Effect of Reduced Nitrogen and Supplemented Amino Acids Nutrient Solution on the Nutritional Quality of Baby Green and Red Lettuce Grown in a Floating System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Nutritional Quality Assessment
2.3. Statistical Analysis
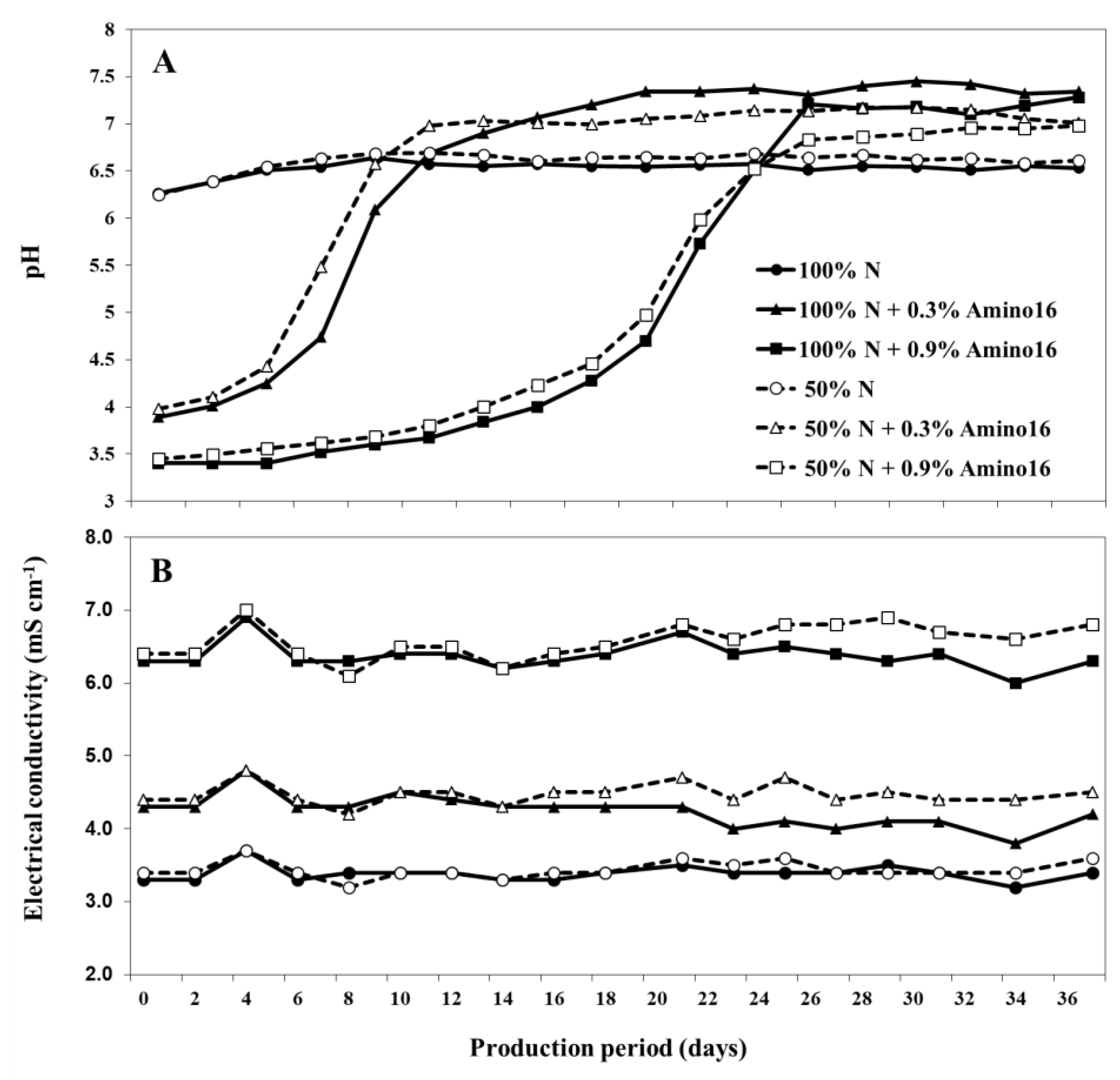
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Broadley, M.R.; Seginer, I.; Burns, A.; Escobar-Gutiérrez, A.J.; Burns, I.G.; White, P.J. The nitrogen and nitrate economy of butterhead lettuce (Lactuca sativa var. capitata L.). J. Exp. Bot. 2003, 54, 2081–2090. [Google Scholar] [CrossRef] [Green Version]
- Salomez, J.; Hofman, G. Nitrogen nutrition effects on nitrate accumulation of soil-grown greenhouse butterhead lettuce. Commun. Soil Sci. Plant Anal. 2009, 40, 620–632. [Google Scholar] [CrossRef]
- Montemurro, F. Are organic fertilizing strategies able to improve lettuce yield, use of nitrogen and N statues? J. Plant Nutr. 2010, 33, 1980–1997. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Liu, W.K.; Yang, Q.C.; Qiu, Z.P. Spatiotemporal changes of nitrate and Vc contents in hydroponic lettuce treated with various nitrogen-free solutions. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 286–290. [Google Scholar] [CrossRef]
- Gonnella, M.; Serio, F.; Conversa, G.; Santamaria, P. Yield and quality of lettuce grown in floating system using different sowing density and plant spatial arrangements. Acta Hortic. 2003, 614, 687–692. [Google Scholar] [CrossRef]
- Zanin, G.; Ponchia, G.; Sambo, P. Yield and quality of vegetables grown in a floating system for ready-to-eat produce. Acta Hortic. 2009, 807, 433–438. [Google Scholar] [CrossRef]
- Rodríguez-Hidalgo, S.; Artés-Hernández, F.; Gómez, P.A.; Fernández, J.A.; Artés, F. Quality of fresh-cut baby spinach grown under a floating trays system as affected by nitrogen fertilisation and innovative packaging. J. Sci. Food Agric. 2010, 90, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.E.; Stivers, L.J.; Warden, B.T.; Tanji, K.K. Crop nitrogen utilization and soil nitrate loss in a lettuce field. Fertil. Sci. Res. 1994, 37, 93–105. [Google Scholar] [CrossRef]
- Owen, A.G.; Jones, D.L. Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol. Biochem. 2001, 33, 651–657. [Google Scholar] [CrossRef]
- Blom-zandstra, M. Nitrate accumulation in vegetables and its relationship to quality. Ann. Appl. Biol. 1989, 115, 553–561. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Ferrante, A.; Magnani, G. Application of biostimulants in floating system for improving rocket quality. J. Food Agric. Environ. 2005, 33, 86–88. [Google Scholar]
