A Gaussian-Process-Based Global Sensitivity Analysis of Cultivar Trait Parameters in APSIM-Sugar Model: Special Reference to Environmental and Management Conditions in Thailand
Abstract
1. Introduction
2. Materials and Methods
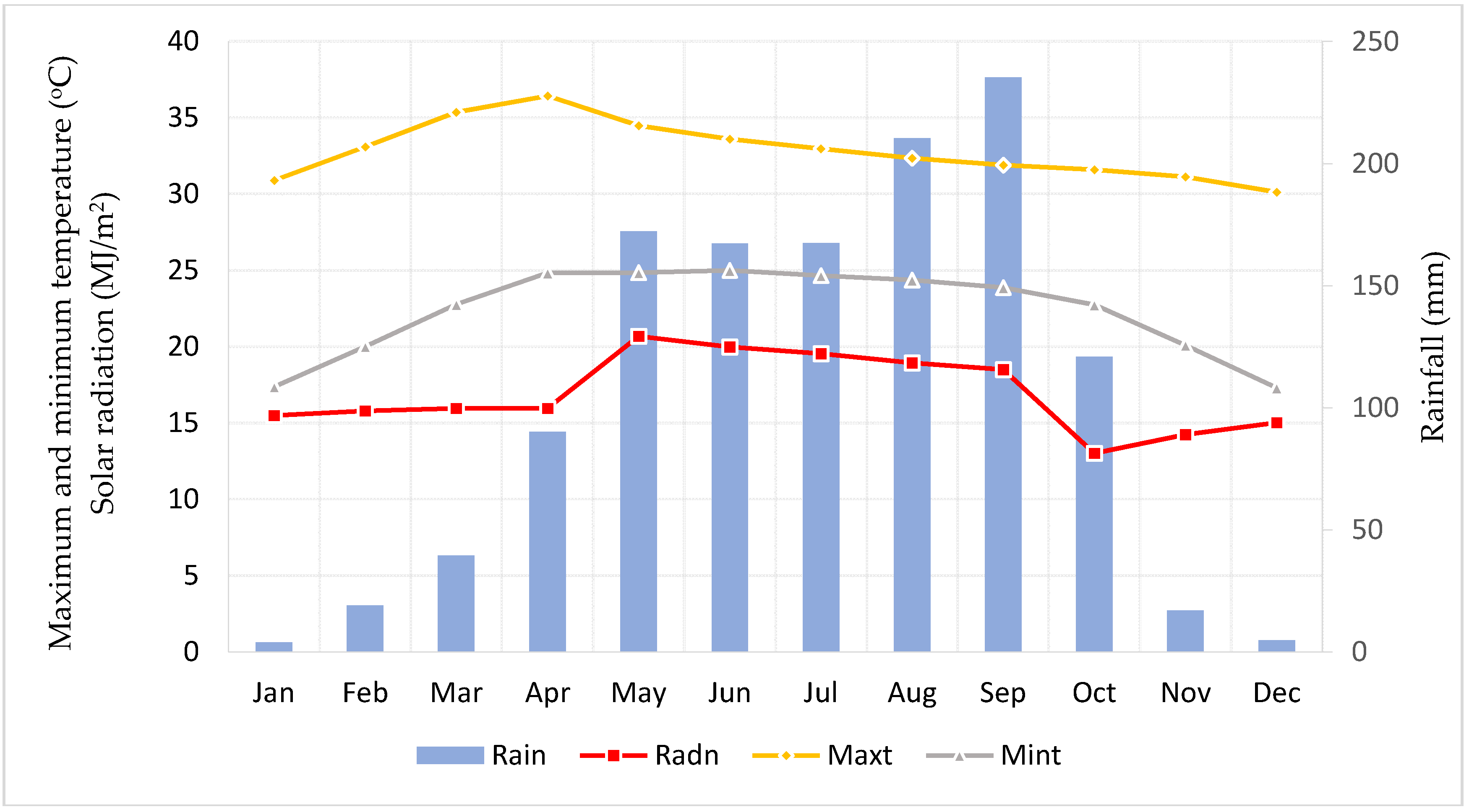
2.1. Study Field
2.2. APSIM Simulation
2.3. Sensitivity Analysis
2.3.1. Preparation of Training Design Points
2.3.2. Gaussian-Process-Based Global Sensitivity Analysis
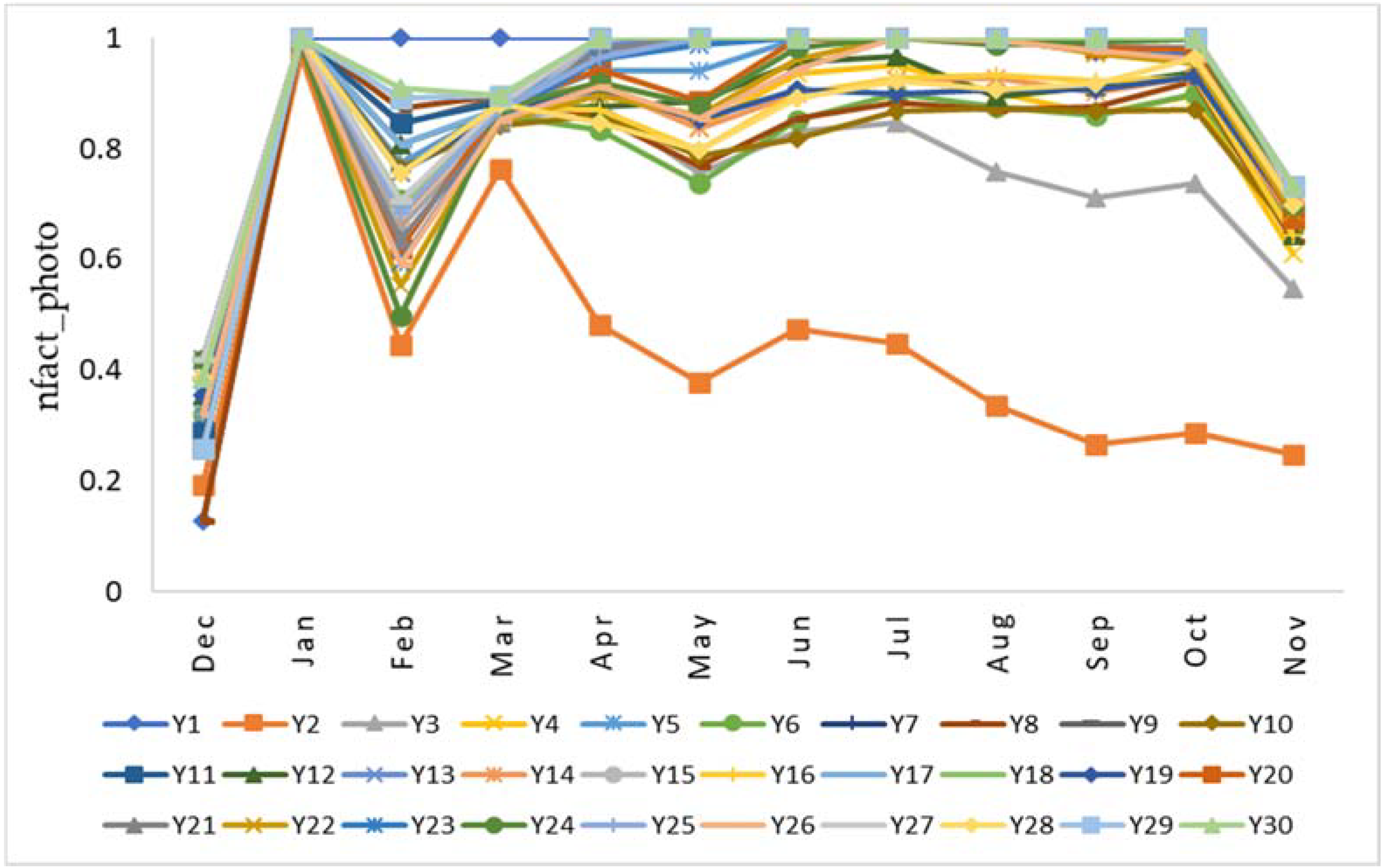
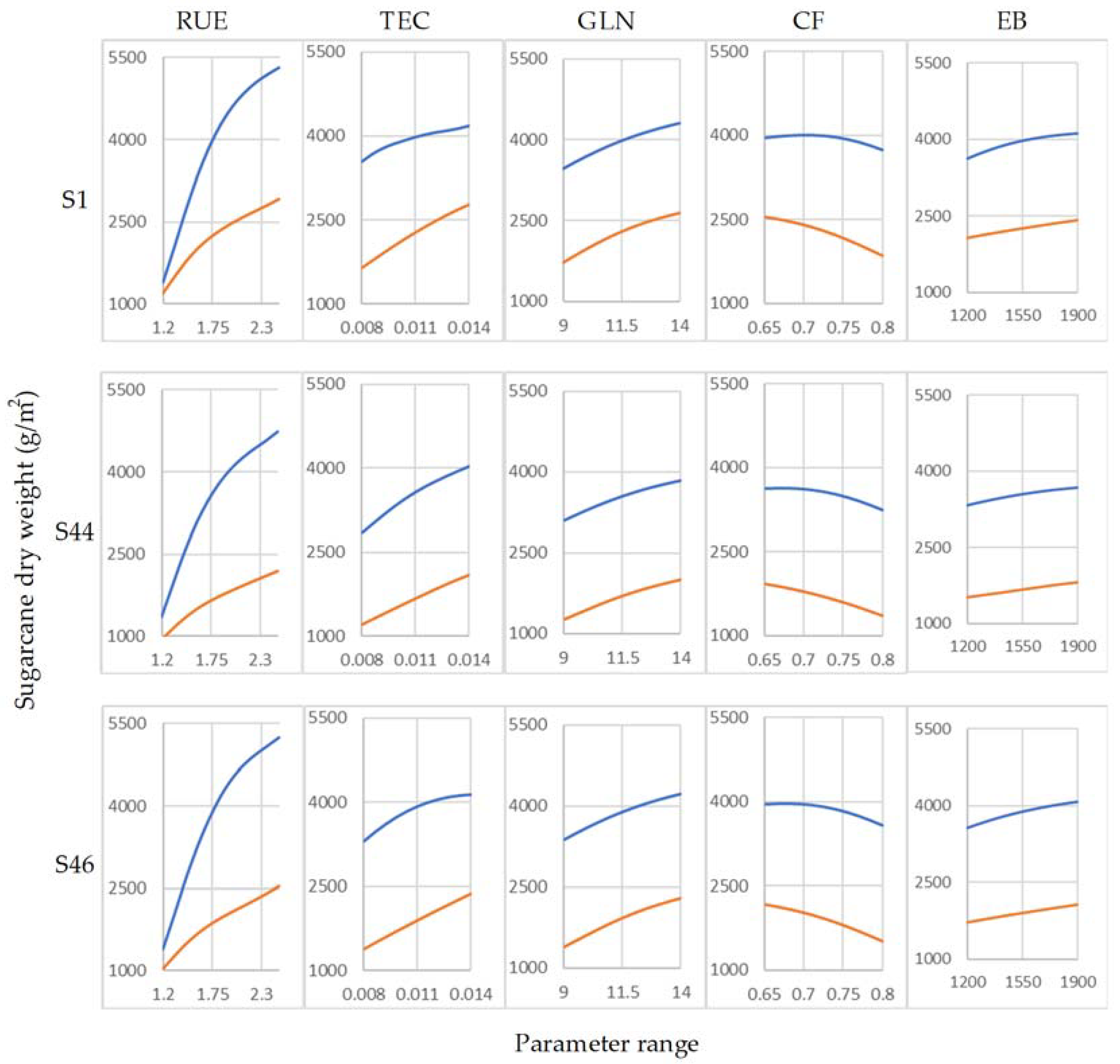
3. Results and Discussion
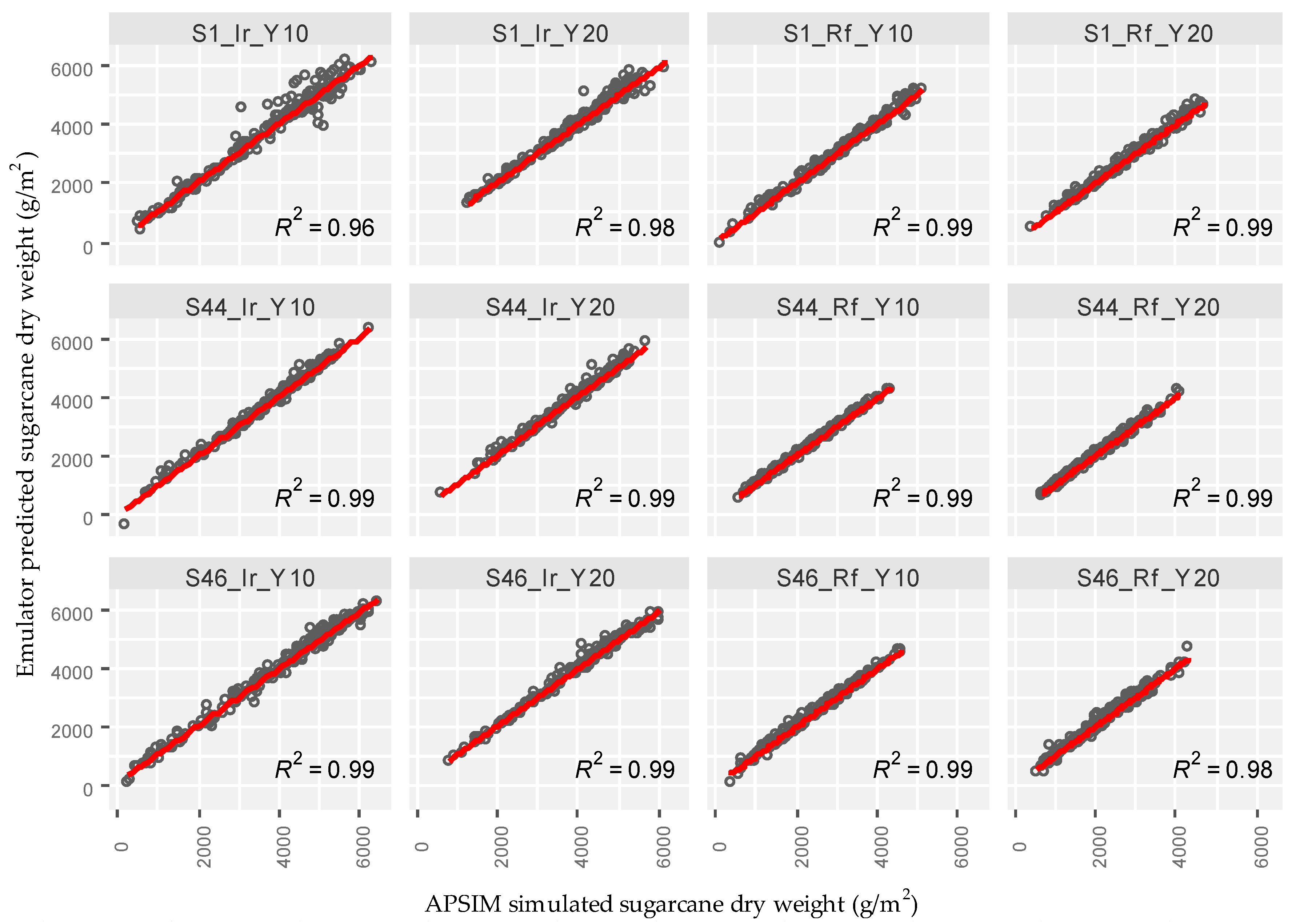
3.1. Emulator Accuracy
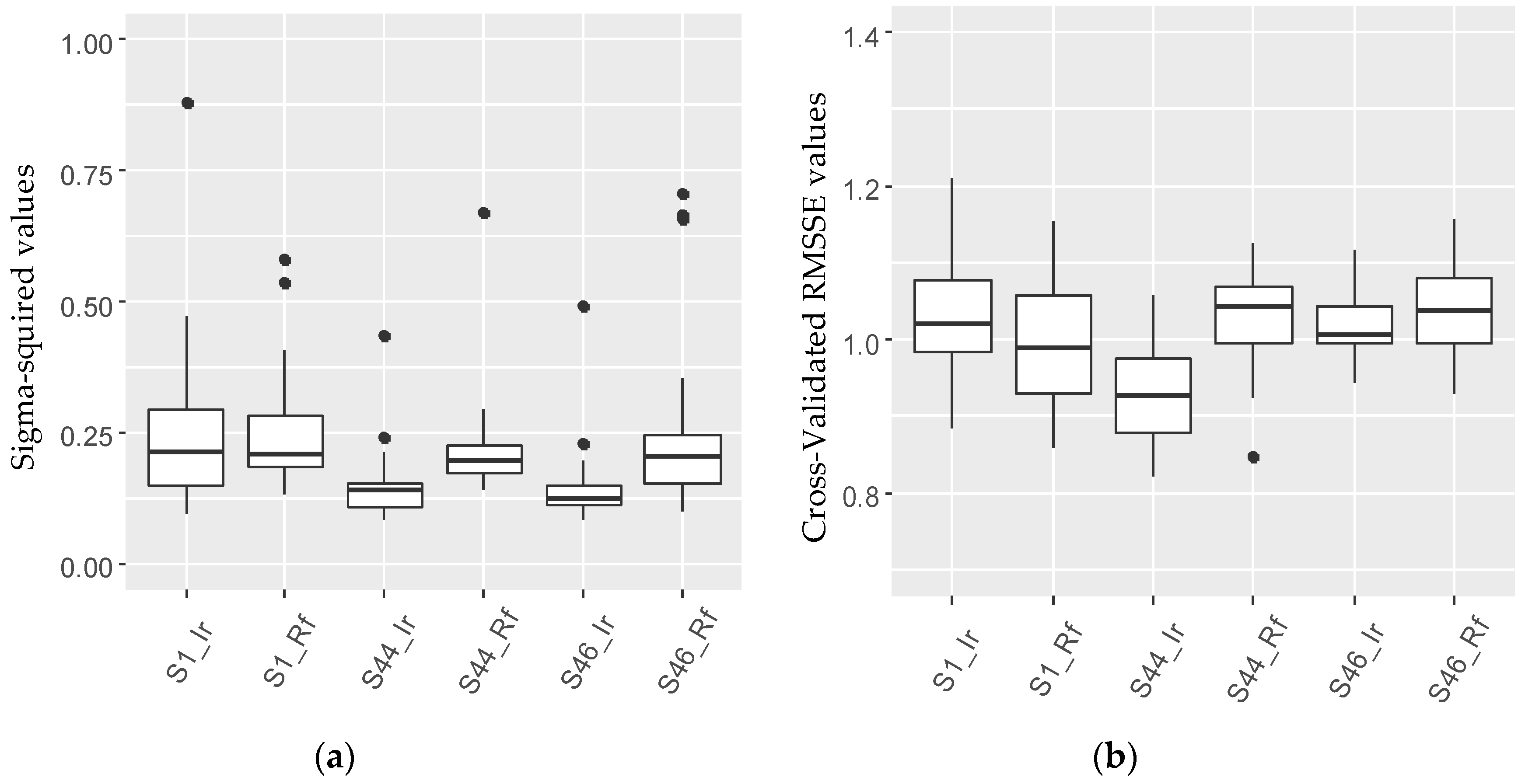
3.2. Determination of Parameter Sensitivity
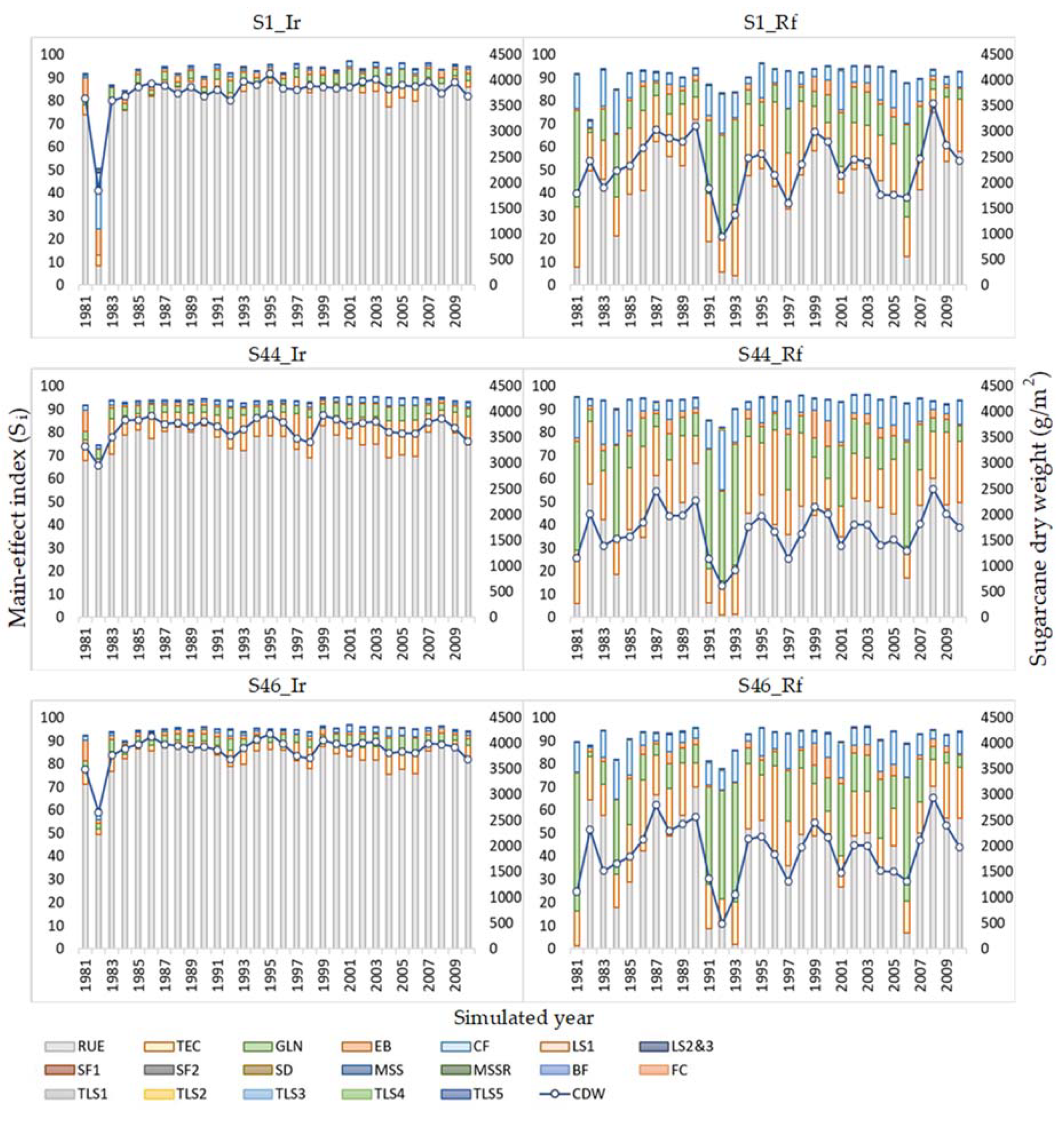
3.3. Sensitivity of Highly Influential Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manivong, P.; Bourgois, E. White Paper: Thai Sugarcane Sector and Sustainability; FairAgora Asia Co. Ltd.: Bangkok, Thailand, 2017. [Google Scholar]
- Hongthong, P.; Patanothai, A. Variations in Sugarcane Yield among Farmers’ Fields and Their Causal Factors in Northeast Thailand. Int. J. Plant Prod. 2017, 11, 533–548. [Google Scholar] [CrossRef]
- Rambo, A.T. The Agrarian Transformation in Northeastern Thailand: A Review of Recent Research. Southeast Asian Stud. 2017, 6, 211–245. [Google Scholar] [CrossRef]
