1. Introduction
Loquat (
Eriobotrya japonica (Thunb.) Lindl.) is a high-value crop mainly produced in Eastern Asia and in the Mediterranean Basin, where the unusual phenology of the plant allows to collect the fruits in late winter. During the last two decades, heavy decay symptoms in orchards located in the production areas of Southeastern Spain have been observed and associated to the replant disease syndrome also known as soil fatigue. Both abiotic and biotic factors can cause decay symptoms in trees. Among the biotic agents of the replant syndrome, the soilborne plant pathogenic fungus
Armillaria mellea (Vahl:Fr) P Kumm causes white root rot in woody crops, including loquat [
1] and great losses in Mediterranean orchards and vineyards [
2]. Orchards infested by other fungi of the genera
Rosellinia [
3],
Phytophthora [
1],
Cylindrodendrum,
Dactylonectria [
4] and
Diplodia [
5] have also been described at replant sites.
Armillaria mellea is a facultative necrotrophic pathogen, whose behavior is characterized by colonizing live radicular tissue in its parasitic phase and finally, in its saprophytic phase, using dead tissue as a nutrient source. The symptoms of this disease in the aerial part are typically nonspecific, such as wilting, chlorosis, loss of turgor in the leaves, which develop late and fall, and the gradual or sudden death of the tree [
6]. The specific symptoms of white rot are observed in the root system and collar of the plant, where the bark is detached and below it, in the cambial zone, white creamy mycelial masses in the form of fingers colonize the tissue. When the environmental conditions and the amount of inoculum allow it, the honey-colored fruiting bodies can appear at the baseline of the affected plants, a characteristic that gives the common name to the species mellea, “honey fungus”.
Traditional control methods against
A. mellea are not very effective. The mycelium of the fungus can persist for long periods, even decades, in the infected dead roots remaining in the soil, which represents a source of primary inoculum. Such capacity to grow saprophytically makes the disease difficult to prevent and eradicate [
7]. The presence of rhizomorphs and their ability to extend deep into the soil and the mycelium, protected under the bark, also limit the control and eradication of the disease [
8]. In addition, in the case of loquat plants, there are no commercial rootstocks able to confer tolerance or resistance to the disease. In this context, the use of microorganisms recognized as key components in the development of a sustainable agriculture, could be considered as an alternative method in the control of soil pathogens [
9]. The initial increase in tolerance of white root rot caused by
A. mellea through the application of arbuscular mycorrhizal fungi (AMF) has already been observed in cultivars from several plant species such as grapevine [
10,
11] and lavender [
12]. The mechanisms involved in plant protection by AMF are not fully understood. It has been observed that the induction of resistance against pathogens depends on several mechanisms that can operate simultaneously [
13]. It appears that the mode of action of arbuscular mycorrhizae on pathogens is not directly linked to the elimination of the latter, but rather through a series of described mechanisms that confer tolerance to the host plant [
14], including the alteration of the plant metabolome [
15]. Nowadays, metabolomics is an emerging tool for the study of plant interactions, but few studies are involved in the metabolomic changes in response to arbuscular mycorrhizal colonization. Schliemann et al. (2008) [
16] quantified the relative abundance of metabolites in
Medicago trunculata Gaertn. transformed roots colonized or not with
Rhizoglomus irregulare (Blaszk, Wubet, Renker and Buscot) Sieverd, G.A. Silva and Oehl comb.nov., and observed the accumulation of certain amino acids, fatty acids, apocarotenoids and isoflavonoids in the colonized roots. Laparre et al. (2014) [
17] detected 71 mycorrhiza-associated metabolites exclusively present in mycorrhizal roots, a majority of which with no particular regulatory functions. Moreover, information about the metabolomic changes induced in a plant by AMF in front of root pathogen infections is scarce.
In the field, loquat is known to establish the mutualistic symbiosis with AMF, and the benefits of plant inoculation with selected AMF under severe drought have been reported [
18]. In the present study, the use of AMF as a preventive control strategy against loquat white root rot was evaluated after defining the species mycorrhizal dependence in low fertility soils. An estimation of morphological pathogenicity symptoms in plant tissues and the quantification of plant growth parameters were analyzed to investigate the plant response. The screening of plant metabolites, using liquid chromatography coupled with mass spectrometry, was performed to elucidate the AMF–
A. mellea interaction.
2. Materials and Methods
Seeds of E. japonica were sown in vermiculite as a growing substrate in a greenhouse (April 2015). Seedlings of homogeneous size were selected to be transplanted each into 1 L capacity containers on autoclaved sandy soil. At the time of the transplant, the loquat plants were inoculated with different arbuscular mycorrhizal fungi, by applying 20 mL of bulk inoculum under the roots. Two mycorrhizal treatments and one non-mycorrhizal control treatment were then established. All plants received 20 g of slow release fertilizer (Osmocote®) soon after the transplant.