- Liu, X.-Q.; Ko, K.-Y.; Kim, S.-H.; Lee, K. Effect of amino acid fertilization on nitrate assimilation of leafy radish and soil chemical properties in high nitrate soil. Commun. Soil Sci. Plant Anal. 2008, 39, 269–281. [Google Scholar] [CrossRef]
- Padgett, P.E.; Leonard, R.T. Regulation of nitrate uptake by amino acids in maize cell suspension culture and intact roots. Plant Soil 1993, 155, 159–161. [Google Scholar] [CrossRef]
- Pôrto, M.L.; Alves, J.D.C.; De Souza, A.P.; Araújo, R.D.C.; De Arruda, J.A. Nitrate production and accumulation in lettuce as affected by mineral nitrogen supply and organic fertilization. Hortic. Bras. 2008, 26, 227–230. [Google Scholar] [CrossRef]
- Maynard, D.N.; Barker, A.V.; Minotti, P.L.; Peck, N.H. Nitrate accumulation in vegetables. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press Inc.: Cambridge, MA, USA, 1976; pp. 71–118. [Google Scholar]
- Santamaria, P.; Elia, A.; Gonnella, M. Changes in nitrate accumulation and growth of endive plants during light period as affected by nitrogen level and form 1. J. Plant Nutr. 1997, 20, 1255–1266. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Li, S.-X.; Malhi, S. Effects of fertilization and other agronomic measures on nutritional quality of crops. J. Sci. Food Agric. 2008, 88, 7–23. [Google Scholar] [CrossRef]
- Elwan, M.W.M.; Abd El-Hamed, K.E. Influence of nitrogen form, growing season and sulfur fertilization on yield and the content of nitrate and vitamin C of broccoli. Sci. Hortic. 2011, 127, 181–187. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Gent, M.P.N. Solution electrical conductivity and ratio of nitrate to other nutrients affect accumulation of nitrate in hydroponic lettuce. HortScience 2003, 38, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Burns, I.G.; Zhang, K.; Turner, M.K.; Meacham, M.; Al-Redhiman, K.; Lynn, J.; Broadley, M.R.; Hand, P.; Pink, D. Screening for genotype and environment effects on nitrate accumulation in 24 species of young lettuce. J. Sci. Food Agric. 2011, 91, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Gutiérrez, A.J.; Burns, I.G.; Lee, A.; Edmondson, R.N. Screening lettuce cultivars for low nitrate content during summer and winter production. J. Hortic. Sci. Biotechnol. 2002, 77, 232–237. [Google Scholar] [CrossRef]
- Burns, I.G.; Durnford, J.; Lynn, J.; Mcclement, S.; Hand, P.; Pink, D. The influence of genetic variation and nitrogen source on nitrate accumulation and iso-osmotic regulation by lettuce. Plant Soil 2012, 352, 321–339. [Google Scholar] [CrossRef]