- Preecha, K.; Sakai, K.; Pisanjaroen, K.; Sansayawichai, T.; Cho, T.; Nakamura, S.; Nakandakari, T. Calibration and Validation of Two Crop Models for Estimating Sugarcane Yield in Northeast Thailand. Trop. Agric. Dev. 2016, 60, 31–39. [Google Scholar] [CrossRef]
- Jeuffroy, M.H.; Barbottin, A.; Jones, J.W.; Lecoeur, J. Crop Models with Genotype Parameters. In Working with Crop Models, 1st ed.; Wallach, D., Makowski, D., Jones, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 281–308. [Google Scholar]
- Ojeda, J.J.; Rezaei, E.E.; Remenyi, T.A.; Webb, M.A.; Webber, H.A.; Kamali, B.; Harris, R.M.B.; Brown, J.N.; Kidd, D.B.; Mohammed, C.L.; et al. Effects of Soil and Climate Data Aggregation on Simulated Potato Yield and Irrigation Water Requirement. Sci. Total Environ. 2020, 710, 135589. [Google Scholar] [CrossRef]
- Kennedy, M.C.; O’Hagan, A. Bayesian Calibration of Computer Models. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2001, 63, 425–464. [Google Scholar] [CrossRef]
- Song, X.; Zhan, C.; Kong, F.; Xia, J. Advances in the Study of Uncertainty Quantification of Large-Scale Hydrological Modeling System. J. Geogr. Sci. 2011, 21, 801–819. [Google Scholar] [CrossRef]
- Ewert, F.; van Ittersum, M.K.; Heckelei, T.; Therond, O.; Bezlepkina, I.; Andersen, E. Scale Changes and Model Linking Methods for Integrated Assessment of Agri-Environmental Systems. Agric. Ecosyst. Environ. 2011, 142, 6–17. [Google Scholar] [CrossRef]