The mycorrhizal treatments consisted of two fungal isolates: a native arbuscular mycorrhizal fungus isolated from commercial loquat plantations of the Spanish Mediterranean area, classified as
Rhizoglomus sp., and an IRTA (Institut de Recerca i Tecnologia Agroalimentaries) collection fungus
Rhizoglomus irregulare registered in the International Bank of the Glomeromycota as
Glomus intraradices s.l. BEG 72. This fungus was isolated from a citrus nursery in eastern Spain [
19] and its effectiveness in plant growth promotion has been demonstrated in several agricultural systems [
20,
21,
22]. The inocula of both mycorrhizal fungi were obtained from leek (
Allium porrum L.) cultures on sterilized sand.
Loquat plants were kept for 12 months in 1 L volume containers in autoclaved sandy soil poor in nutrients, under greenhouse conditions with periodical irrigation. During this period, the growth of the plants was measured monthly and the relative dependence on mycorrhizae (RMD) was evaluated [
23]. At harvest, ten plants from each treatment were used to measure the biomass parameters and to assess the mycorrhizal colonization extent in the roots [
24,
25].
The rest of the plants (sixteen plants per treatment) were transplanted into 5 L containers filled with a mixture of autoclaved sandy soil, silica sand and sphagnum peat (3:2:1 v/v) and then transferred to a greenhouse with shading mesh. After four months of growth in the greenhouse, the plants were inoculated with an A. mellea isolated from a loquat orchard.
The inoculum production of
A. mellea was previously produced on chestnuts in an agar-malt medium [
12]. To inoculate the plants with the pathogen, one chestnut colonized by
A. mellea was placed in a 10 cm deep hole made in the growing substrate near the roots. Six treatments were established, each of them with eight plants with a factorial combination of mycorrhizal inoculation (three levels: control,
R. irregulare, and native AMF isolate) and
A. mellea (two levels: with and without).
One year after inoculation with
A. mellea, the plants were uprooted to evaluate the damage caused by the pathogen, to measure the parameters of plant biomass and to estimate the mycorrhizal colonization in the roots [
24,
25]. Damage caused by the pathogen was estimated using a rate of disease symptoms in the neck and the roots, together with the percentage of root necrosis, the presence of visible rhizomorphs, and the observation of aerial decay symptoms (defoliation). Data from different treatments were analyzed by two-way analysis of variance and the means were compared using the Tukey test.
For the metabolomic analyses, the samples of five leaves were collected from each plant in the morning at harvest and quenched using liquid nitrogen. The samples were then transferred at −80 °C until analyzed in the lab. Frozen leaf samples (0.5 g) were extracted in 5 mL of acidified (0.1% HCOOH) 80% methanol using an Ultra-Turrax (Ika T-25, Staufen, Germany), filtered through a 0.2 μm cellulose membrane and transferred into amber vials for analysis. The screening of the plant metabolites was carried out using a liquid chromatography coupled to quadrupole-time-of-flight mass spectrometer through a JetStream electrospray ionization system (UHPLC–ESI–QTOF–MS) as previously reported [
26]. A 1290 series liquid chromatograph was interfaced to a G6550 iFunnel mass spectrometer (all from Agilent technologies Santa Clara, CA, USA). Chromatographic separation was carried out under a water and methanol gradient, using an Agilent Poroshell 120 EC-C18 column (150 × 2.1 mm i.d., 1.9 μm). The elution gradient ranged from 5% to 95% ACN (acetonitrile) within 60 min, and the flow rate was 220 μL min
−1. The injection volume was 2 μL, whereas the QTOF was operated in positive SCAN mode (100–1200 m/z
+ range) and extended dynamic range mode. Deconvolution, mass and retention time alignment, as well as annotations and filtering were carried out using the software Profinder B.08.02 (Agilent Technologies, adopting a mass accuracy of <5 ppm). Post-acquisition processing was conducted according to Rouphael et al. 2016, [
27]: compounds’ annotation was based on monoisotopic accurate mass, isotope spacing and isotope ratio, against the database exported from PlantCyc 12.5 (Plant Metabolic Network,
http://www.plantcyc.org; accessed on 16 April 2018). Finally, the compounds were filtered by frequency: those compounds that were not present in 100% of replications within at least one treatment were discarded [
28]. Therefore, based on COSMOS Metabolomics Standards Initiative (
http://cosmos-fp7.eu/msi; accessed on 3 April 2019), a Level 2 identification (i.e., putatively annotated compounds) was achieved in our experiment.
