Pitaya, an Attractive Alternative Crop for Mediterranean Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
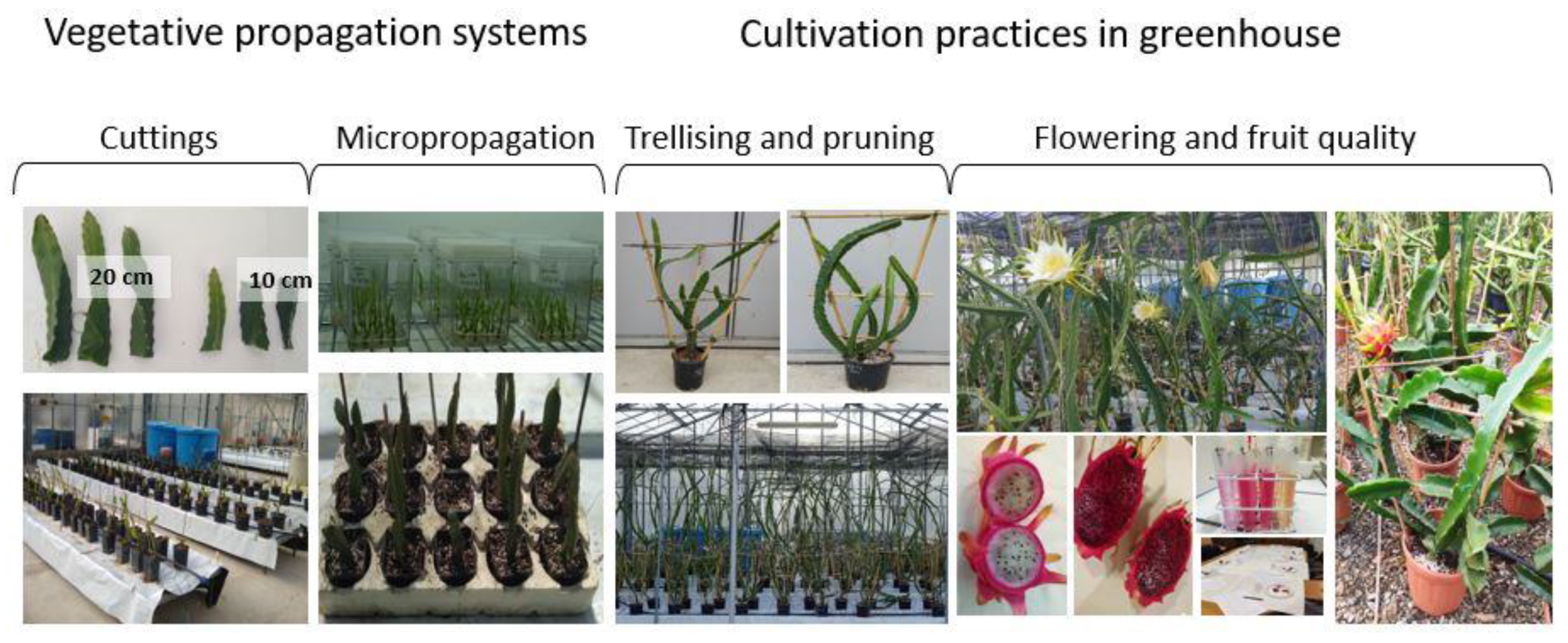
2.2. Vegetative Propagation Systems
2.2.1. Propagation by Cuttings
2.2.2. In Vitro Propagation
2.3. Greenhouse Cultivation Practices
2.3.1. Trellising and Pruning
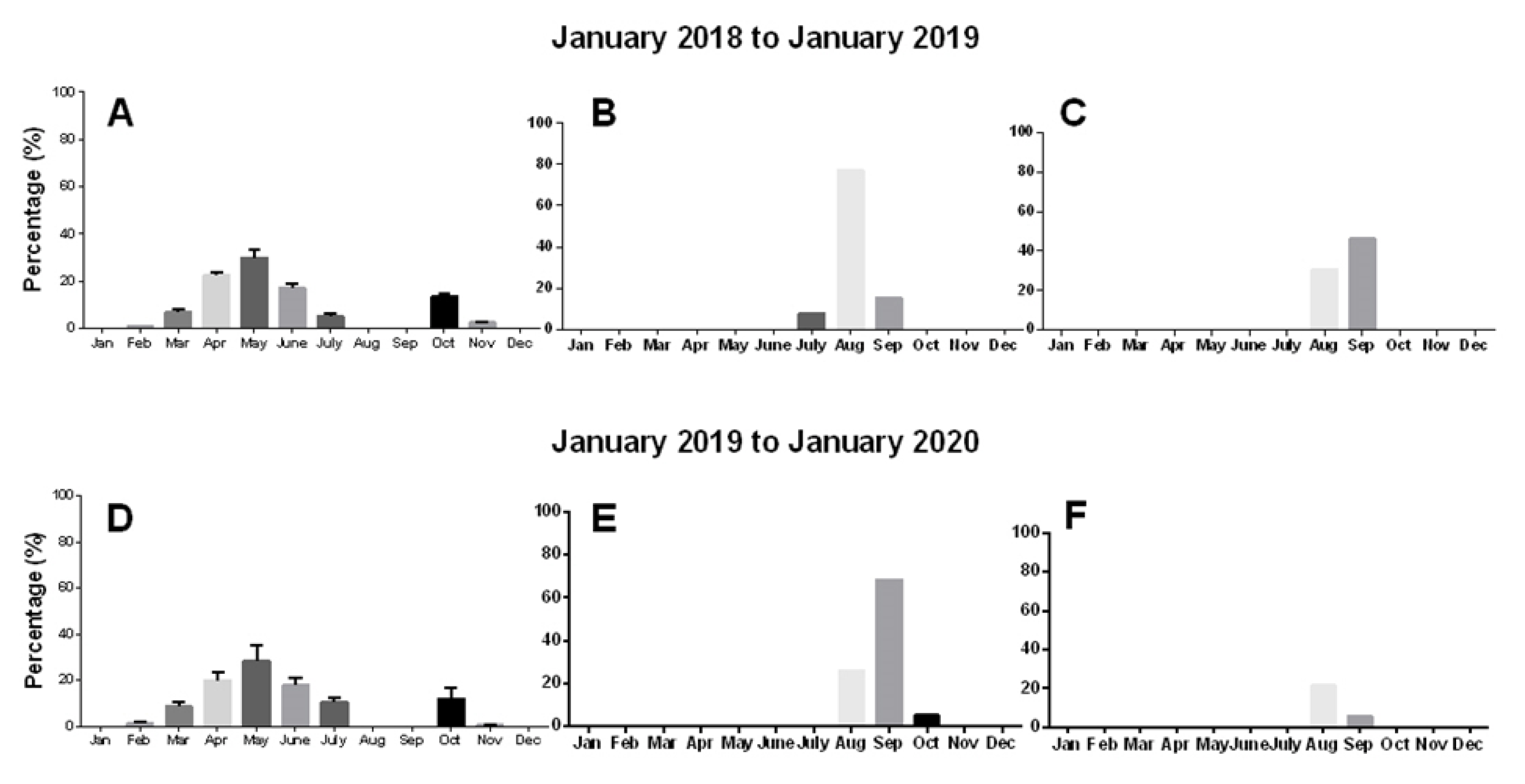
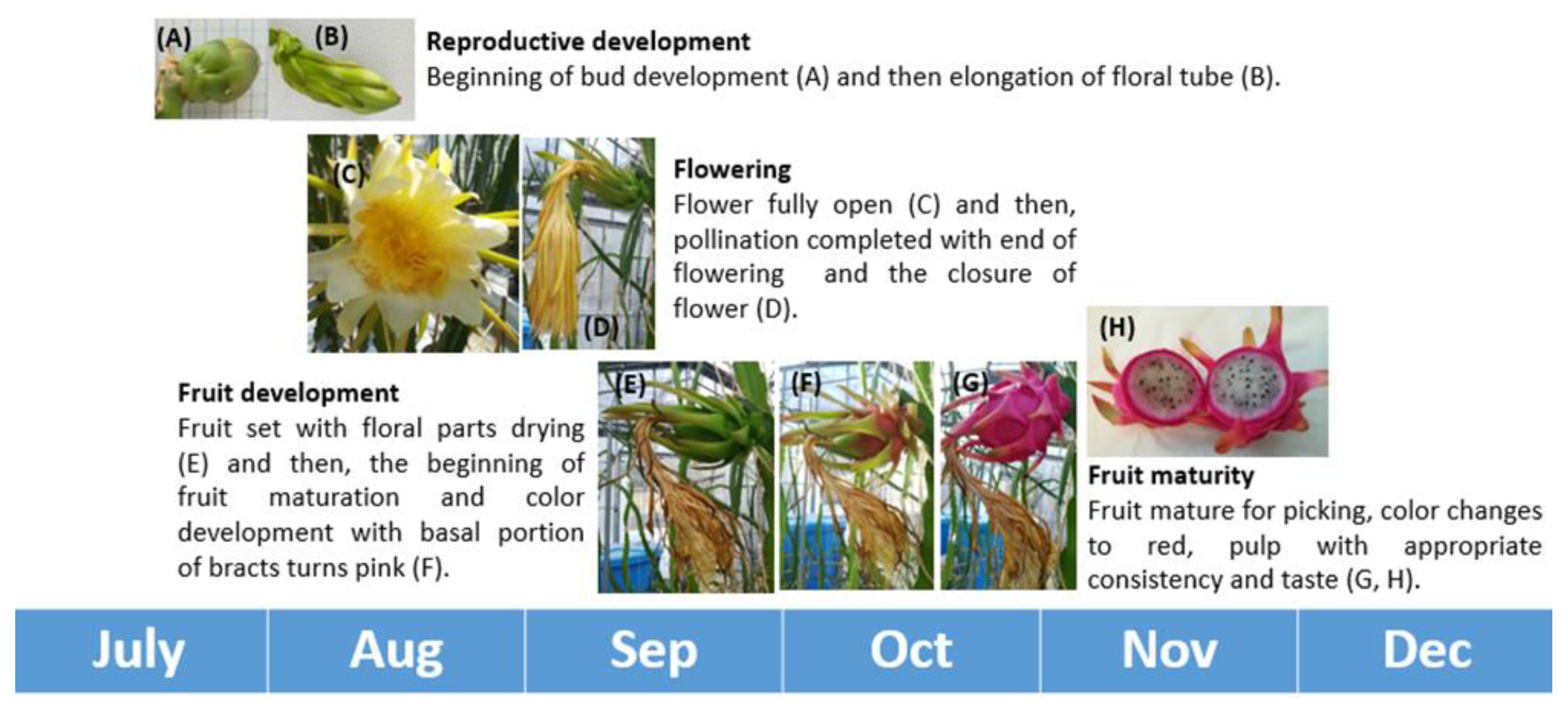