- Song, X.M.; Kong, F.Z.; Zhan, C.S.; Han, J.W.; Zhang, X.H. Parameter Identification and Global Sensitivity Analysis of Xin’anjiang Model Using Meta-Modeling Approach. Water Sci. Eng. 2013, 6, 1–17. [Google Scholar] [CrossRef]
- Sexton, J.; Everingham, Y.L.; Inman-Bamber, G. A Global Sensitivity Analysis of Cultivar Trait Parameters in a Sugarcane Growth Model for Contrasting Production Environments in Queensland, Australia. Eur. J. Agron. 2017, 88, 96–105. [Google Scholar] [CrossRef]
- Muñoz-Carpena, R.; Zajac, Z.; Kuo, Y.M. Global Sensitivity and Uncertainty Analyses of the Water Quality Model VFSMOD-W. Trans. ASABE 2007, 50, 1719–1732. [Google Scholar] [CrossRef]
- Cukier, R.I.; Fortuin, C.M.; Shuler, K.E.; Petschek, A.G.; Schaibly, J.H. Study of the Sensitivity of Coupled Reaction Systems to Uncertainties in Rate Coefficients. I Theory. J. Chem. Phys. 1973, 59, 3873–3878. [Google Scholar] [CrossRef]
- Mara, T.A.; Tarantola, S. Application of Global Sensitivity Analysis of Model Output to Building Thermal Simulations. Build. Simul. 2008, 1, 290–302. [Google Scholar] [CrossRef]
- Saltelli, A. Making Best Use of Model Evaluations to Compute Sensitivity Indices. Comput. Phys. Commun. 2002, 145, 280–297. [Google Scholar] [CrossRef]
- Homma, T.; Saltelli, A. Importance Measures in Global Sensitivity Analysis of Nonlinear Models. Reliab. Eng. Syst. Saf. 1996, 52, 1–17. [Google Scholar] [CrossRef]