Interpretation of metabolomics was carried out in Mass Profiler Professional B.14.9.1 (Agilent technologies). Compounds’ abundance was Log2 transformed and normalized (75th percentile), then baselined against the median in the dataset. An unsupervised hierarchical cluster analysis was then carried out using an “Euclidean” similarity measure and “Wards” as the linkage rule. The differential metabolites were next gained using a Volcano plot analysis, i.e., combining the analysis of variance (moderated t-test, p < 0.01, Bonferroni multiple testing correction) with fold-change analysis (cut-off value = 2). Thereafter, partial least square discriminant analysis (PLS-DA, N-fold validation with N = 4) as further supervised modeling, was carried out. The most discriminant compounds were finally exported from the PLS-DA loading plot, according to their weight in the class prediction model.
3. Results
At the end of the first year, the growth curve of the loquat plants from the three treatments was obtained (
Figure 1). All mycorrhizal plants were larger in height than the non-mycorrhizal plants (
p < 0.05), those inoculated with
R. irregulare (BEG 72) being the largest. The significant differences between the treatments were apparent after nine months’ growth (
Figure 1).
All the biomass parameters evaluated (height, shoot diameter, shoot and root dry weights) were significantly lower in the non-mycorrhizal plants compared to the mycorrhizal plants. Among both inoculation treatments, the plants inoculated with
R. irregulare produced a slightly higher biomass than plants inoculated with the native loquat fungus (
Table 1). After one-year growth, both AMF had achieved a very high-root colonization of loquat plant roots, above 85% (
Table 1), while the plants from the control treatment remained non mycorrhizal. By the end of the experiment, the loquat plants showed a similar high relative mycorrhizal dependency on
R. irregulare and to the native AMF in a low-nutrient substrate (
Table 1).
In relation to the growth response of the mycorrhizal plants against
A. mellea colonization, the higher biomass of plants from the three treatments, non inoculated with the pathogen, indicate a more vigorous development tendency of the plants in both mycorrhizal treatments compared to the control (
