Unraveling Ecophysiological Mechanisms in Potatoes under Different Irrigation Methods: A Preliminary Field Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Material
2.2. Experimental Design and Crop Management
2.3. Water Management
2.4. Ecophysiological Monitoring and Tuber Yield Assessment
2.5. Statistical Analysis
3. Results
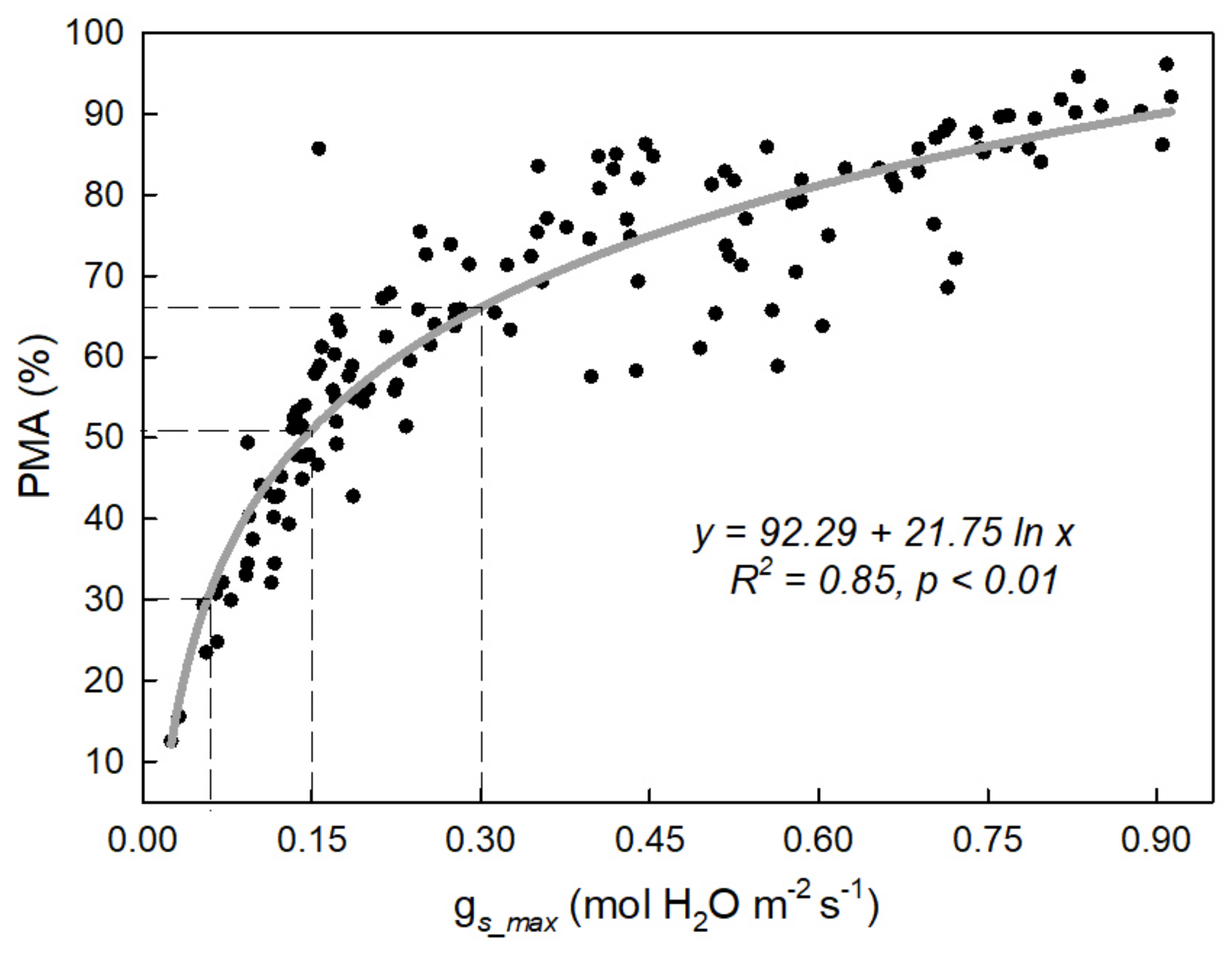
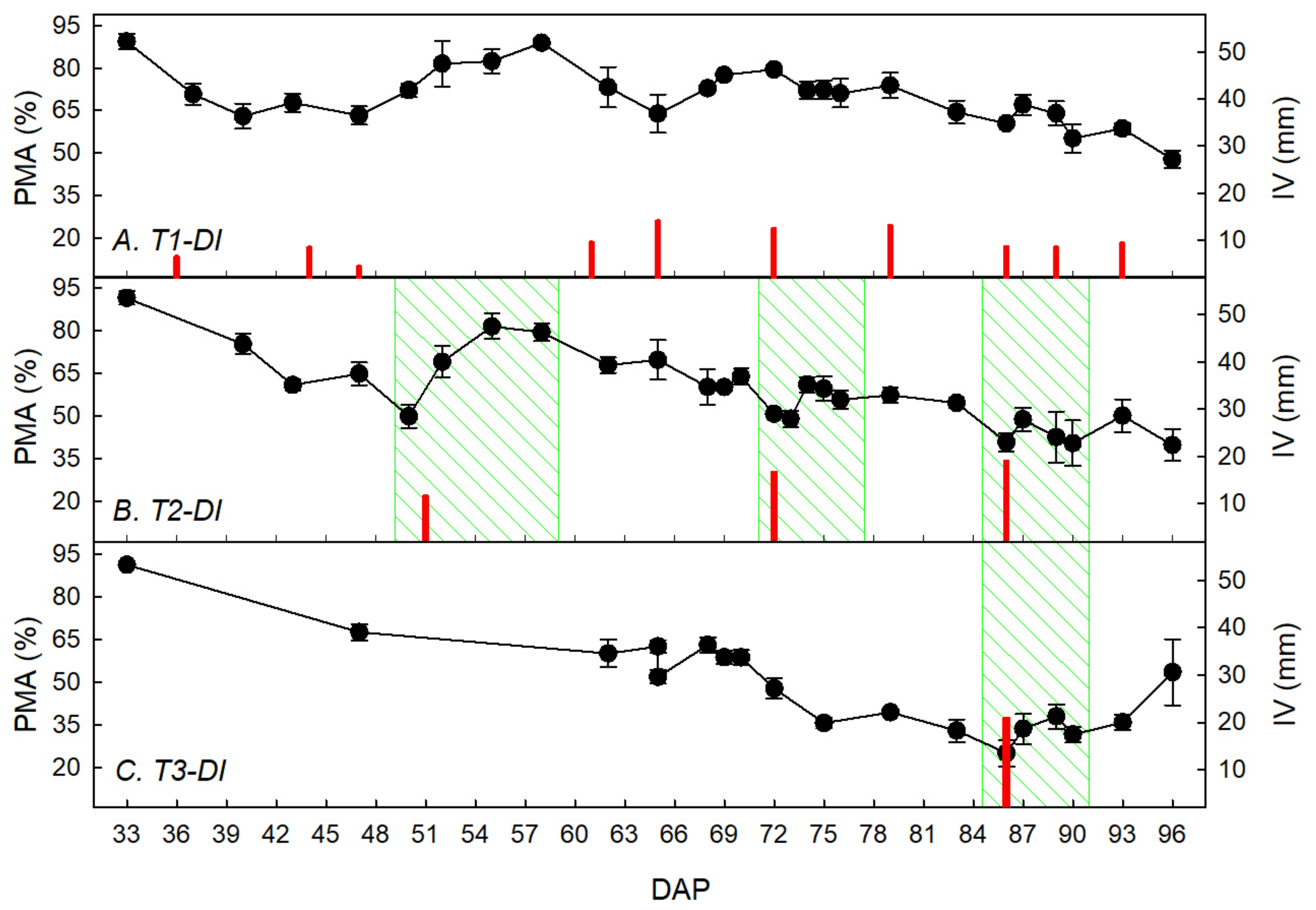
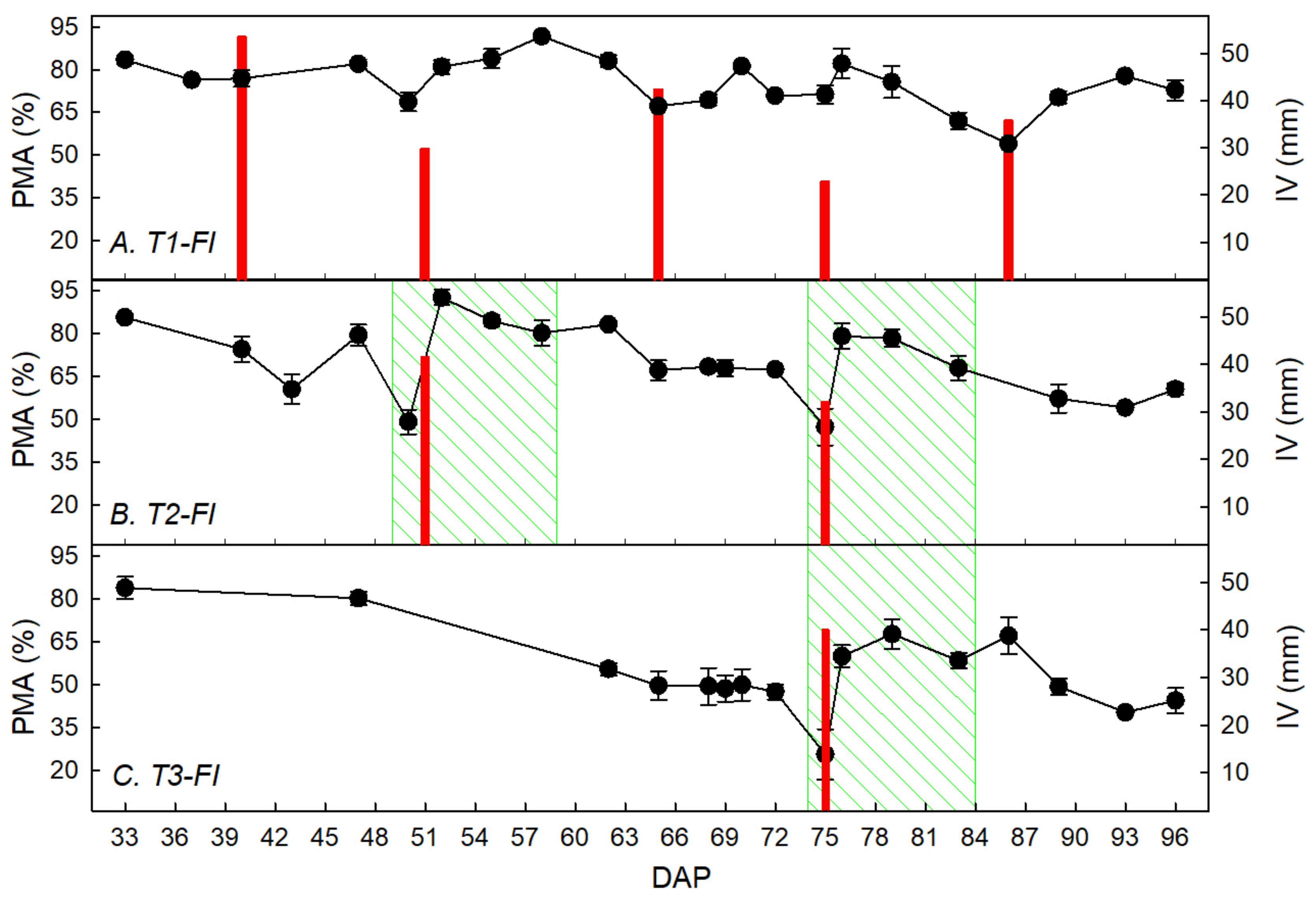
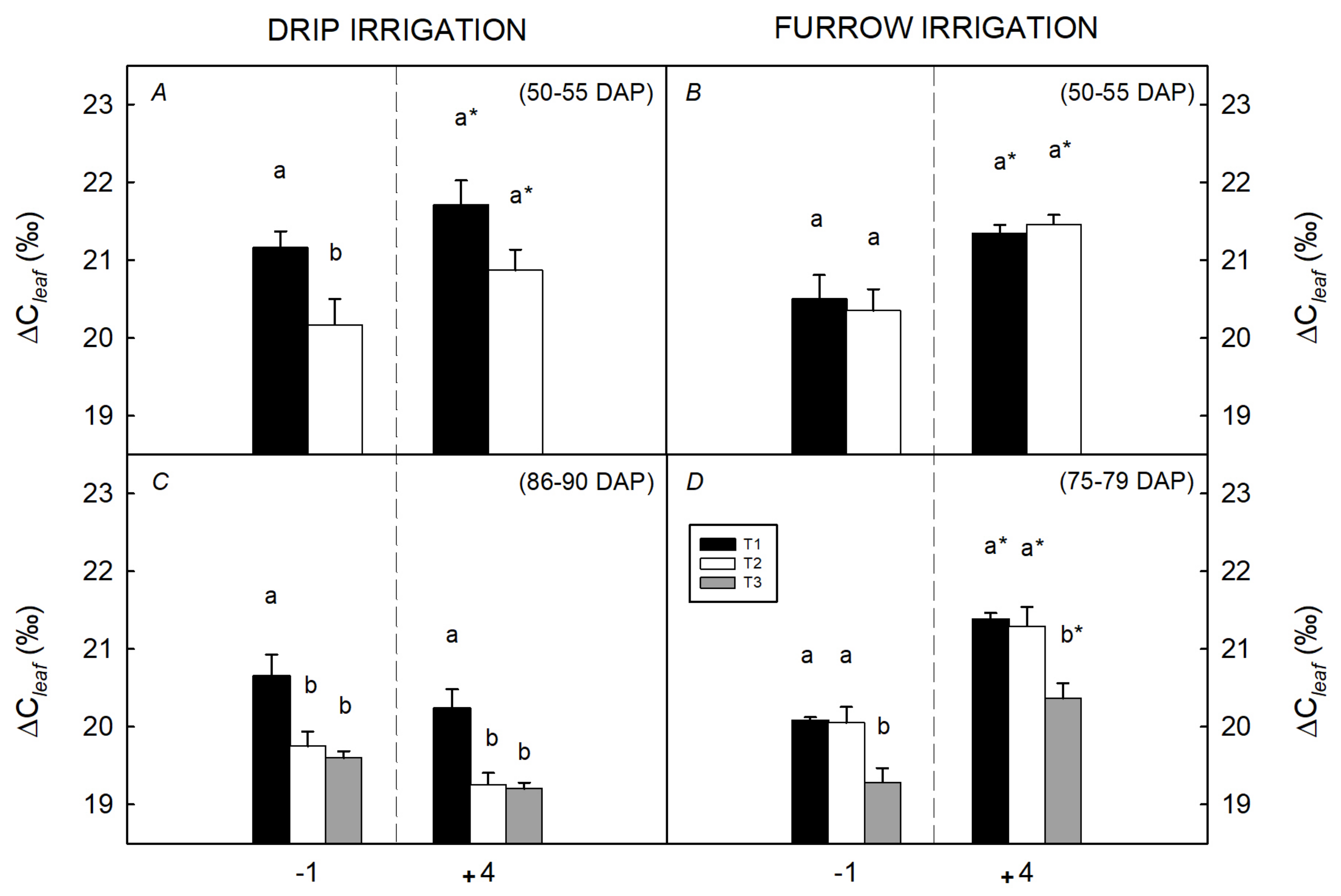
3.1. Water Restriction Effects on Tuber Yield and Ecophysiological Recovery Capacity
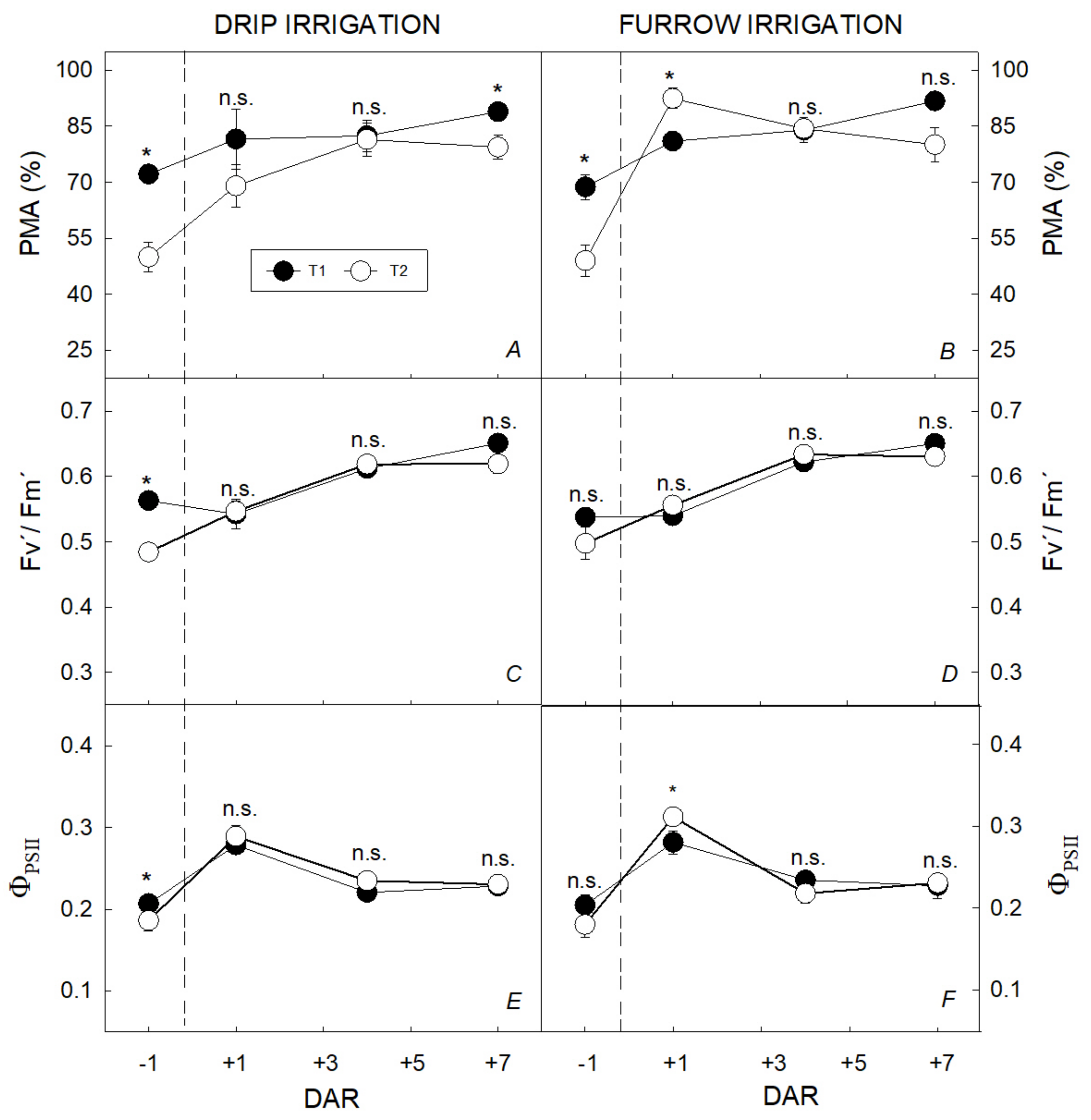
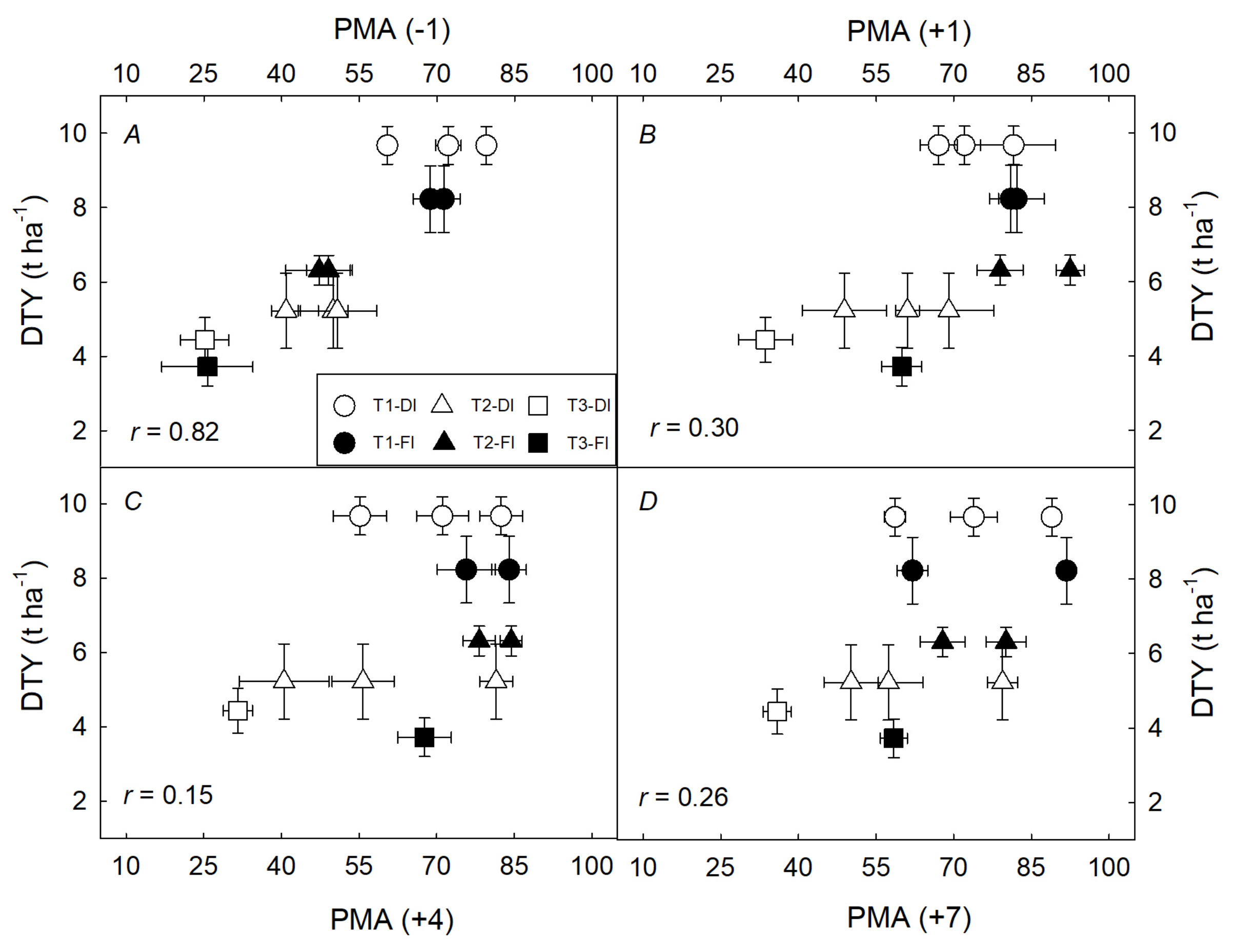