- Eppendorfer, W.H. Free and total amino acid composition of edible parts of beans, kale, spinach, cauliflower and potatoes as influenced by nitrogen fertilisation and phosphorus and potassium deficiency. J. Sci. Food Agric. 1996, 71, 449–458. [Google Scholar] [CrossRef]
- Fontes, P.C.R.; Pereira, P.R.G.; Conde, R.M. Critical chlorophyll, total nitrogen, and nitrate-nitrogen in leaves associated to maximum lettuce yield. J. Plant Nutr. 1997, 20, 1061–1068. [Google Scholar] [CrossRef]
- Abu-Rayyan, A.; Kharawish, B.H.; Al-Ismail, K. Nitrate content in lettuce (Lactuca sativa L) heads in relation to plant spacing, nitrogen form and irrigation level. J. Sci. Food Agric. 2004, 84, 931–936. [Google Scholar] [CrossRef]
- Burns Ian, G.; Kefeng, Z.; Mary, K.T.; James, L.; Sandy, M.C.; Paul, H.; David, P. Genotype and environment effects on nitrate accumulation in a diversity set of lettuce accessions at commercial maturity: The influence of nitrate uptake and assimilation, osmotic interactions and shoot weight and development. J. Sci. Food Agric. 2011, 91, 2217–2233. [Google Scholar] [CrossRef] [Green Version]
- Ferentinos, K.P.; Albright, L.D.; Ramani, D.V. Optimal light integral and carbon dioxide concentration combinations for lettuce in ventilated greenhouses. J. Agric. Eng. Res. 2000, 77, 309–315. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Koukounaras, A.; Siomos, A.S. Application of amino acids improves lettuce crop uniformity and inhibits nitrate accumulation induced by the supplemental inorganic nitrogen fertilization. Int. J. Agric. Biol. 2014, 16, 951–955. [Google Scholar]
- Santamaria, P.; Elia, A. Producing nitrate-free endive heads: Effect of nitrogen form on growth, yield, and ion composition of endive. J. Am. Soc. Hortic. Sci. 1997, 122, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Quattrini, E.; Penati, M.; Alberici, A.; Martinetti, L.; Marino Gallina, P.; Ferrante, A.; Schiavi, M. Effect of the reduction of nutrient solution concentration on leafy vegetables quality grown in floating system. Acta Hortic. 2008, 801, 1167–1176. [Google Scholar]
- Konstantopoulou, E.; Kapotis, G.; Salachas, G.; Petropoulos, S.A.; Karapanos, I.C.; Passam, H.C. Nutritional quality of greenhouse lettuce at harvest and after storage in relation to N application and cultivation season. Sci. Hortic. 2010, 125, 93.e1–93.e5. [Google Scholar] [CrossRef]