Table 2 and
Figure S1). Treatments inoculated with AMF, both
R. irregulare and native AMF, reached higher biomass values, with significant differences in height, shoot diameter, and shoot/root dry weights than the control plants. In the plants infected by
A. mellea, the growth of the control plants and those colonized by the native AMF was not affected by the pathogen. However, the
A. mellea infection reduced the growth of the
R. irregulare-colonized plants (
Table 2).
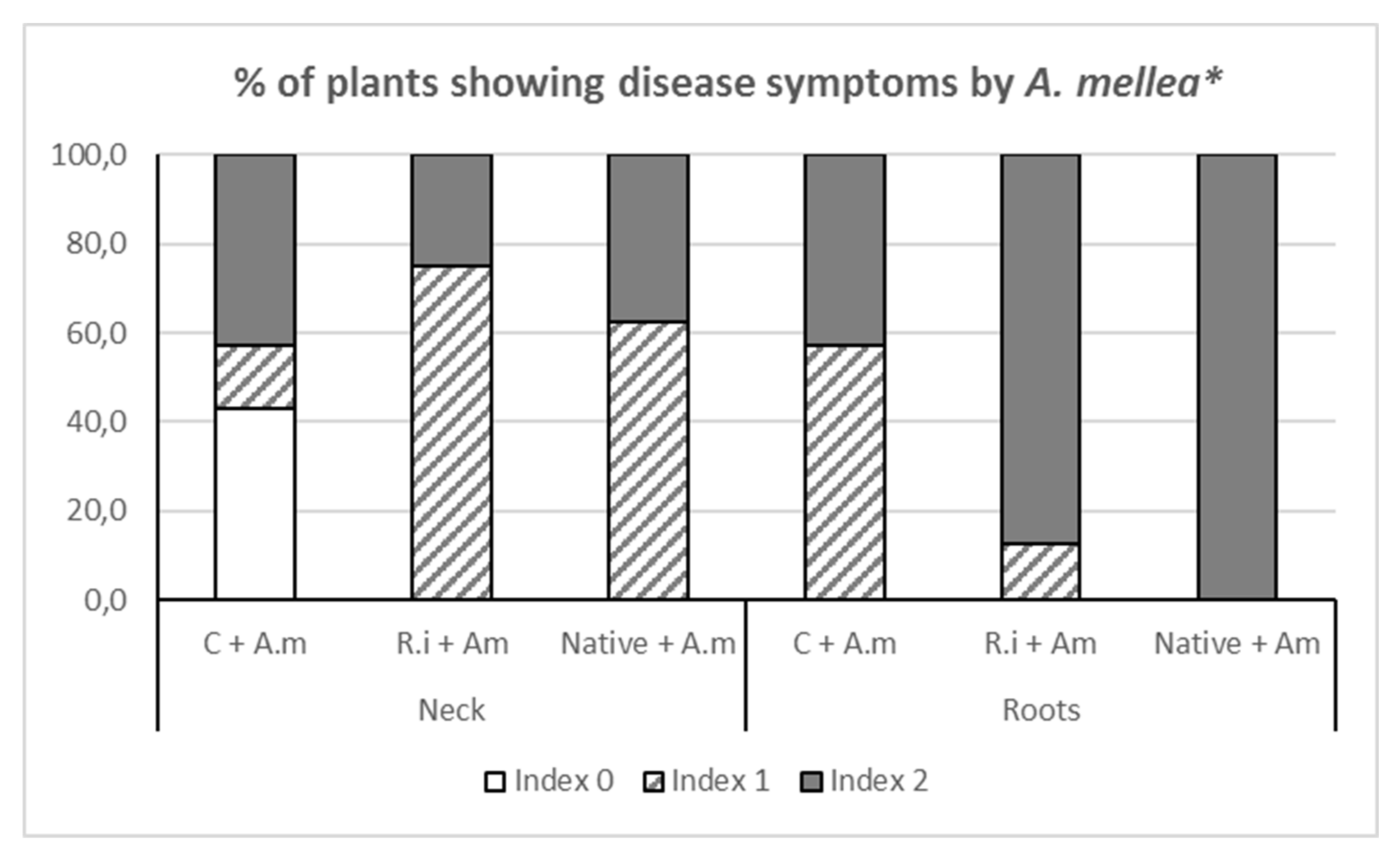
Regarding the disease symptoms produced in loquat the roots by
A. mellea one year after inoculation, they were observed in the plants of all the pathogen inoculation treatments. Plants of the Control +
A. mellea treatment had a lower extent of the root system with visible rot symptoms. In plants from all the treatments, necrosis with and without mycelium in the roots and crown was observed (
Figure 2) but no compact mycelium was ever found.
In the roots of both mycorrhizal treatments with
A. mellea, the presence of necrosis with mycelium predominated (index 2), whereas in the collar the most common rot symptom was necrosis without any visible mycelium. In both cases, the symptoms were more severe than in the control plants inoculated with
A. mellea (
Figure 2). Concerning the rhizomorphs, they were also observed in plants from all the pathogen inoculation treatments achieving percentages above 85%.
The observation of the decay plant symptoms indicated that the control plants inoculated with
A. mellea achieved a very poor development of root and shoot systems (
Table 2). Mycorrhizal plants colonized by
R. irregulare with
A. mellea infection were heterogeneous in size and had visible lesions in the crown. Plants from both treatments (Control +
A. mellea and
R. irregulare +
A. mellea) suffered from apparent defoliation and were characterized by smaller leaves. None of these symptoms were observed in mycorrhizal plants colonized by the native AMF despite the infection of
A. mellea (Native AMF +
A. mellea treatment).
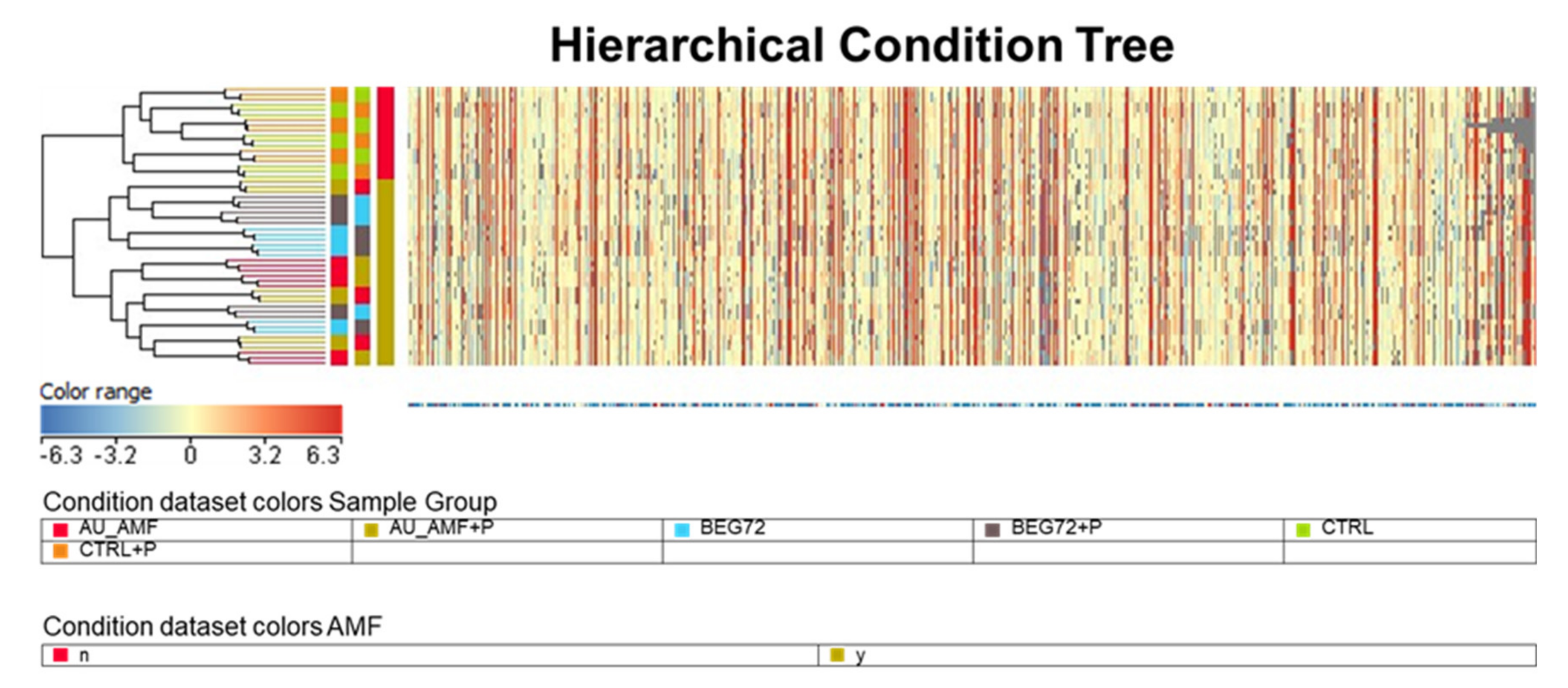