3.2. Integrated Characterization of Water Pulses Effect on Physiological Descriptors under Both Irrigation Methods
4. Discussion
4.1. Physiological Thresholds for Watering Affected Photosynthetic Recovery Depending on the Irrigation Method
4.2. The Photosynthetic Recovery Mainly Depends on the Timing and Frequency of Water Pulses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, Z.; Zhou, G.; Shimizu, H. Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J. Exp. Bot. 2009, 60, 3737–3749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khordadi, M.J.; Olesen, J.E.; Alizadeh, A.; Nassiri Mahallati, M.; Ansari, H.; Sanaeinejad, H. Climate change impacts and adaptation for crop management of winter wheat and maize in the semi-arid region of Iran. Irrig. Drain. 2019, 68, 841–856. [Google Scholar] [CrossRef]
- Zeng, X.; Evans, M.N.; Liu, X.; Wang, W.; Xu, G.; Wu, G.; Zhang, L. Spatial Patterns of Precipitation-Induced Moisture Availability and their Effects on the Divergence of Conifer Stem Growth in the Western and Eastern Parts of China’s Semi-Arid Region. For. Ecol. Manag. 2019, 451, 117524. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Water Relations. In Plant Physiological Ecology; Springer: New York, NY, USA, 2008; pp. 163–216. [Google Scholar]
- Faostat. Available online: http://www.fao.org/faostat/en/ (accessed on 4 June 2019).