2.3.2. Phenology of Sprouting and Floral Bud Emergence
2.3.3. Hand-Pollination and Assessment of Fruit Set
2.4. Fruits Nutraceutical Characteristics
2.5. Sensory Evaluation of Harvested Fruits
2.6. Statistical Analysis
3. Results
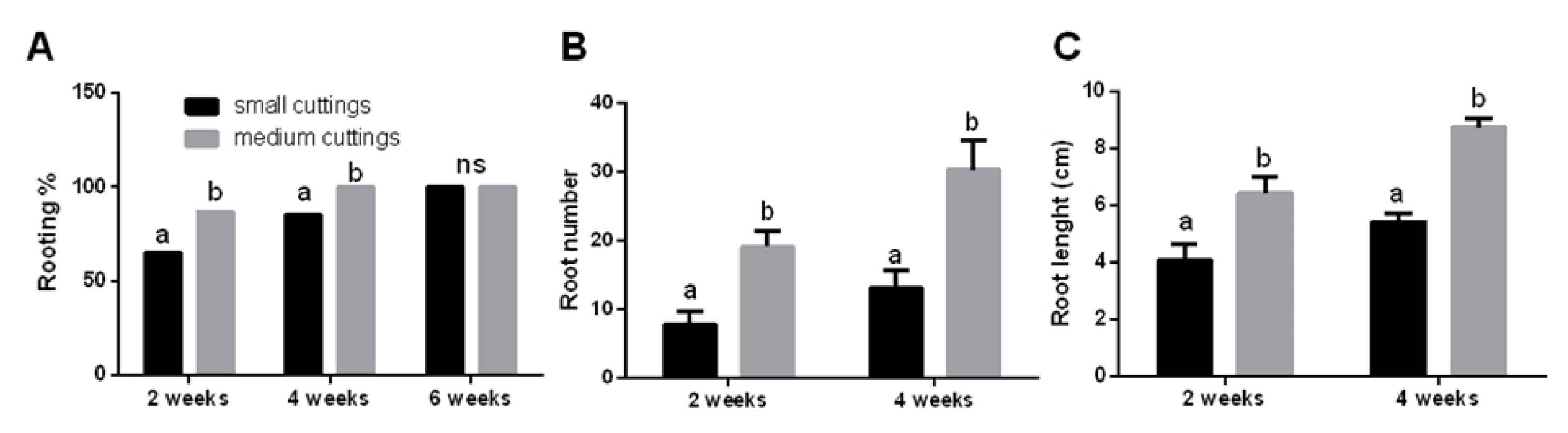
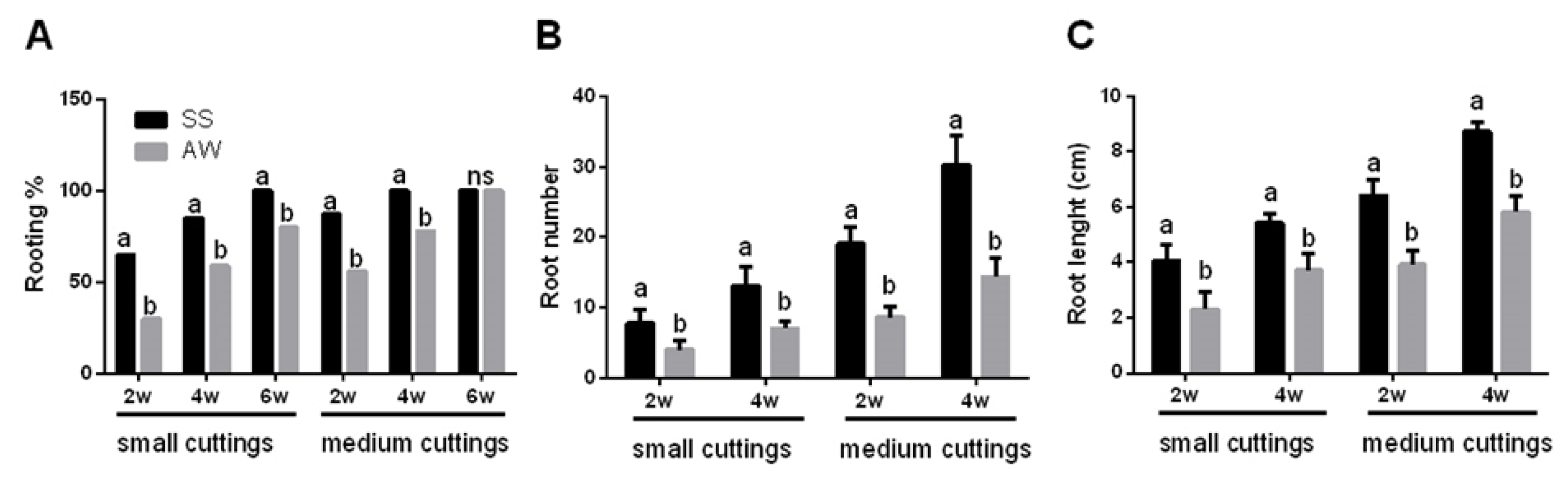
3.1. Propagation of Pitaya Cuttings Depends on Their Size and Season