The untargeted UHPLC–ESI–QTOF–MS analysis allowed annotating about 940 putative metabolites. The unsupervised hierarchical clustering based on the heat map produced from the fold-change values highlighted two main clusters, where the AMF inoculation represented the hierarchically more relevant factor for classification (
Figure 3). Within the sub-cluster without AMF, the control and the control in the presence of the pathogen did not show distinct profiles. However, distinct profiles could be gained for mycorrhized plants (from either native or
R. irregulare) in the presence of the pathogen. This unsupervised multivariate analysis allowed defining the pivotal role played by AMF colonization in determining the actual metabolic profile of loquat.
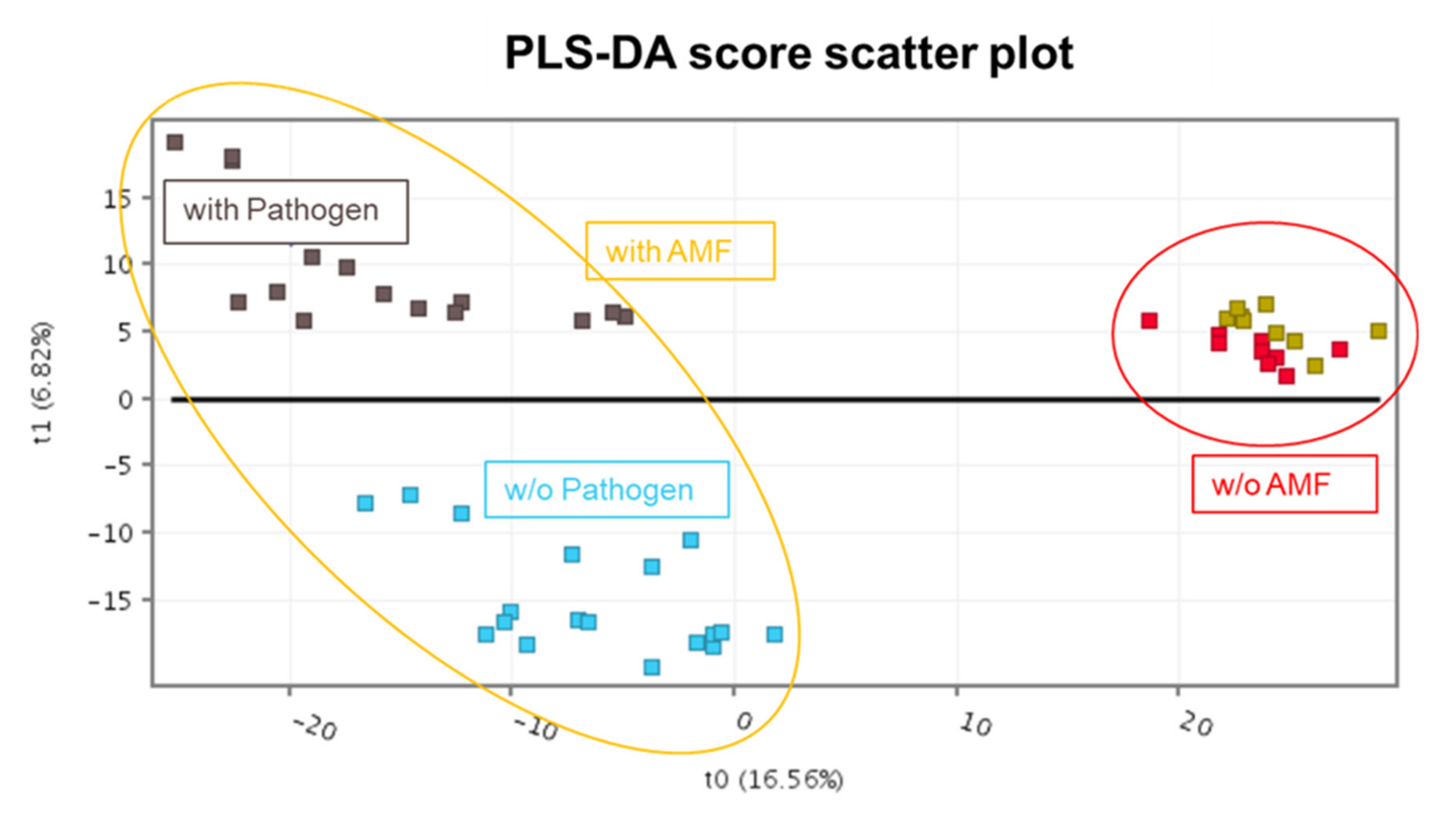
To identify the metabolites better characterizing the changes modulated by the tripartite interaction between the plant, AMF and the pathogen, a supervised multivariate analysis (i.e., PLS-DA) was carried out. Looking at the PLS-DA score scatter plots (
Figure 4), the modeling highlighted a clear separation of the treatments according to the AMF colonization on the first latent vector. The effect arising from the presence of the pathogen could be highlighted in the second latent vector, but only in mycorrhized plants. The PLS-DA model fitting parameters were more than adequate, with a goodness-of-fit R
2Y = 0.92 and a goodness-of-prediction Q
2Y 0.70.
The multivariate modeling highlighted a clear separation of the treatments according to AMF colonization on the first latent vector. The effect arising from the presence of the pathogen could be highlighted in the second latent vector, but only in mycorrhized plants. Thereafter, the metabolomic reprogramming imposed by the presence of pathogens in the loquat was evaluated following inoculation with either
R. irregulare (BEG 72) or the native loquat AMF (
Rhizoglomus sp.). With this aim, analysis of variance (AVOVA
p < 0.05) and fold-change analysis (FC, threshold = 2) were combined into the Volcano Plot analysis. Differential metabolites were provided as