- Konstantopoulou, E.; Kapotis, G.; Salachas, G.; Petropoulos, S.A.; Chatzieustratiou, E.; Karapanos, I.C.; Passam, H.C. Effect of nitrogen application on growth parameters, yield and leaf nitrate content of greenhouse lettuce cultivated during three seasons. J. Plant Nutr. 2012, 35, 1246–1254. [Google Scholar] [CrossRef]
- Gent, M.P.N. Rate of change of composition of lettuce in response to nitrogen depletion or re-supply. J. Sci. Food Agric. 2012, 92, 3007–3015. [Google Scholar] [CrossRef]
- Stefanelli, D.; Brady, S.; Winkler, S.; Jones, R.B.; Tomkins, B.T. Lettuce (Lactuca sativa L.) growth and quality response to applied nitrogen under hydroponic conditions. Acta Hortic. 2012, 353–359. [Google Scholar] [CrossRef]
- Fischer, W.N.; André, B.; Rentsch, D.; Krolkiewicz, S.; Tegeder, M.; Breitkreuz, K.; Frommer, W.B. Amino acid transport in plants. Trends Plant Sci. 1998, 3, 188–195. [Google Scholar] [CrossRef]
- El-Naggar, A.; El-Araby, A.; De Neergaard, A.; Høgh-Jensen, H. Crop responses to 15N-labelled organic and inorganic nitrogen sources. Nutr. Cycl. Agroecosyst. 2008, 80, 49–60. [Google Scholar] [CrossRef]
- El-Naggar, A.; De Neergaard, A.; El-Araby, A.; Høgh-Jensen, H. Simultaneous uptake of multiple amino acids by Wheat. J. Plant Nutr. 2009, 32, 725–740. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Ni, Q.; Seung, L.K. Evaluation of the role of mixed amino acids in nitrate uptake andassimilation in leafy radish by using 15 N-labeled nitrate. Agric. Sci. China 2008, 7, 1196–1202. [Google Scholar] [CrossRef]
- Güneş, A.; İnal, A.; Aktaş, M. Reducing nitrate content of NFT grown winter onion plants (Allium cepa L.) by partial replacement of NO3 with amino acid in nutrient solution. Sci. Hortic. 1996, 65, 203–208. [Google Scholar] [CrossRef]
- Gunes, A.; Post, W.H.K.; Kirkby, E.A.; Aktas, M. Influence of partial replacement on nitrate by amino acid nitrogen or urea in the nutrient medium on nitrate accumulation in NFT grown winter lettuce. J. Plant Nutr. 1994, 17, 1929–1938. [Google Scholar] [CrossRef]
- Aslam, M.; Travis, R.L.; Rains, D.W. Differential effect of amino acids on nitrate uptake and reduction systems in barley roots. Plant Sci. 2001, 160, 219–228. [Google Scholar] [CrossRef]
- Wang, H.-J.; Wu, L.-H.; Wang, M.-Y.; Zhu, Y.-H.; Tao, Q.-N.; Zhang, F.-S. Effects of amino acids replacing nitrate on growth, nitrate accumulation, and macroelement concentrations in pak-choi (Brassica chinensis L.). Pedosphere 2007, 17, 595–600. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- European Patent Office. European Patent Application No. 11386012.6. of Aminoacids of Plant Origin and Method of Production. Patent No. EP 2 537 823 A1, 26 December 2012.