3.2. Efficiency of Micropropagation System in Pitaya (or Efficient Pitaya Propagation Methodology)
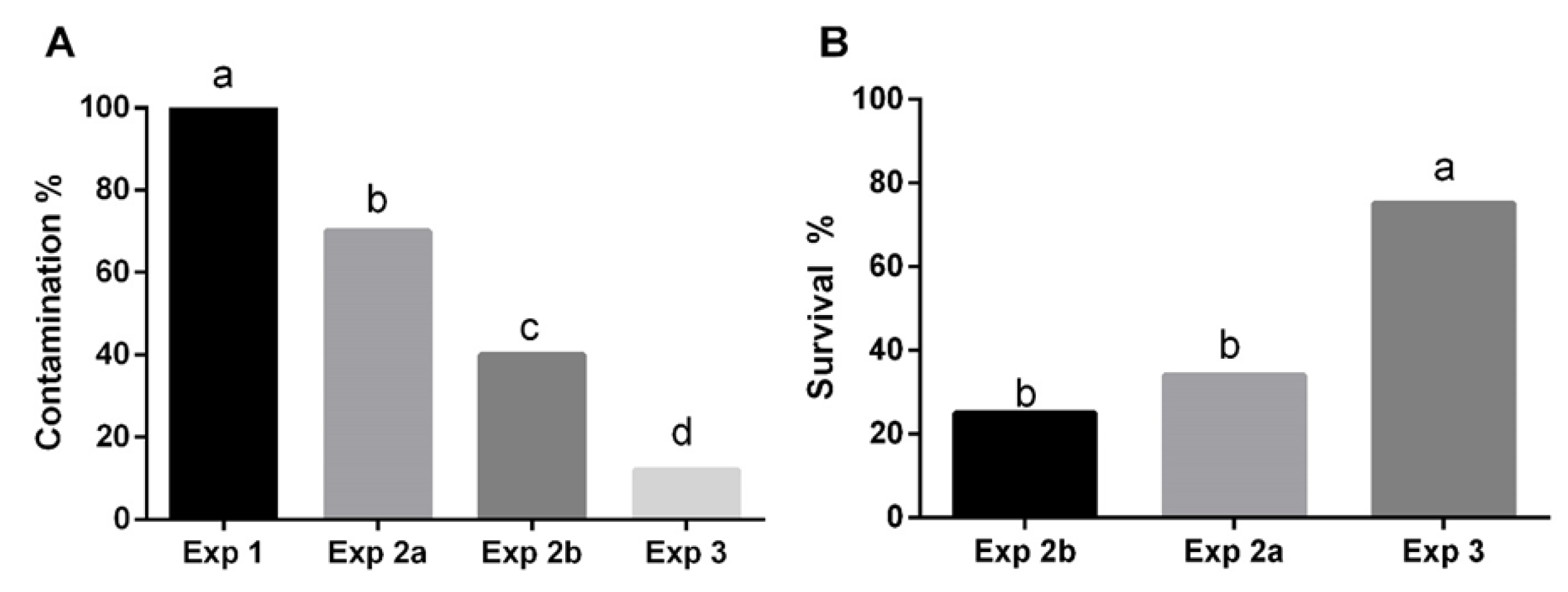
3.2.1. Establishment of Aseptic Cultures
3.2.2. Factors Affecting Shoot Proliferation, Rhizogenesis and Acclimatization Process
3.3. Growing Potted Pitaya in Greenhouse
3.3.1. Phenology of Sprouting and Floral Bud Emergence
3.3.2. Pollination and Assessment of Fruit Set
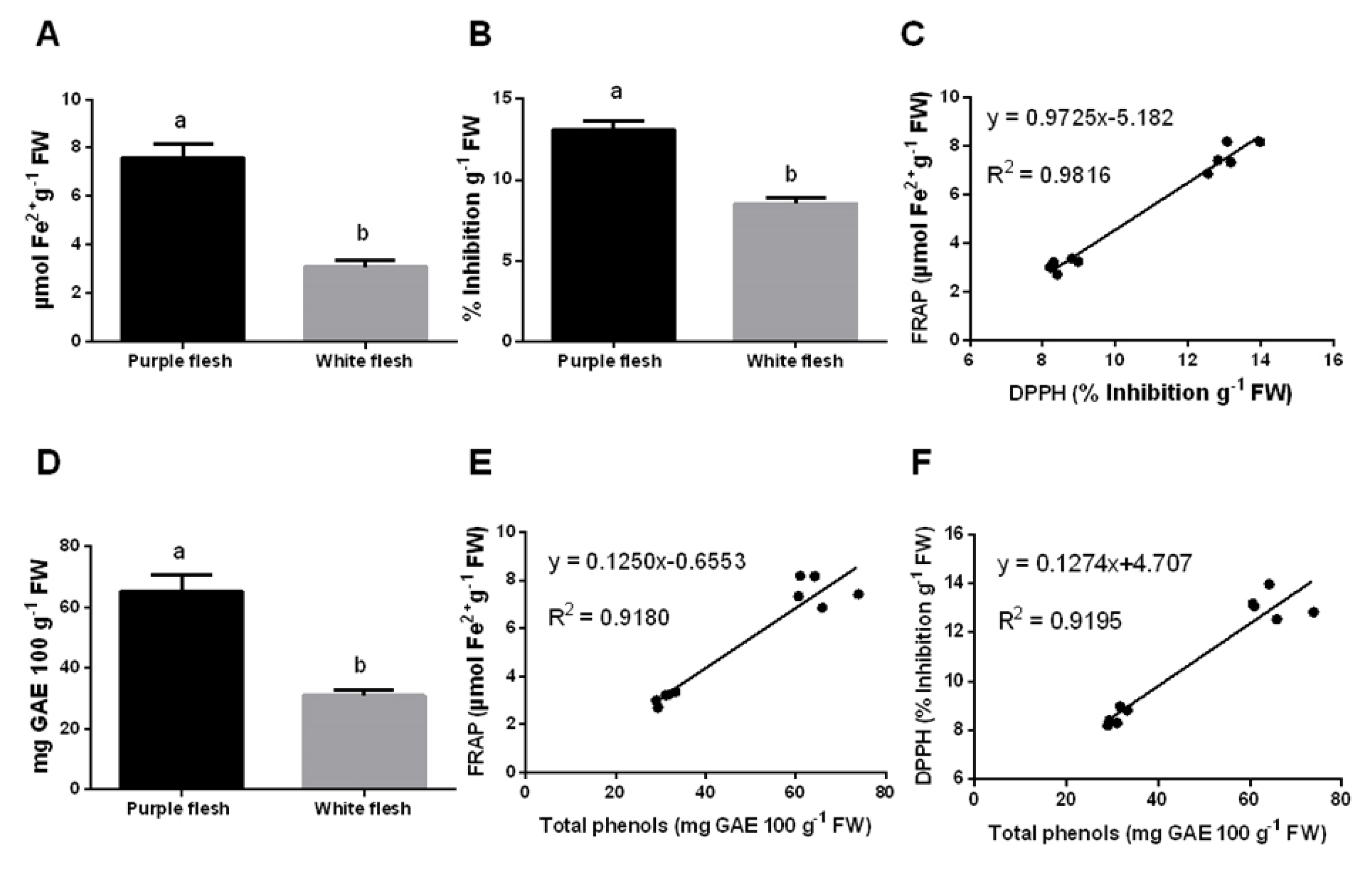