Supplementary Materials (Supplementary Table S1). These latter compounds were loaded into the online Omic Dashboard of the Plant Metabolic Network to be classified into metabolic classes. This data reduction approach allowed pointing out the biochemical classes involved at most in the loquat response to pathogen following the AMF colonization. The classes highlighted are fully provided in
Supplementary Table S1. Despite the distinctive responses that could be outlined between the two AMF considered, several common AMF-mediated responses could be outlined in the presence of the pathogen. A general up-accumulation of brassinosteroids and their related hydroxysterols could be observed, with campest-5-en-3-one having a Log FC of 1.44. Another hormone being accumulated was the conjugated auxin indole-3-acetyl-phenylalanine, having a Log FC = 1.03. Interestingly, glucosinolates and their precursors (e.g.,
S-8-methylthiooctylhydroximoyl-
l-cysteine) could also be identified in response to the pathogen, with a general up accumulation. With this regard, 3-sinapoyloxypropylglucosinolate exhibited the largest increase (Log FC = 1.14). On the contrary, oxidative stress-related compounds such as hydroperoxy fatty acids, polyunsaturated fatty acids (PUFA) and phenolics (lignans, catechin and other flavonol-derivatives, as well as glucoside-derivatives of flavonoids) were all down accumulated.
4. Discussion
The results indicated that the mycorrhizal inoculation of loquat plants with both AMF isolates significantly enhanced growth and development in low-nutrient soils. When plants were mycorrhized with
R. irregulare (BEG 72), the shoot and root dry weights increased by up to 500% and 560%, respectively, when compared with non-mycorrhizal plants. The largest root colonization extent (above 90%) was achieved with the native AMF, which suggests that the fungus isolated from the loquat orchards is well adapted to achieve a symbiotic association with the plant species. This observation sustains the idea that in general, native fungi show a high capacity to colonize the roots of host plants [
29]. Loquat plants showed dependence on mycorrhizae with both fungi,
R. irregulare and the native loquat AMF (
Rhizoglomus sp.), which according to the MD categories proposed by Habte and Manjunath, 1991 [
30], loquat has turned out to be a “very highly dependent” (RMD above 75%) on mycorrhizae in a low-fertility sandy soil.
