||||
| δ15N | –0.56 ** | –0.36 NS | 1 | |||
| BNF | 0.62 *** | 0.34 NS | –0.99 *** | 1 | ||
| N | 0.44 * | 0.37 * | –0.52 ** | 0.54 *** | 1 | |
| iWUE | 0.54 ** | 0.81 *** | –0.58 ** | 0.58 *** | 0.40 * | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berriel, V.; Monza, J.; Perdomo, C.H. Cover Crop Selection by Jointly Optimizing Biomass Productivity, Biological Nitrogen Fixation, and Transpiration Efficiency: Application to Two Crotalaria Species. Agronomy 2020, 10, 1116. https://doi.org/10.3390/agronomy10081116
Berriel V, Monza J, Perdomo CH. Cover Crop Selection by Jointly Optimizing Biomass Productivity, Biological Nitrogen Fixation, and Transpiration Efficiency: Application to Two Crotalaria Species. Agronomy. 2020; 10(8):1116. https://doi.org/10.3390/agronomy10081116
Chicago/Turabian StyleBerriel, Verónica, Jorge Monza, and Carlos H. Perdomo. 2020. "Cover Crop Selection by Jointly Optimizing Biomass Productivity, Biological Nitrogen Fixation, and Transpiration Efficiency: Application to Two Crotalaria Species" Agronomy 10, no. 8: 1116. https://doi.org/10.3390/agronomy10081116
APA StyleBerriel, V., Monza, J., & Perdomo, C. H. (2020). Cover Crop Selection by Jointly Optimizing Biomass Productivity, Biological Nitrogen Fixation, and Transpiration Efficiency: Application to Two Crotalaria Species. Agronomy, 10(8), 1116. https://doi.org/10.3390/agronomy10081116