- Hijmans, R.J. The effect of climate change on global potato production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Sanabria, J.; Lhomme, J.P. Climate change and potato cropping in the Peruvian Altiplano. Theor. Appl. Climatol. 2013, 112, 683–695. [Google Scholar] [CrossRef]
- Fleisher, D.H.; Condori, B.; Quiroz, R.; Alva, A.; Asseng, S.; Barreda, C.; Bindi, M.; Boote, K.J.; Ferrise, R.; Franke, A.C.; et al. A potato model intercomparison across varying climates and productivity levels. Glob. Chang. Biol. 2017, 23, 1258–1281. [Google Scholar] [CrossRef]
- Quiroz, R.; Ramírez, D.A.; Kroschel, J.; Andrade-Piedra, J.; Barreda, C.; Condori, B.; Mares, V.; Monneveux, P.; Perez, W. Impact of climate change on the potato crop and biodiversity in its center of origin. Open Agric. 2018, 3, 273–283. [Google Scholar] [CrossRef]
- Harris, P.M. Impact of climate change on the potato crop. In The Potato Crop; Springer: Dordrecht, The Netherlands, 1978; p. 909. [Google Scholar]
- Vincent, C.; Rowland, D.; Schaffer, B.; Bassil, E.; Racette, K.; Zurweller, B. Primed acclimation: A physiological process offers a strategy for more resilient and irrigation-efficient crop production. Plant Sci. 2019, 3, 110240. [Google Scholar] [CrossRef]
- Byrd, S.A.; Rowland, D.L.; Bennett, J.; Zotarelli, L.; Wright, D.; Alva, A.; Nordgaard, J. Reductions in a Commercial Potato Irrigation Schedule during Tuber Bulking in Florida: Physiological, Yield, and Quality Effects. J. Crop Improv. 2014, 28, 660–679. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Luo, H.H.; Zhang, Y.L.; Zhang, W.F. Effects of water stress and rewatering on photosynthesis, root activity, and yield of cotton with drip irrigation under mulch. Photosynthetica 2016, 54, 65–73. [Google Scholar] [CrossRef]
- Kirby, M.; Bark, R.; Connor, J.; Qureshi, M.E.; Keyworth, S. Sustainable irrigation: How did irrigated agriculture in Australia’s Murray–Darling Basin adapt in the Millennium Drought? Agric. Water Manag. 2014, 145, 154–162. [Google Scholar] [CrossRef]
- Van der Kooij, S.; Zwarteveen, M.; Boesveld, H.; Kuper, M. The efficiency of drip irrigation unpacked. Agric. Water Manag. 2013, 123, 103–110. [Google Scholar] [CrossRef]
- Alcon, F.; Navarro, N.; de-Miguel, M.D.; Balbo, A.L. Drip Irrigation Technology. In Sustainable Solutions for Food Security Combating Climate Change by Adaptation; Springer: Cham, Switzerland, 2019; pp. 262–285. [Google Scholar]
- Amali, S.; Rolston, D.E.; Fulton, A.E.; Hanson, B.R.; Phene, C.J.; Oster, J.D. Soil water variability under subsurface drip and furrow irrigation. Irrig. Sci. 1997, 17, 151–155. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Plauborg, F.; Andersen, M.N.; Sepaskhah, A.R.; Jensen, C.R.; Hansen, S. Effects of irrigation strategies and soils on field grown potatoes: Root distribution. Agric. Water Manag. 2011, 98, 1280–1290. [Google Scholar] [CrossRef]
- Tagar, A.; Chandio, F.A.; Mari, I.A.; Wagan, B. Comparative Study of drip and furrow irrigation methods at farmer’s field in Umarkot. Int. J. Agric. Biosyst. Eng. 2012, 69, 863–867. [Google Scholar]
- Perry, C. Does Improved Irrigation Technology Save Water? A Review of the Evidence; FAO (Food and Agriculture Organization of the United Nations): Rome, Italy, 2017; Volume 42. [Google Scholar]
- Ward, F.A.; Pulido-Velazquez, M. Water conservation in irrigation can increase water use. Proc. Natl. Acad. Sci. USA 2008, 105, 18215–18220. [Google Scholar] [CrossRef] [Green Version]
- Perry, C.; Steduto, P.; Allen, R.G.; Burt, C.M. Increasing productivity in irrigated agriculture: Agronomic constraints and hydrological realities. Agric. Water Manag. 2009, 105, 1517–1524. [Google Scholar] [CrossRef] [Green Version]
- van Loon, C.D. The effect of water stress on potato growth, development, and yield. Am. Potato J. 1981, 58, 51–69. [Google Scholar] [CrossRef]