- Sobol’, I.M. On Sensitivity Estimation for Nonlinear Mathematical Models. Matem. Mod. 1990, 2, 112–118. [Google Scholar]
- Saltelli, A.; Chan, K.; Scott, M. Sensitivity Analysis; Probability and Statistics Series; John Wiley Sons: Chichester, UK, 2000. [Google Scholar]
- Specka, X.; Nendel, C.; Wieland, R. Temporal Sensitivity Analysis of the MONICA Model: Application of Two Global Approaches to Analyze the Dynamics of Parameter Sensitivity. Agriculture 2019, 9, 37. [Google Scholar] [CrossRef]
- O’Hagan, A. Bayesian Analysis of Computer Code Outputs: A Tutorial. Reliab. Eng. Syst. Saf. 2006, 91, 1290–1300. [Google Scholar] [CrossRef]
- Sacks, J.; Welch, W.J.; Mitchell, T.J.; Wynn, H.P. Design and Analysis of Computer Experiments. Stat. Sci. 1989, 4, 409–423. [Google Scholar] [CrossRef]
- Oakley, J.E.; O’Hagan, A. Probabilistic Sensitivity Analysis of Complex Models: A Bayesian Approach. J. R. Stat. Soc. Ser. B Stat. Methodol. 2004, 66, 751–769. [Google Scholar] [CrossRef]
- Uusitalo, L.; Lehikoinen, A.; Helle, I.; Myrberg, K. An Overview of Methods to Evaluate Uncertainty of Deterministic Models in Decision Support. Environ. Model. Softw. 2015, 63, 24–31. [Google Scholar] [CrossRef]
- Boukouvalas, A.; Cornford, D.; Maniyar, D.; Singer, A. Gaussian Process Emulation of Stochastic Models: Developments and Application to Rabies Modelling. In Proceedings of the RSS 2008 Conference, Nottingham, UK, 1–5 September 2008. [Google Scholar]