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood: Comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. Extraction and Determination of Total Anthocyanin in Cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Troll, W.; Lindsley, J. A photometric method for the determination of proline. J. Biol. Chem. 1955, 215, 655–660. [Google Scholar] [PubMed]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis Part 3 Chemical Methods; Helmke, P.A., Loeppert, R.H., Eds.; SSSA Book Ser 53; SSSA: Madison, WI, USA; ASA: Schaumburg, IL, USA, 1996; pp. 1085–1122. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. Methods of Soil Analysis Part 2 Chemical and Microbiological Properties—Agronomy Monograph No. 9, 2nd ed.; Page, A.L., Ed.; ASA: Schaumburg, IL, USA; SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Scuderi, D.; Giuffrida, F.; Noto, G. Effects of nutrient solution EC on yield, quality andshelf-life of lettuce grown in floating system. Acta Hortic. 2009, 807, 221–226. [Google Scholar] [CrossRef]
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby Romaine lettuce (Lactuca sativa L. cv. Duende). Food Chem. 2010, 119, 1502–1506. [Google Scholar] [CrossRef]
- Scuderi, D.; Restuccia, C.; Chisari, M.; Barbagallo, R.N.; Caggia, C.; Giuffrida, F. Salinity of nutrient solution influences the shelf-life of fresh-cut lettuce grown in floating system. Postharvest Biol. Technol. 2011, 59, 132–137. [Google Scholar] [CrossRef]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Nutritional, biophysical and physiological characteristics of wild rocket genotypes as affected by soilless cultivation system, salinity level of nutrient solution and growing period. Front. Plant Sci. 2017, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Vidmar, J.J.; Zhuo, D.; Siddiqi, M.Y.; Schjoerring, J.K.; Touraine, B.; Glass, A.D.M. Regulation of high-affinity nitrate transporter genes and high-affinity nitrateinflux by nitrogen pools in roots of barley. Plant Physiol. 2000, 123, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
| Nitrogen (%) | Amino16 (%) | Plant Weight (g) | Root Length (cm) | ||
|---|---|---|---|---|---|
| Source of variation | p | % TV s | p | % TV | |
| (N) Nitrogen v | *** | 34 | |||
| (A) Amino16 u | *** r | 85 | *** | 45 | |
| N × A | *** | 20 | |||
| 100 | 13.38 z | 1.51 b | |||
| 50 | 12.85 | 3.70 a | |||
| 0 | 14.61 a y | 3.68 a | |||
| 0.3 | 13.80 a | 4.15 a | |||
| 0.9 | 10.95 b | 0.00 b | |||
| 100 | 0 | 14.55 | 3.14 c | ||
| 0.3 | 14.41 | 1.41 d | |||
| 0.9 | 11.19 | 0.00 e | |||
| 50 | 0 | 14.67 | 4.22 b | ||
| 0.3 | 13.19 | 6.89 a | |||
| 0.9 | 10.70 | 0.00 e | |||