3.4. Nutraceutical Potential and Antioxidant Benefits of Pitaya
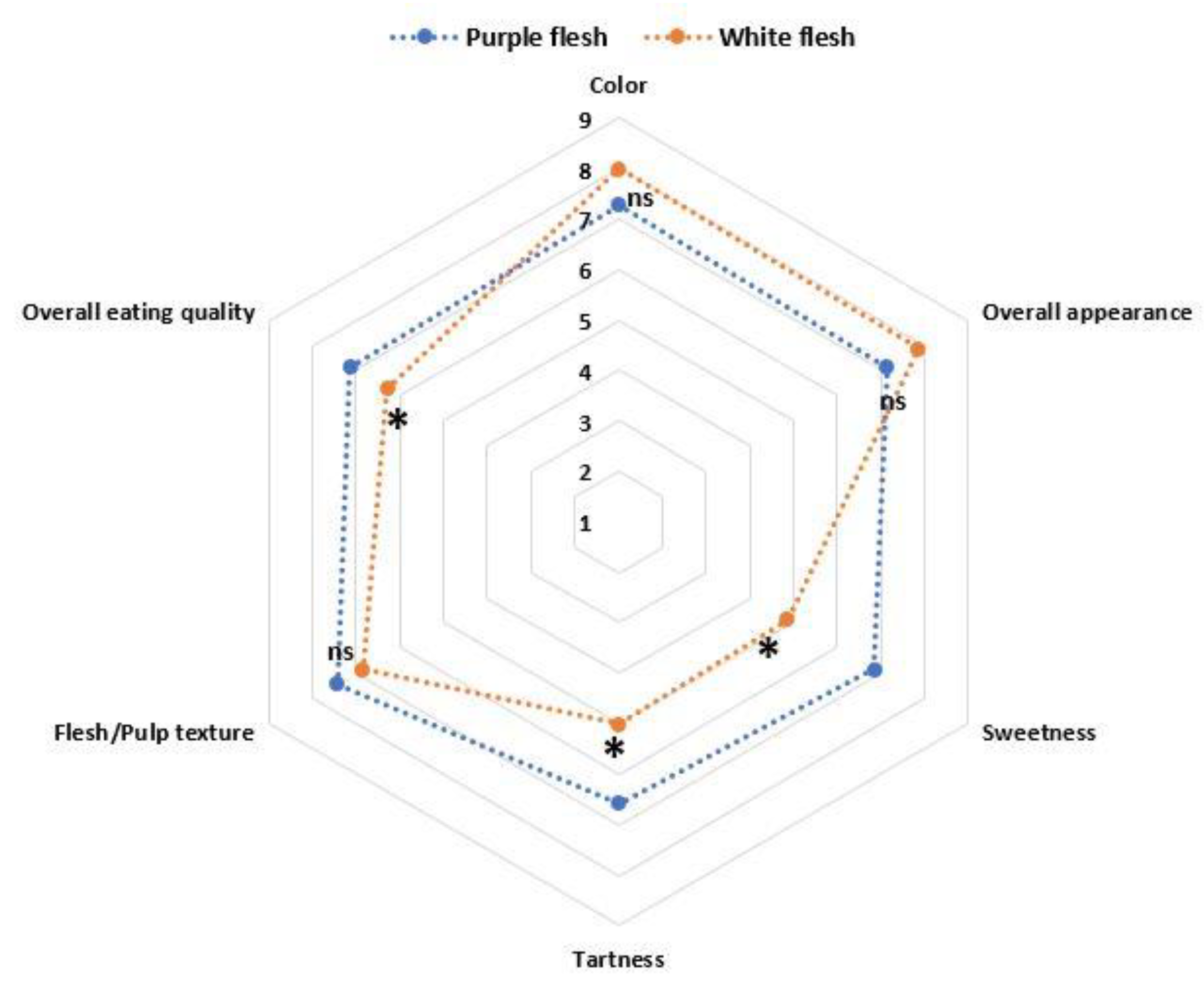
3.5. Fruit Quality Evaluation by Sensory Panel
4. Discussion
5. Conclusions
- (i)
- Efficient vegetative propagation methodologies were developed for both pitaya cultivars (Hylocereous undatus and Hylocereous spp.) to support the cultivation and expansion of pitaya crop in Italy.