- Rasmussen, C.E.; Williams, C.K.I. Gaussian Processes for Machine Learning Cambridge; MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Sexton, J.; Everingham, Y. Global Sensitivity Analysis of Key Parameters in A Process-Based Sugarcane Growth Model—A Bayesian Approach. In Proceedings of the 7th International Congress on Environmental Modelling and Software, San Diego, CA, USA, 15–19 June 2014. [Google Scholar]
- Gunarathna, M.H.J.P.; Sakai, K.; Nakandakari, T.; Momii, K.; Kumari, M.K.N. Sensitivity Analysis of Plant and Cultivar-Specific Parameters of APSIM-Sugar Model: Variation between Climates and Management Conditions. Agronomy 2019, 9, 242. [Google Scholar] [CrossRef]
- Khon Kaen Climate. Available online: https://en.climate-data.org/asia/thailand/khon-kaen-province/khon-kaen-4291/ (accessed on 19 November 2019).
- USDA. Soil Texture Calculator. Available online: https://www.nrcs.usda.gov (accessed on 15 November 2019).
- Holzworth, D.P.; Huth, N.I.; de Voil, P.G.; Zurcher, E.J.; Herrmann, N.I.; McLean, G.; Chenu, K.; van Oosterom, E.J.; Snow, V.; Murphy, C.; et al. APSIM–evolution towards a new generation of agricultural systems simulation. Environ. Model. Softw. 2014, 62, 327–350. [Google Scholar] [CrossRef]