| Nitrogen (%) | Amino16 (%) | Dry Matter (%) | Nitrates (mg/kg FW) | SSC (%) | Phenols (μg GAE/g FW) | Antioxidant Capacity (mg AEAC/100 g FW) | Chlorophyll (μg/g FW) | Carotenoids (μg/g FW) | Prolines (mM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | p | % TV s | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | |
| Nitrogen (N) v | *** r | 47 | *** | 71 | *** | 82 | |||||||||||
| Amino16 (A16) u | *** | 35 | *** | 80 | * | 64 | *** | 88 | |||||||||
| N × A16 | *** | 17 | *** | 58 | * | 20 | |||||||||||
| 100 | 4.63 z | 469 a y | 3.02 | 0.050 b | 2.91 b | 304 | 67.7 | 0.00370 | |||||||||
| 50 | 4.75 | 269 b | 3.14 | 0.064 a | 4.93 a | 292 | 65.7 | 0.00394 | |||||||||
| 0 | 4.75 | 519 a | 3.06 | 0.058 | 4.13 | 258 b | 62.1 b | 0.00280 b | |||||||||
| 0.3 | 4.60 | 233 c | 2.97 | 0.054 | 3.83 | 308 a | 67.1 b | 0.00335 b | |||||||||
| 0.9 | 4.71 | 355 b | 3.22 | 0.060 | 3.80 | 328 a | 70.8 a | 0.00531 a | |||||||||
| 100 | 0 | 4.69 | 729 a | 2.78 b | 0.044 c | 2.49 | 256 | 61.0 | 0.00298 | ||||||||
| 0.3 | 4.72 | 320 b | 3.10 ab | 0.052 bc | 3.42 | 314 | 68.6 | 0.00321 | |||||||||
| 0.9 | 4.47 | 358 b | 3.17 ab | 0.053 bc | 2.84 | 343 | 73.3 | 0.00491 | |||||||||
| 50 | 0 | 4.81 | 308 b | 3.33 a | 0.072 a | 5.78 | 261 | 63.2 | 0.00262 | ||||||||
| 0.3 | 4.48 | 147 c | 2.83 b | 0.055 bc | 4.25 | 303 | 65.6 | 0.00349 | |||||||||
| 0.9 | 4.96 | 352 b | 3.27 a | 0.066 ab | 4.77 | 313 | 68.3 | 0.00570 | |||||||||
| Nitrogen (%) | Amino16 (%) | N (% DW) | K (% DW) | P (% DW) | Na (% DW) | Ca (μg/g DW) | Mg (μg/g DW) | Fe (μg/g DW) | Mn (μg/g DW) | Cu (μg/g DW) | Zn (μg/g DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | p | % TV s | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | |
| Nitrogen (N) v | ** r | 68 | ** | 39 | *** | 13 | |||||||||||||||
| Amino16 (A16) u | ** | 61 | *** | 95 | *** | 51 | *** | 78 | *** | 47 | |||||||||||
| N × A16 | *** | 8 | *** | 75 | *** | 41 | |||||||||||||||
| 100 | 4.61 z a y | 7.15 | 0.643 | 0.763 | 0.867 | 0.428 | 32.73 a | 4.76 a | 3.14 | 7.51 | |||||||||||
| 50 | 4.20 b | 6.94 | 0.621 | 0.791 | 0.830 | 0.322 | 26.36 b | 4.24 b | 2.72 | 7.78 | |||||||||||
| 0 | 4.20 | 7.20 | 0.594 b | 0.313 b | 0.888 | 0.313 | 24.41 b | 3.71 b | 2.89 | 7.04 b | |||||||||||
| 0.3 | 4.47 | 6.83 | 0.617 b | 0.483 b | 0.769 | 0.343 | 27.28 b | 3.95 b | 3.26 | 7.93 a | |||||||||||
| 0.9 | 4.54 | 7.10 | 0.685 a | 1.536 a | 0.888 | 0.469 | 36.95 a | 5.84 a | 2.64 | 7.96 a | |||||||||||
| 100 | 0 | 4.55 | 7.36 | 0.603 | 0.329 | 0.857 | 0.327 | 27.24 | 3.78 c | 3.22 ab | 7.39 bcd | ||||||||||
| 0.3 | 4.57 | 6.64 | 0.604 | 0.339 | 0.758 | 0.384 | 28.49 | 3.96 c | 2.39 bc | 7.33 cd | |||||||||||
| 0.9 | 4.71 | 7.43 | 0.723 | 1.622 | 0.985 | 0.573 | 42.47 | 6.55 a | 3.81 ab | 7.81 abc | |||||||||||
| 50 | 0 | 3.85 | 7.03 | 0.586 | 0.297 | 0.919 | 0.299 | 21.58 | 3.63 c | 2.55 abc | 6.69 d | ||||||||||