The PLS-DA-supervised modeling (
Figure 4) confirmed that the loquat metabolome is surely affected by the AMF colonization, as indicated by the presence of two main groups, namely non-colonized and colonized plants. However, going into a hierarchically detailed evaluation, the presence of
A. mellea induced more distinctive shifts in the metabolomic signatures in AMF-colonized loquat plants. This data reduction approach indicates that AMF played a pivotal role in shaping the plant metabolic response, and this modulation was even more evident in presence of the pathogen. There is little published information on the role of arbuscular mycorrhizae on white root rot caused by
A. mellea. Nogales et al., 2009 [
31], in a study of the vine rootstock Richter 110, found that the aerial symptoms of the disease in mycorrhizal plants developed more slowly than in non-mycorrhizal plants and demonstrated that mycorrhizal plants were more tolerant to the pathogen, confirming previous results obtained by Aguín et al., 2006 [
10] also in vine rootstocks. The evaluation of the results in both works was in the short term after inoculation with
A. mellea, as in our study. The mycorrhizal response to the incidence of white root rot shows very different results depending on the AMF used as an inoculant [
32]. In our experiments, the growth of mycorrhizal plants infected by the pathogen was not significantly different from the growth of non-mycorrhizal control plants inoculated with the pathogen only, and the severe damage of
A. mellea root rot was observed. Hence, in our findings, the fungus did not confer resistance against the native
A. mellea isolate attacking loquat plants.
On the other hand, the inoculation with the native fungus isolated from the loquat orchards in the presence of the pathogen caused a highly significant increase in plant biomass. However, the low root/shoot ratio revealed that the roots were deeply affected by
A. mellea and in fact the evaluation of the disease symptom estimation (
Figure 2) confirms the results. Therefore, it is suggested that although the inoculation with the native AMF did not inhibit
A. mellea’s infection, mycorrhizal plants in this case showed tolerance to the pathogen, since no decay symptoms and no decrease in the plants’ vegetative development were observed. In this study, tolerance to
A. mellea seems to be linked to an indirect mechanism of damage compensation since mycorrhizal plants were better nourished and could compensate the loss of functionality of the roots infected with the pathogen [
14]. The lack of disease damage in the shoots of mycorrhizal plants colonized by the native fungus can be associated to improve plant growth. However, this hypothesis does not explain why in plants colonized with
R. irregulare, the mycorrhizal fungus has not conferred a similar tolerance against the pathogen, a fact that encourages further discussions. It must be pointed out that in the present work,
A. mellea and the native AMF share the geographical origin and the plant host species (loquat) from where they had been isolated. The results also highlighted that the native fungus had an intrinsic capacity to overgrow the damage caused by the closely related
A. mellea isolate.
In studies concerning the influence of AMF on the development and incidence of the diseases, the interactions between the pathogen, the symbiotic fungus, the host plant and the environmental conditions must be taken into account [
33]. Harrier and Watson 2004 [
14] proposed that AMF does not directly interact with the pathogen, but it would rather be an indirect mechanism of the bioprotection of AM symbionts against pathogenic soilborne fungi. The results obtained indicate that only the mycorrhizal fungus, isolated from a loquat orchard soil in the same geographical area where the
A. mellea isolate was found, was able to confer an initial tolerance to the pathogen, although no resistance was acquired as the mycorrhizal plants still had severe rot symptoms in the roots and crown.
Looking at the molecular basis of the observed improvement in growth following AMF colonization, a wide and diverse response could be observed using metabolomics. Indeed, several response pathways are invoked following pathogen interaction, and the corresponding signaling has several interconnections [
34]. The complex response induced by pathogens finally involves the synthesis of reactive oxygen species (ROS), the induction of resistance genes, the alterations of the cell wall and the production of bioactive metabolites (e.g., phytoalexins). In fact, the plants’ response involves a diversity of secondary metabolites which prominently function to protect them and repel pathogens. Consistently, AMF-colonized plants in the presence of the pathogen, exhibited a down accumulation of hydroxyperoxy fatty acids, of polyunsaturated fatty acids and of several classes of phenolics. All together, these clues corroborate the involvement of an oxidative stress-mediated response, in comparison to plants without the pathogen.