- (ii)
- A detailed analysis of the greenhouse cultivation practices and the effect of controlled environment for Hylocereous cultivars were provided. The clarification of the sprouting and flowering phenology as well as the fruit maturation period of pitaya crop grown in Italian greenhouse will enable the grower to define the agronomic management required to create a new profitable alternative horticultural/ornamental product.
- (iii)
- From the results obtained in the sensory and nutraceutical properties evaluations of Hylocereous fruits it can be concluded that the red-flesh fruits represent a promising source of natural antioxidants with a superior overall eating quality perceived by the panelists.
Author Contributions
Funding
Conflicts of Interest
References
- Ferrante, A.; Trivellini, A.; Scuderi, D.; Romano, D.; Vernieri, P. Post-production physiology and handling of ornamental potted plants. Postharvest Biol. Technol. 2015, 100, 99–108. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L. Adaptation strategies for agricultural water management under climate change in europe. Agric. Water Manag. 2015, 155, 113–124. [Google Scholar] [CrossRef]
- Costes, E.; Khadari, B.; Zaher, H.; Moukhli, A.; Morillon, R.; Legave, J.; Regnard, J. Adaptation of Mediterranean fruit tree cultivation to climate change. In The Mediterranean Region under Climate Change; Moatti, J., Thiébault, S., Eds.; AllEnvi, IRD Éditions: Marseille, France, 2016; pp. 503–510. [Google Scholar]
- Nobel, P.S.; Barrera, E.D.L. Stem water ralations and net CO2 uptake for a hemiepiphytic cactus during short-term drought. J. Exp. Bot. 2002, 48, 129–137. [Google Scholar] [CrossRef]
- Keiichi, N.; Mai, I.; Takuya, A.; Yoshimi, Y. Seasonal differences in diurnal patterns of metabolities and enzyme activities in pitaya (Hylocereus undatus) grown in a temperate zone. J. Jpn. Soc. Hortic. Sci. 2010, 79, 135–140. [Google Scholar]
- Wang, L.; Zhang, X.; Ma, Y.; Qing, Y.; Wang, H.; Huang, X. The highly drought-tolerant pitaya (Hylocereusundatus) is a non-facultative CAM plant under both well-watered and drought conditions. J. Hortic. Sci. Biotechnol. 2019, 94, 643–652. [Google Scholar] [CrossRef]
- Lüttge, U. Ecophysiology of crassulacean acid metabolism (CAM). Ann. Bot. 2004, 93, 629–652. [Google Scholar] [CrossRef]
- Herrera, A. Crassulacean acid metabolism and fitness under water deficit stress: If not for carbon gain, what is facultative CAM good for? Ann. Bot. 2009, 103, 645–653. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Betalain-emerging prospect for food scientist. Trends Food Sci. Technol. 2007, 18, 514–525. [Google Scholar] [CrossRef]
- Nurliyana, R.; Syed Zahir, I.; Mustapha Suleiman, K.; Aisyah, M.R.; Kamarul Rahim, K. Antioxidant study of pulps and peels of dragon fruits: A comparative study. Int. Food Res. J. 2010, 17, 367–375. [Google Scholar]
- Wichienchot, S.; Jatupornpipat, M.; Rastall, R.A. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 2010, 120, 850–857. [Google Scholar] [CrossRef]
- Cos, P.; Bruyne, D.T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef]
- Wybranice, S.; Mizrahi, Y. Fruit flesh betacyanin pigments in hyloceruus Cacti. J. Agric. Food Chem. 2002, 50, 6086–6089. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, H.; Moon, J.; Kim, Y.; Mosaddik, A.; Cho, S. Comparative antioxidant and antiproliferative activities of red and white pitayas and their correlation with flavonoid and polyphenol content. J. Food Sci. 2010, 76, C38–C45. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Stintzing, F.C.; Carle, R. Pigment pattern and expression of colour in fruits from different Hylocereus sp. Genotypes. Innov. Food Sci. Emerg. Technol. 2007, 8, 451–457. [Google Scholar] [CrossRef]