| 0.3 | 4.37 | 7.01 | 0.630 | 0.627 | 0.779 | 0.302 | 26.07 | 3.94 c | 4.12 a | 8.52 a | |||||||||||
| 0.9 | 4.37 | 6.77 | 0.648 | 1.449 | 0.791 | 0.366 | 31.42 | 5.14 b | 1.48 c | 8.11 ab | |||||||||||
| Nitrogen (%) | Amino16 (%) | Plant Weight (g) | Root Length (cm) | ||
|---|---|---|---|---|---|
| Source of variation | p | % TV s | p | % TV | |
| (N) Nitrogen v | *** | 9 | |||
| (A) Amino16 u | *** r | 93 | *** | 79 | |
| N × A | *** | 11 | |||
| 100 | 10.99 z | 3.80 b | |||
| 50 | 11.30 | 5.51 a | |||
| 0 | 13.03 b y | 8.78 a | |||
| 0.3 | 11.89 b | 5.19 b | |||
| 0.9 | 8.51 c | 0.00 c | |||
| 100 | 0 | 12.97 | 8.86 a | ||
| 0.3 | 11.25 | 2.55 b | |||
| 0.9 | 8.76 | 0.00 c | |||
| 50 | 0 | 13.10 | 8.70 a | ||
| 0.3 | 12.53 | 7.83 a | |||
| 0.9 | 8.27 | 0.00 c | |||
| Nitrogen (%) | Amino16 (%) | Dry Matter (%) | Nitrates (mg/kg FW) | SSC (%) | Phenols (μg GAE/g FW) | Antioxidant Capacity (mg AEAC/100 g FW) | Chlorophyll (μg/g FW) | Carotenoids (μg/g FW) | Anthocyanins (μg/g FW) | Prolines (mM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | p | % TV s | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | |
| Nitrogen (N) v | ** r | 28 | *** | 20 | ** | 30 | * | 8 | |||||||||||
| Amino16 (A16) u | *** | 45 | *** | 80 | *** | 57 | *** | 77 | *** | 76 | *** | 86 | *** | 81 | * | 69 | ** | 82 | |
| N × A16 | *** | 25 | * | 17 | * | 19 | |||||||||||||
| 100 | 4.38 z b y | 398 a | 2.72 b | 0.157 | 10.69 | 453 b | 81.83 | 0.026 | 0.00326 | ||||||||||
| 50 | 5.15 a | 321 b | 2.90 a | 0.163 | 11.18 | 496 a | 83.99 | 0.030 | 0.00317 | ||||||||||
| 0 | 4.16 b | 499 a | 2.67 b | 0.105 c | 6.01 b | 355 c | 76.86 b | 0.036 a | 0.00290 b | ||||||||||
| 0.3 | 4.36 b | 250 b | 2.70 b | 0.168 b | 11.96 a | 466 b | 83.28 a | 0.027 ab | 0.00308 b | ||||||||||
| 0.9 | 5.77 a | 330 c | 3.07 a | 0.206 a | 14.83 a | 604 a | 88.59 a | 0.020 b | 0.00367 a | ||||||||||
| 100 | 0 | 4.20 b | 545 | 2.53 | 0.095 c | 5.23 c | 348 | 75.74 | 0.035 | 0.00294 | |||||||||
| 0.3 | 4.30 b | 291 | 2.70 | 0.191 ab | 14.16 ab | 460 | 81.36 | 0.024 | 0.00319 | ||||||||||
| 0.9 | 4.64 b | 358 | 2.93 | 0.184 ab | 12.66 ab | 551 | 88.39 | 0.017 | 0.00365 | ||||||||||
| 50 | 0 | 4.13 b | 453 | 2.80 | 0.116 c | 6.79 c | 361 | 77.98 | 0.036 | 0.00286 | |||||||||
| 0.3 | 4.41 b | 208 | 2.70 | 0.144 bc | 9.76 bc | 471 | 85.20 | 0.029 | 0.00298 | ||||||||||
| 0.9 | 6.90 a | 302 | 3.20 | 0.228 a | 16.99 a | 657 | 88.78 | 0.024 | 0.00369 | ||||||||||
| Nitrogen (%) | Amino16 (%) | N (% DW) | K (% DW) | P (% DW) | Na (% DW) | Ca (μg/g DW) | Mg (μg/g DW) | Fe (μg/g DW) | Mn (μg/g DW) | Cu (μg/g DW) | Zn (μg/g DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | p | % TV s | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | p | % TV | |