- Wang, E.; Robertson, M.J.; Hammer, G.L.; Carberry, P.S.; Holzworth, D.; Meinke, H.; Chapman, S.C.; Hargreaves, J.N.G.; Huth, N.I.; McLean, G. Development of a Generic Crop Model Template in the Cropping System Model APSIM. Eur. J. Agron. 2002, 18, 121–140. [Google Scholar] [CrossRef]
- Ojeda, J.J.; Pembleton, K.G.; Caviglia, O.P.; Islam, M.R.; Agnusdei, M.G.; Garcia, S.C. Modelling Forage Yield and Water Productivity of Continuous Crop Sequences in the Argentinian Pampas. Eur. J. Agron. 2018, 92, 84–96. [Google Scholar] [CrossRef]
- Keating, B.A.; Robertson, M.J.; Muchow, R.C.; Huth, N.I. Modelling sugarcane production systems. I. Description and validation of the sugarcane module. F. Crop. Res. 1999, 61, 253–271. [Google Scholar] [CrossRef]
- Dias, H.B.; Inman-Bamber, G.; Bermejo, R.; Sentelhas, P.C.; Christodoulou, D. New APSIM-Sugar Features and Parameters Required to Account for High Sugarcane Yields in Tropical Environments. F. Crop. Res. 2019, 235, 38–53. [Google Scholar] [CrossRef]
- Sexton, J.; Everingham, Y.; Inman-Bamber, G. A Theoretical and Real-World Evaluation of Two Bayesian Techniques for the Calibration of Variety Parameters in a Sugarcane Crop Model. Environ. Model. Softw. 2016, 83, 126–142. [Google Scholar] [CrossRef]
- Keating, B. The APSIM Sugar Model. Available online: http://apsrunet.apsim.info/svn/development/trunk/apsim/sugar/docs/sugar_pseudo.html#sugar_dm_partition_pot (accessed on 23 November 2019).