- Wu, L.C.; Hsu, H.W.; Chen, Y.C.; Chiu, C.C.; Lin, Y.I.; Ho, J.A. Antioxidantand antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Guimarães, D.A.B.; De Castro, D.D.S.B.; de Oliveira, F.L.; Nogueira, E.M.; da Silva, M.A.M.; Teodoro, A.J. Pitaya extracts induce growth inhibition and proapoptotic effects on human cell lines of breast cancer via downregulation of estrogen receptor gene expression. Oxid. Med. Cell. Longev. 2017, 2017, 7865073. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods LWT. Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Tsuda, S.; Murakami, M.; Matsusaka, N.; Kano, K.; Taniguchi, K.; Sasaki, Y.F. DNA damage induced by red food dyes orally administered to pregnant and male mice. Toxicol. Sci. 2001, 61, 92–99. [Google Scholar] [CrossRef]
- Le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Antioxidant capacity of lettuce leaf tissue increases after wounding. J. Agri. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power: The FRAP Assa. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Marion Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Dembitsky, V.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Vearasilp, S.; Trakhtenberg, S.; Gorinstein, S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Food Res. Int. 2001, 44, 1671–1701. [Google Scholar] [CrossRef]
- Mizrahi, Y. Vine-cacti Pitayas the New Crop of the World. Rev. Bras. Frutic. 2014, 36, 124–138. [Google Scholar] [CrossRef]
- Cornara, L.; Xiao, J.; Smeriglio, A.; Trombetta, D.; Burlando, B. Emerging Exotic Fruits: New Functional Foods in the European Market. EFood 2020, 1, 126–139. [Google Scholar] [CrossRef]
- Obeidy, A.A.E. Mass propagation of pitaya (dragon fruit). Fruits 2006, 61, 313–319. [Google Scholar]
- Kakade, V.; Dinesh, D.; Singh, D.; Bhatnagar, P.R.; Kadam, D. Influence of length of cutting on root and shoot growth in dragon fruit (Hylocereus undatus). Indian J. Agric. Sci. 2019, 89, 1895–1904. [Google Scholar]
- Naidu, R.D.; Jones, N.B. The effect of cutting length on the rooting and growth of subtropical Eucalyptus hybrid clones in South Africa, Southern Forests. J. For. Sci. 2009, 71, 297–301. [Google Scholar]
- Bona, C.M.; Biasetto, I.R.; Masetto, M.; Deschamps, C.; Biasi, L.A. Influence of cutting type and size on rooting of Lavandula dentata L. Rev. Bras. Planta Med. Botucatu 2012, 14, 8–11. [Google Scholar] [CrossRef]
- Okunlola, A.I. The effects of cutting types and length on rooting of Duranta repens in the nursery. Glob. J. Hum. Soc. Sci. Geogr. Geo-Sci. Environ. Disaster Manag. 2013, 13, 380–390. [Google Scholar]
- Aminah, H.M.; Ahmad Fauzi, S.; Tariq Mubarak, H.; Hamzah, M. Effect of Hormone and Cutting Length on the Rooting of Tinospora crispa. Int. J. Sci. Res. Publ. 2015, 5, 1–4. [Google Scholar]
- Philipson, J. Root growth in Sitka spruce and Douglas-fir transplants: Dependence on the shoot and stored carbohydrates. Tree Physiol. 1998, 4, 101–108. [Google Scholar] [CrossRef]
- Francis, D.; Barlow, P. Temperature and the cell cycle. Symp. Soc. Exp. Biol. 1988, 42, 181–201. [Google Scholar]
- Feraru, E.; Feraru, M.I.; Barbez, E.; Waidmann, S.; Sun, L.; Gaidora, A.; Kleine-Vehn, J. PILS6 is a temperature-sensitive regulator of nuclear auxin input and organ growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2019, 116, 3893–3898. [Google Scholar] [CrossRef]
- Mohamed-Yasseen, Y. Micropropagation of pitaya (Hylocereus undatus Britton et rose). In Vitro Cell Dev. Biol-Plant. 2002, 38, 427–429. [Google Scholar] [CrossRef]
- Dahanayake, N.; Ranawake, A.L. Regeneration of dragon fruit (Hylocereus undatus) plantlets from leaf and stem explants. Trop. Agric. Res. Ext. 2011, 14, 85–89. [Google Scholar] [CrossRef]
- Vinas, M.; Ferna’ndez-Brenes, M.; Azofeifa, A.; Jime´nez, V.M. In vitro propagation of purple pitahaya (Hylocereus costaricensis [F.A.C. Weber] Britton & Rose) cv. Cebra. In Vitro Cell Dev. Biol. Plant 2012, 48, 469–477. [Google Scholar]
- Fan, Q.J.; Zheng, S.C.; Yan, F.X.; Zhang, B.X.; Qiao, G.; Wen, X.P. Efficient regeneration of dragon fruit (Hylocereus undatus) and an assessment of the genetic fidelity of in vitro: Derived plants using ISSR markers. J. Hortic. Sci. Biotechnol. 2013, 88, 631–637. [Google Scholar] [CrossRef]
- Hua, Q.; Chen, P.; Liu, W.; Ma, Y.; Liang, R.; Wang, L.; Wang, Z.; Hu, G.; Qin, Y. A Protocol for Rapid in Vitro Propagation of Genetically Diverse Pitaya. Plant. Cell Tissue Organ. Cult. 2015, 120, 741–745. [Google Scholar] [CrossRef]