| Nitrogen (N) v | * r | 25 | *** | 5 | ** | 84 | *** | 42 | |||||||||||||
| Amino16 (A16) u | *** | 66 | *** | 95 | ** | 64 | *** | 52 | *** | 76 | |||||||||||
| N × A16 | * | 1 | * | 18 | * | 60 | |||||||||||||||
| 100 | 5.37 z | 9.00 a y | 0.637 | 0.657 a | 0.649 | 0.52 a | 37.28 a | 5.55 | 2.36 | 7.51 | |||||||||||
| 50 | 5.17 | 8.44 b | 0.557 | 0.577 b | 0.633 | 0.48 b | 29.08 b | 5.41 | 1.97 | 7.63 | |||||||||||
| 0 | 5.27 | 9.59 a | 0.641 | 0.302 c | 0.714 a | 0.50 | 27.32 b | 5.89 a | 2.03 | 7.75 | |||||||||||
| 0.3 | 5.29 | 8.51 b | 0.546 | 0.609 b | 0.629 b | 0.49 | 29.49 b | 4.72 b | 2.22 | 7.63 | |||||||||||
| 0.9 | 5.25 | 8.06 b | 0.603 | 0.940 a | 0.580 b | 0.52 | 42.74 a | 5.84 a | 2.26 | 7.33 | |||||||||||
| 100 | 0 | 5.27 | 9.95 | 0.638 | 0.316 e | 0.734 | 0.52 | 29.45 | 5.68 ab | 1.96 | 8.07 a | ||||||||||
| 0.3 | 5.36 | 8.55 | 0.636 | 0.648 c | 0.594 | 0.52 | 33.30 | 5.12 b | 2.38 | 7.28 b | |||||||||||
| 0.9 | 5.47 | 8.50 | 0.636 | 1.006 a | 0.620 | 0.54 | 49.09 | 5.85 ab | 2.75 | 7.18 b | |||||||||||
| 50 | 0 | 5.28 | 9.22 | 0.644 | 0.288 e | 0.694 | 0.47 | 25.18 | 6.11 a | 2.09 | 7.42 ab | ||||||||||
| 0.3 | 5.22 | 8.47 | 0.456 | 0.570 d | 0.665 | 0.46 | 25.67 | 4.31 c | 2.07 | 7.99 ab | |||||||||||
| 0.9 | 5.02 | 7.63 | 0.570 | 0.873 b | 0.540 | 0.49 | 36.39 | 5.82 ab | 1.77 | 7.48 ab | |||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsouvaltzis, P.; Kasampalis, D.S.; Aktsoglou, D.-C.; Barbayiannis, N.; Siomos, A.S. Effect of Reduced Nitrogen and Supplemented Amino Acids Nutrient Solution on the Nutritional Quality of Baby Green and Red Lettuce Grown in a Floating System. Agronomy 2020, 10, 922. https://doi.org/10.3390/agronomy10070922
Tsouvaltzis P, Kasampalis DS, Aktsoglou D-C, Barbayiannis N, Siomos AS. Effect of Reduced Nitrogen and Supplemented Amino Acids Nutrient Solution on the Nutritional Quality of Baby Green and Red Lettuce Grown in a Floating System. Agronomy. 2020; 10(7):922. https://doi.org/10.3390/agronomy10070922
Chicago/Turabian StyleTsouvaltzis, Pavlos, Dimitrios S. Kasampalis, Danai-Christina Aktsoglou, Nikolaos Barbayiannis, and Anastasios S. Siomos. 2020. "Effect of Reduced Nitrogen and Supplemented Amino Acids Nutrient Solution on the Nutritional Quality of Baby Green and Red Lettuce Grown in a Floating System" Agronomy 10, no. 7: 922. https://doi.org/10.3390/agronomy10070922
APA StyleTsouvaltzis, P., Kasampalis, D. S., Aktsoglou, D.-C., Barbayiannis, N., & Siomos, A. S. (2020). Effect of Reduced Nitrogen and Supplemented Amino Acids Nutrient Solution on the Nutritional Quality of Baby Green and Red Lettuce Grown in a Floating System. Agronomy, 10(7), 922. https://doi.org/10.3390/agronomy10070922