- Stanfill, B. Apsimr: Edit, Run and Evaluate APSIM Simulations Easily Using R. Available online: https://cran.r-project.org/web/packages/apsimr/index.html (accessed on 23 November 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 25 November 2019).
- Sinclair, T.R. Is Transpiration Efficiency a Viable Plant Trait in Breeding for Crop Improvement? Funct. Plant Biol. 2012, 39, 359–365. [Google Scholar] [CrossRef]
- Jackson, P.A.; Basnayake, J.; Inman-Bamber, G.; Lakshmanan, P. Selecting Sugarcane Varieties with Higher Transpiration Efficiency. In Proceedings of the Australian Society of Sugar Cane Technologists, Broadbeach, Australia, 28 April–1 May 2014; Volume 36. [Google Scholar]
- Park, S.E.; Robertson, M.; Inman-Bamber, N.G. Decline in the Growth of a Sugarcane Crop with Age under High Input Conditions. F. Crop. Res. 2005, 92, 305–320. [Google Scholar] [CrossRef]
- Ferreira, R.A.; de Souza, J.L.; Lyra, G.B.; Escobedo, J.F.; Santos, M.V.C. Energy Conversion Efficiency in Sugarcane under Two Row Spacings in Northeast of Brazil. Rev. Bras. Eng. Agrícola e Ambient. 2015, 19, 741–747. [Google Scholar] [CrossRef]
- Olivier, F.C.; Singels, A.; Eksteen, A.B. Water and Radiation Use Efficiency of Sugarcane for Bioethanol Production in South Africa, Benchmarked against Other Selected Crops. S. Afr. J. Plant Soil 2016, 33, 1–11. [Google Scholar] [CrossRef]
- Meki, M.N.; Kiniry, J.R.; Youkhana, A.H.; Crow, S.E.; Ogoshi, R.M.; Nakahata, M.H.; Tirado-Corbalá, R.; Anderson, R.G.; Osorio, J.; Jeong, J. Two-Year Growth Cycle Sugarcane Crop Parameter Attributes and Their Application in Modeling. Agron. J. 2015, 107, 1310–1320. [Google Scholar] [CrossRef]
- Villa-Vialaneix, N.; Follador, M.; Ratto, M.; Leip, A. A Comparison of Eight Metamodeling Techniques for the Simulation of N2O Fluxes and N Leaching from Corn Crops. Environ. Model. Softw. 2012, 34, 51–66. [Google Scholar] [CrossRef]
- O’Hagan, A. Probabilistic Uncertainty Specification: Overview, Elaboration Techniques and Their Application to a Mechanistic Model of Carbon Flux. Environ. Model. Softw. 2012, 36, 35–48. [Google Scholar] [CrossRef]
- Kennedy, M.C.; Anderson, C.W.; Conti, S.; O’Hagan, A. Case Studies in Gaussian Process Modelling of Computer Codes. Reliab. Eng. Syst. Saf. 2006, 91, 1301–1309. [Google Scholar] [CrossRef]
- Kennedy, M.C.; Petropoulos, G.P. GEM-SA: The Gaussian Emulation Machine for Sensitivity Analysis. In Sensitivity Analysis in Earth Observation Modelling; George, P.P., Prashant, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 341–361. ISBN 978-0-12-803011-0. [Google Scholar]
- Qin, X.; Wang, H.; Li, Y.; Li, Y.; McConkey, B.; Lemke, R.; Li, C.; Brandt, K.; Gao, Q.; Wan, Y.; et al. A Long-Term Sensitivity Analysis of the Denitrification and Decomposition Model. Environ. Model. Softw. 2013, 43, 26–36. [Google Scholar] [CrossRef]
- Sexton, J. Bayesian Statistical Calibration of Variety Parameters in Asugarcane Crop Model. Master’s Thesis, James Cook University, Townsville, Australia, April 2015. [Google Scholar]
- Petropoulos, G.; Wooster, M.J.; Carlson, T.N.; Kennedy, M.C.; Scholze, M. A Global Bayesian Sensitivity Analysis of the 1d SimSphere Soil-Vegetation-Atmospheric Transfer (SVAT) Model Using Gaussian Model Emulation. Ecol. Modell. 2009, 220, 2427–2440. [Google Scholar] [CrossRef]
- Ojeda, J.J.; Volenec, J.J.; Brouder, S.M.; Caviglia, O.P.; Agnusdei, M.G. Evaluation of Agricultural Production Systems Simulator as Yield Predictor of Panicum Virgatum and Miscanthus x Giganteus in Several US Environments. GCB Bioenergy 2017, 9, 796–816. [Google Scholar] [CrossRef]
- Smit, M.A.; Singels, A.; van Antwerpen, A. Differences in Canopy Development of Two Sugarcane Cultivars under Conditions of Water Stress: Preliminary Results. Proc. S. Afr. Sugar Technol. Assoc. 2004, 78, 149–152. [Google Scholar]
- Ojeda, J.J.; Pembleton, K.G.; Islam, M.R.; Agnusdei, M.G.; Garcia, S.C. Evaluation of the Agricultural Production Systems Simulator Simulating Lucerne and Annual Ryegrass Dry Matter Yield in the Argentine Pampas and South-Eastern Australia. Agric. Syst. 2016, 143, 61–75. [Google Scholar] [CrossRef]