- Pérez-Molphe-Balch, E.; Santos-Díaz, M.d.S.; Ramírez-Malagón, R.; Ochoa-Alejo, N. Tissue culture of ornamental cacti. Sci. Agric. 2015, 72, 540–561. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Kulus, D.; Zhang, X.; Zeng, S.; Ma, G.; Piqueras, A. Disinfection of explants for saffron (Crocus sativus L.) tissue culture. Environ. Exp. Biol. 2016, 14, 183–198. [Google Scholar] [CrossRef]
- Lucchesini, M.; Mensuali-Sodi, A. Influence of medium composition and vessel ventilation on in vitro propagation of Phillyrea latifolia L. Sci. Hortic. 2004, 100, 117–125. [Google Scholar] [CrossRef]
- D’Utra Vaz, F.B.; dos Santos, A.V.P.; Manders, G.; Cocking, E.C.; Davey, M.R.; Power, J.B. Plant regeneration from leaf mesophyll protoplasts of the tropical woody plant, passionfruit (Passiflora edulis fv flavicarpa Degener.): The importance of the antibiotic cefotaxime in the culture medium. Plant Cell Rep. 1993, 12, 220–225. [Google Scholar] [CrossRef]
- Zaerr, J.B.; Mapes, M.O. Action of growth regulators. In Tissue Culture in Forestryl; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhoff: Dordrecht, The Netherlands; Boston, MA, USA, 1982; pp. 231–255. [Google Scholar]
- Moebius, K.G.; Mata, M.; Chávez, V.M. Organogenesis and somatic embryogenesis in Ariocarpus kotschoubeyanus (Lem.) K. Schum. (Cactaceae), an endemic and endangered mexican species. In Vitro Cell. Dev. Biol.-Plant 2003, 39, 388–393. [Google Scholar] [CrossRef]
- Burdyn, L.; Luna, C.; Tarrago, J.; Sansberro, P.; Dudit, N.; González, A.; Mroginski, I. Direct shoot regeneration from leaf and internode explants of Aloysia polystachya [Gris.] Mold. (Verbenaceae). In Vitro Cell. Dev. Biol.-Plant. 2006, 42, 235–239. [Google Scholar] [CrossRef]
- Drew, R.A.; Azimi, M. Micropropagation of red pitaya (Hylocereous undatus). Acta Hortic. 2002, 575, 93–98. [Google Scholar] [CrossRef]
- De Medeiros, L.A.; de Ribeiro, R.C.S.; Gallo, L.A.; de Oliveira, E.T.; Demattê, M.E.S.P. In vitro propagation of Notocactus magnificus. Plant Cell Tissue Organ Cult. 2006, 84, 165–169. [Google Scholar] [CrossRef][Green Version]
- Nerd, A.; Sitrit, Y.; Kaushik, R.A.; Mizrahi, Y. High summer temperatures inhibit flowering in vine pitaya crops (Hylocereus spp.). Sci. Hortic. 2002, 96, 343–350. [Google Scholar] [CrossRef]
- Raveh, E.; Nerd, A.; Mizrahi, Y. Responses of two hemiepiphytic fruit crop cacti to different degrees of shade. Sci. Hortic. 1998, 73, 151–164. [Google Scholar] [CrossRef]
- Grant, V.; Grant, K.A. The pollination spectrum in the southwestern American cactus flora. Plant. Sytematics Evol. 1979, 133, 29–37. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Santos-Gally, R.; Arizmendi, M.C.; Casas, A. Pollination biology of the hemiepiphytic cactus Hylocereus undatus in the Tehuacán Valley, Mexico. J. Arid Environ. 2007, 68, 1–8. [Google Scholar] [CrossRef]
- Weiss, J.; Nerd, A.; Mizrahi, Y. Flowering behavior and pollination requirements in climbing cacti with fruit crop potential. HortScience 1994, 29, 1487–1492. [Google Scholar] [CrossRef]
- Lichtenzveig, J.; Abbo, S.; Nerd, A.; Tel-Zur, N.; Mizrahi, Y. Cytology and mating system in the climbing cacti Hylocereus and Selenicereus. Am. J. Bot. 2000, 87, 1058–1065. [Google Scholar] [CrossRef]
- Tran, D.H.; Yen, C.R.; Chen, Y.K.H. Effect of pollination method and pollen source on fruit set and growth of red-peel pitaya (Hylocereus spp.) in Taiwan. J. Hortic. Sci. Biotechnol. 2015, 90, 254–258. [Google Scholar] [CrossRef]
- Ha, T.D.; Oanh, L.T.K.; Yen, C.R. Flowering phenology and mating system of a red skin pitaya (Hylocereus spp.) germplasm collection in Taiwan. Asian J. Adv. Agric. Res. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Freitas, S.T.D.; Mitcham, E.J. Quality of pitaya fruit (Hylocereus undatus) as influenced by storage temperature and packaging. Sci. Agric. 2013, 70, 257–262. [Google Scholar] [CrossRef]
- To, L.V.; Ngu, N.; Duc, N.D.; Trinh, D.T.K.; Thanh, N.C.; Mien, D.V.H.; Hai, C.N.; Long, T.N. Quality assurance system for dragon fruit. ACIAR Proc. 2000, 100, 101–114. [Google Scholar]