Consistently with the modulation of loquat response to infection under AMF colonization, a reprogramming of plant biochemical profiles could be observed. Although specific differences could be observed with R. irregulare rather than the native loquat AMF (Rhizoglomus sp.) were present, some common responses could be outlined.
Phytohormones were confirmed to be involved in the modulation of plant response to infection. Jasmonate exhibited an opposite trend between the native or
R. irregulare colonized plants (the former showing an up accumulation while the latter showing down accumulation). Notably, jasmonate is known to play a pivotal role in coordinating both the plant growth and development or defense [
35]. In plants colonized by the native AMF, cytokinins were negatively modulated following interaction with the pathogen. However, an increase in auxins and brassinosteroids, together with a decrease in abscisic acid, could be observed in
R. irregulare-colonized plants. Current knowledge has described the roles of auxin, abscisic acid, cytokinins and brassinosteroids together with classical defense hormones (salicylic acid, jasmonate and ethylene) in molding plant–pathogen interactions [
36]. Indeed, as in our experiments, such hormonal crosstalk represents a crucial factor in disease and defense modulation.
The imbalance of hormone profile in plant–pathogen interaction is connected to the modulation of ROS, these latter being produced via the incomplete reduction of oxygen (O
2−) and acting as signaling molecules integrated with hormone-signaling networks. Unlike mammals (where ROS are mainly produced in mitochondria), plants produce singlet oxygen mainly in thylakoids. In this sense, photosynthesis and chlorophyll intermediates play a pivotal role in ROS production and signaling in the presence of light. With this regard, the negative modulation of porphyrins and phenyl quinones (naphto- and phylloquinol) we observed, can be related to their function in chloroplast-to-nucleus signal transduction and is involved in plant acclimation to stress [
37]. Consistently, chlorophyll degradation was reduced in plants mycorrhized by the native AMF.
Taking into account the pivotal role of phenolics as antioxidants [
34], the previously mentioned concurrent reduction in phenylpropanoids (more marked in plants mycorrhized by the native AMF) could be related to the improved management of oxidative stress. The altered regulation of the antioxidant betacyanins betanin and betanidin (its aglycone) in both native and
R. irregulare-colonized plants confirms the possible involvement of redox balance. Notably, the regulation of oxidative balance is a key process in plant–pathogen interaction [
34]. Indeed, the production of ROS via the oxidative burst, is one of the earliest responses following successful pathogen recognition [
38].
Interestingly, the loquat response to infection following
R. irregulare-colonization specifically involved compounds acting as phytoalexins. The increased accumulation of DIMBOA, a cyclic hydroxamic acid, is involved in the defense of plants against a wide range of pathogens and insects. The plant stores its glucoside derivative, DIMBOA-glucoside, and forms the autotoxic aglycone through a specific glucosidase following infection [
39]. The induction of DIMBOA accumulation has been specifically reported as a mechanism promoted by AMF to reduce disease incidence and severity in roots [
40]. Similarly, the conjugated isoflavonoid phytoalexins biochanin A glucoside (possibly acting as phytoanticipin), and maackiain glucoside, are known to be induced under stress and to be elicitated under plant–pathogen interaction [
41], particularly in roots [
42] following jasmonate signaling [
43]. Similarly, the increased accumulation of glucosinolates in the mycorrhizal plant, in the presence of the pathogen, represented an additional strategy to contrast the infection. Indeed, glucosinolates are well known primary chemical defense compounds having a pivotal role in plant innate immunity [
44].
Finally, pathways involving carbohydrates and lipids were highlighted by the interpretation of differential metabolites gained from the plants colonized by the native AMF following the pathogen infection. Regarding the formers, no clear trends could be observed, with the cell wall-related fucose biosynthesis being increased together with ADP-glucose biosynthesis, as well as with a decrease in UDP-glucose. Regarding the latter, it is interesting to note that plants with a decreased UDP-glucose were insensitive to pathogen-induced programmed cell death, likely because of its role as an extracellular signaling molecule [
45]. As far as lipids are concerned, a decrease in fatty acids and lipids biosynthesis was outlined. Lipids are part of the fundamental architecture of cells and are involved in several fundamental processes. Indeed, lipid-associated plant-defense responses, involving cleavage or the transformation of lipid substrates have been reported in various plant compartments [
46]. Besides its structural role, the plant cell membrane can serve as reservoirs from which biologically active lipids are released [
47]. Such a role of lipids in plant–pathogen interaction might involve also their emerging role as signaling compounds [
48].
Overall, a complex and diverse metabolic response appeared to be involved in the improved resistance to pathogens in the loquat following AMF-mediated elicitation. Induced systemic resistance acts as an important mechanism by which AMF in the rhizosphere primed the whole plant body for enhanced defense against pathogens [
49].