| Soil Group | Soil Depth (cm) | Texture Class * | Wilting Point (mm/mm) | Field Capacity (mm/mm) | Hydraulic Conductivity (cm/h) | Bulk Density (g/cm3) | Clay % | Silt % | Sand % | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0–100 | Clay soil | 0.328 | 0.461 | 0.06 | 1.44 | 68.0 | 29.0 | 3 | 5.4 |
| S44 | 0–100 | Sandy soil | 0.038 | 0.120 | 13.34 | 1.7 | 1 | 9.5 | 89.5 | 5.6 |
| S46 | 0–100 | Clay loam | 0.133 | 0.231 | 0.36 | 1.52 | 29.2 | 29 | 41.8 | 5.1 |
| Criteria | Value |
|---|---|
| Planting date | November 28 |
| Crop duration | 360 days |
| Stalk density | 6.8 stalks/m2 |
| Planting depth | 100 mm |
| Fertilizer application | |
| Fertilization at planting | Urea_N—46.75 kg/ha |
| Fertilization at 100 days after planting | Urea_N—46.75 kg/ha |
| Water supply by irrigation * | |
| Rainfed condition (Rf) | 24 mm of irrigation at 7, 14, 21 and 28 days after planting a |
| Irrigation condition (Ir) | 24 mm of irrigation with 7 days’ time interval from planting to end date of the simulation b |
| Parameter Name | Description | Level | Code | Units | Range |
|---|---|---|---|---|---|
| leaf_size | Leaf area of the respective leaf | leaf_size_no = 1 | LS1 | mm2 | 500–2000 |
| leaf_size_no = 14 and 20 | LS2&3 | mm2 | 25,000–70,000 | ||
| cane_fraction | Fraction of accumulated biomass partitioned to cane | CF | g/g | 0.65–0.80 | |
| sucrose_fraction_stalk | Fraction of accumulated biomass partitioned to sucrose | SF1 | g/g | 0.50–0.70 | |
| stress_factor_stalk | Stress factor for sucrose accumulation | SF2 | n/a | 0.2–1.0 | |
| sucrose_delay | Sucrose accumulation delay | SD | g/m2 | 0–600 | |
| min_sstem_sucrose | Minimum stem biomass before partitioning to sucrose commences | MSS | g/m2 | 450–1500 | |
| min_sstem_sucrose_redn | Reduction to minimum stem sucrose under stress | MSSR | g/m2 | 0–20 | |
| tt_emerg_to_begcane | Accumulated thermal time from emergence to beginning of cane | EB | °C day | 1200–1900 | |
| tt_begcane_to_flowering | Accumulated thermal time from beginning of cane to flowering | BF | °C day | 5500–6500 | |
| tt_flowering_to_crop_end | Accumulated thermal time from flowering to end of the crop | FC | °C day | 1750–2250 | |
| green_leaf_no | Maximum number of fully expanded green leaves | GLN | No. | 9–14 | |
| tillerf_leaf_size | Tillering factors according to the leaf numbers | Tiller_leaf_size_no = 1 | TLS1 | mm2/mm2 | 1–6 |
| Tiller_leaf_size_no = 4 | TLS2 | mm2/mm2 | 1–6 | ||
| Tiller_leaf_size_no = 10 | TLS3 | mm2/mm2 | 1–6 | ||
| Tiller_leaf_size_no = 16 | TLS4 | mm2/mm2 | 1–6 | ||
| Tiller_leaf_size_no = 26 | TLS5 | mm2/mm2 | 1–6 | ||
| transp_eff_cf | Transpiration efficiency coefficient | TEC | kg kPa/kg | 0.008–0.014 | |
| rue | Radiation use efficiency | RUE | g/MJ | 1.2–2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandara, W.B.M.A.C.; Sakai, K.; Nakandakari, T.; Kapetch, P.; Rathnappriya, R.H.K. A Gaussian-Process-Based Global Sensitivity Analysis of Cultivar Trait Parameters in APSIM-Sugar Model: Special Reference to Environmental and Management Conditions in Thailand. Agronomy 2020, 10, 984. https://doi.org/10.3390/agronomy10070984
Bandara WBMAC, Sakai K, Nakandakari T, Kapetch P, Rathnappriya RHK. A Gaussian-Process-Based Global Sensitivity Analysis of Cultivar Trait Parameters in APSIM-Sugar Model: Special Reference to Environmental and Management Conditions in Thailand. Agronomy. 2020; 10(7):984. https://doi.org/10.3390/agronomy10070984
Chicago/Turabian StyleBandara, W. B. M. A. C., Kazuhito Sakai, Tamotsu Nakandakari, Preecha Kapetch, and R. H. K. Rathnappriya. 2020. "A Gaussian-Process-Based Global Sensitivity Analysis of Cultivar Trait Parameters in APSIM-Sugar Model: Special Reference to Environmental and Management Conditions in Thailand" Agronomy 10, no. 7: 984. https://doi.org/10.3390/agronomy10070984
APA StyleBandara, W. B. M. A. C., Sakai, K., Nakandakari, T., Kapetch, P., & Rathnappriya, R. H. K. (2020). A Gaussian-Process-Based Global Sensitivity Analysis of Cultivar Trait Parameters in APSIM-Sugar Model: Special Reference to Environmental and Management Conditions in Thailand. Agronomy, 10(7), 984. https://doi.org/10.3390/agronomy10070984