- Ortiz, T.A.; Takahashi, L.S.A. Physical and chemical characteristics of pitaya fruits at physiological maturity Genet. Mol. Res. 2015, 14, 14422–14439. [Google Scholar] [CrossRef]
- Hoa, T.T.; Clark, C.J.; Waddell, B.C.; Woolf, A.D. Postharvest quality of dragon fruit (Hylocereus undatus) following disinfesting hot air treatments. Postharvest Biol. Technol. 2016, 41, 62–69. [Google Scholar] [CrossRef]
- Magalhães, D.S.; da Silva, D.M.; Ramos, J.D.; Pio, L.A.S.; Pasqual, M.; Boas, E.V.B.V.; Galvão, E.C.; de Melo, E.T. Changes in the physical and physico-chemical characteristics of red-pulp dragon fruit during its development. Sci. Hortic. 2019, 253, 180–186. [Google Scholar] [CrossRef]
- Merten, S. A review of Hylocereus production in the United States. J. Prof. Assoc. Cactus Dev. 2003, 5, 98–105. [Google Scholar]
- Zee, F.; Yen, C.-R.; Nishina, M. Pitaya. In Fruits and Nuts; Cooperative Extension Service; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2004. [Google Scholar]
- Nerd, A.; Gutman, F.; Mizrahi, Y. Ripening and postharvest behaviour of fruits of two Hylocereus species (Cactaceae). Postharvest Biol. Technol. 1999, 17, 39–45. [Google Scholar] [CrossRef]
- Choo, W.S.; Yong, W.K. Antioxidant properties of two species of Hylocereus fruits. Adv. Appl. Sci. Res. 2011, 2, 418–425. [Google Scholar]
- Senadheera, S.P.N.M.K.; Abeysinghe, D.C. Bioactive Compounds and Total Antioxidant Capacity of Different Tissues of Two Pitaya (Dragon Fruit) Species Grown in Sri Lanka. J. Food Agric. 2015, 8, 33–40. [Google Scholar] [CrossRef]
- Obenland, D.; Cantwell, M.; Lobo, R.; Collin, S.; Sievert, J.; Arpaia, M.L. Impact of storage conditions and variety on quality attributes and aroma volatiles of pitahaya (Hylocereus spp.). Sci. Hortic. 2016, 199, 15–22. [Google Scholar] [CrossRef]
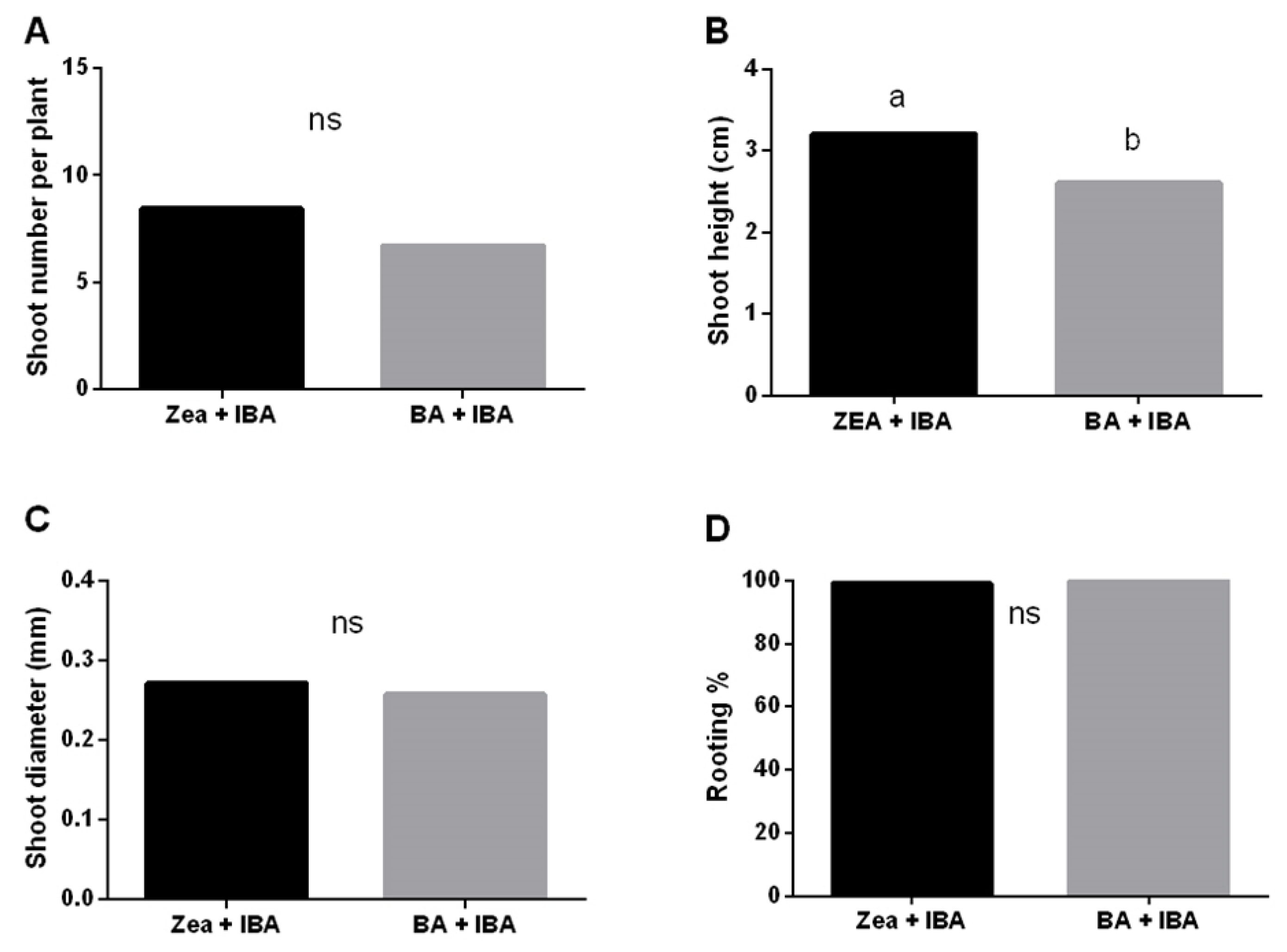
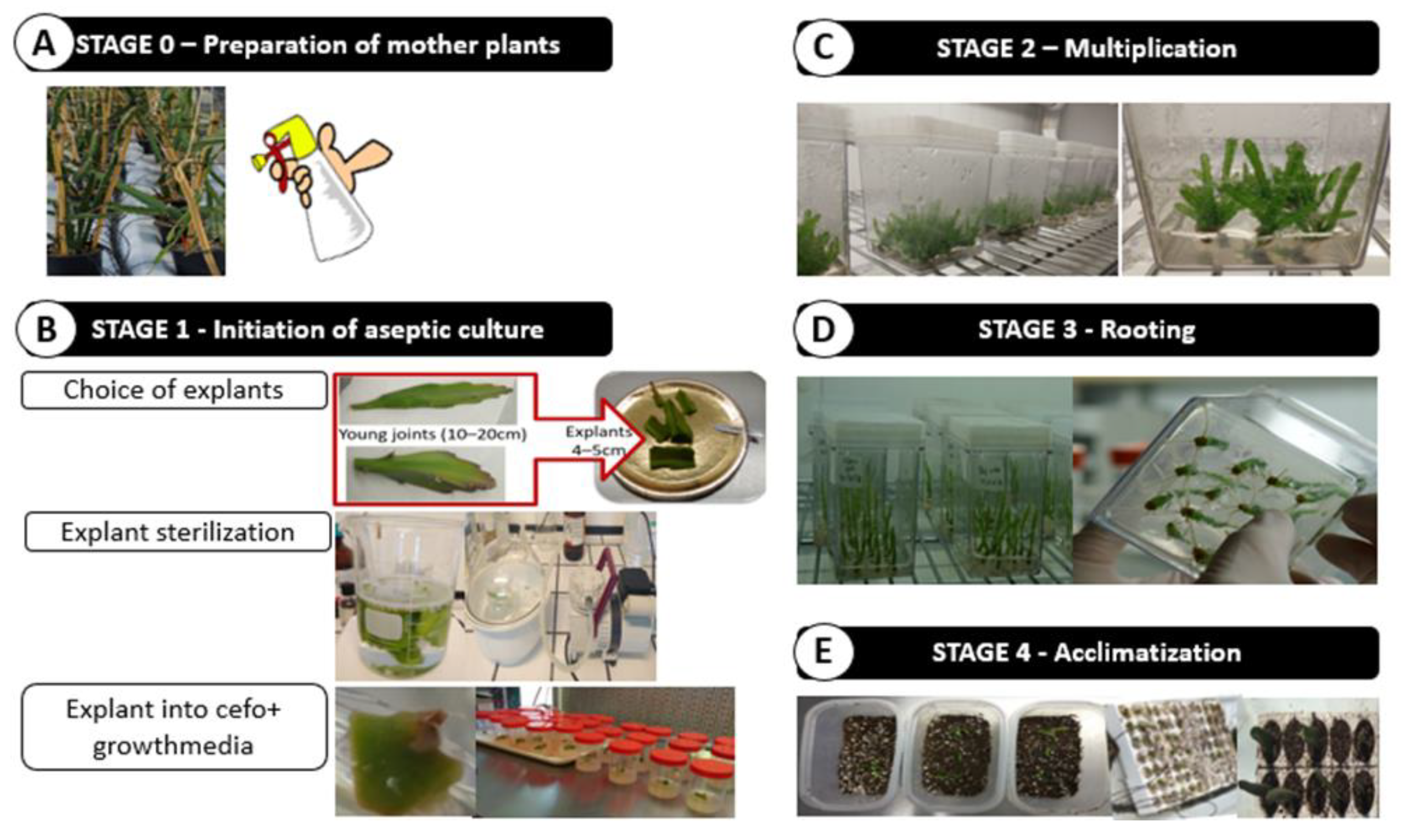
| Disinfection Strategy | EXP 1 | EXP2a | EXP2b | EXP3 |
|---|---|---|---|---|
| Pretreatment | ||||
| 1% AAS 10 min | X | |||
| 1% AAS 5 min vacuum | X | X | ||
| 1g L−1 Benomyl 5 min vacuum | X | |||
| 70% Ethanol 15–30 s | X | X | X | X |
| I disinfection | ||||
| 15% NaOCl 15 min | X | X | X | |
| 3% NaOCl 15 min | X | |||
| II disinfection | ||||
| 4% PPM 5 min vacuum | X | |||
| 0.2% NaOCl 1 min | X | |||
| Culture medium | ||||
| 200mg L−1 ultra-filtered cefotaxime | X | X |
| Proliferation Medium | ZEA + IBA | BA + IBA |
|---|---|---|
| Macroelements | MS | MS |
| Microelements | MS | MS |
| Vitamins | MS | MS |
| Sucrose | 30 g/L | 30 g/L |
| MES | 500 mg/L | 500 mg/L |
| GSH | 300 mg/L | 300 mg/L |
| IBA | 0.25 mg/L | 0.25 mg/L |
| Zeatin | 3 mg/L | |
| BA | 0.5 mg/L | |
| Gelrite | 2.5 g/L | 2.5 g/L |
| pH | 5.9 | 5.9 |
| Hylocereous spp. Clones | Hand Self-Pollination | Hand Cross-Pollination | ||
|---|---|---|---|---|
| Fruit Set % | Fruit Weight (g) | Fruit Set % | Fruit Weight (g) | |
| White flesh | 100 | 140 a | 100 | 273 a |
| Purple flesh | 100 | 113 b | 100 | 315 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivellini, A.; Lucchesini, M.; Ferrante, A.; Massa, D.; Orlando, M.; Incrocci, L.; Mensuali-Sodi, A. Pitaya, an Attractive Alternative Crop for Mediterranean Region. Agronomy 2020, 10, 1065. https://doi.org/10.3390/agronomy10081065
Trivellini A, Lucchesini M, Ferrante A, Massa D, Orlando M, Incrocci L, Mensuali-Sodi A. Pitaya, an Attractive Alternative Crop for Mediterranean Region. Agronomy. 2020; 10(8):1065. https://doi.org/10.3390/agronomy10081065
Chicago/Turabian StyleTrivellini, Alice, Mariella Lucchesini, Antonio Ferrante, Daniele Massa, Matteo Orlando, Luca Incrocci, and Anna Mensuali-Sodi. 2020. "Pitaya, an Attractive Alternative Crop for Mediterranean Region" Agronomy 10, no. 8: 1065. https://doi.org/10.3390/agronomy10081065
APA StyleTrivellini, A., Lucchesini, M., Ferrante, A., Massa, D., Orlando, M., Incrocci, L., & Mensuali-Sodi, A. (2020). Pitaya, an Attractive Alternative Crop for Mediterranean Region. Agronomy, 10(8), 1065. https://doi.org/10.3390/agronomy10081065