- Jefferies, R.A. Physiology of crop response to drought. In Potato Ecology and Modelling of Crops under Conditions Limiting Growth; Haverkort, A.J., MacKerron, D.K.L., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 61–74. [Google Scholar]
- Schneekloth, J.P.; Klocke, N.L.; Davison, D.R.; Payero, J.O. Furrow irrigation management with limited water. Appl. Eng. Agric. 2006, 22, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 30, 239–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Ramírez, D.A.; Xie, K.; Li, W.; Yactayo, W.; Jin, L.; Quiroz, R. Is partial root-zone drying more appropriate than drip irrigation to save water in China? A preliminary comparative analysis for potato cultivation. Potato Res. 2018, 61, 391–406. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought–from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Resco, V.; Ignace, D.D.; Sun, W.; Huxman, T.E.; Weltzin, J.F.; Williams, D.G. Chlorophyll fluorescence, predawn water potential and photosynthesis in precipitation pulse-driven ecosystems—Implications for ecological studies. Funct. Ecol. 2008, 22, 479–483. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Yactayo, W.; Rens, L.R.; Rolando, J.L.; Palacios, S.; de Mendiburu, F.; Mares, V.; Barreda, C.; Loayza, H.; Monneveux, P.; et al. Defining biological thresholds associated with plant water status for monitoring water restriction effects: Stomatal conductance and photosynthesis recovery as key indicators in potato. Agric. Water Manag. 2016, 117, 369–378. [Google Scholar] [CrossRef]
- Chaves, M.M. Effects of Water Deficits on Carbon Assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Cifre, J.; Mariano Escalona, J.; Galmés, J.; Gulías, J.; Lefi, E.; Martinez-Cañellas, S.F.; Moreno, M.T.; Ribas-Carbó, M.; et al. Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Rinza, J.; Ramírez, D.A.; García, J.; de Mendiburu, F.; Yactayo, W.; Barreda, C.; Velasquez, T.; Mejía, A.; Quiroz, R. Infrared Radiometry as a Tool for Early Water Deficit Detection: Insights into Its Use for Establishing Irrigation Calendars for Potatoes Under Humid Conditions. Potato Res. 2019, 62, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Cucho-Padin, G.; Rinza, J.; Ninanya, J.; Loayza, H.; Quiroz, R.; Ramírez, D.A. Development of an open-source thermal image processing software for improving irrigation management in potato crops (Solanum tuberosum L.). Sensors 2020, 20, 472. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Olechowicz, J.; Chomontowski, C.; Olechowicz, P.; Pietkiewicz, S.; Jajoo, A.; Kalaji, M.H. Impact of intraspecific competition on photosynthetic apparatus efficiency in potato (Solanum tuberosum) plants. Photosynthetica 2018, 56, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Ann. Rev. Plant Phys. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Rolando, J.L.; Yactayo, W.; Monneveux, P.; Mares, V.; Quiroz, R. Improving potato drought tolerance through the induction of long-term water stress memory. Plant Sci. 2015, 238, 26–32. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Jompuk, C.; Ribaut, J.M.; Stamp, P.; Leipner, J. Genetic analysis of cold tolerance of photosynthesis in maize. Plant Mol. Biol. 2004, 56, 241–253. [Google Scholar] [CrossRef]
- Anithakumari, A.M.; Nataraja, K.N.; Visser, R.G.; van der Linden, C.G. Genetic dissection of drought tolerance and recovery potential by quantitative trait locus mapping of a diploid potato population. Mol. Breed. 2012, 30, 1413–1429. [Google Scholar] [CrossRef] [Green Version]
- Erdem, T.; Orta, A.H.; Erdem, Y.; Okursoy, H. Crop water stress index for potato under furrow and drip irrigation systems. Potato Res. 2005, 48, 49–58. [Google Scholar] [CrossRef]
- Gutiérrez-Rosales, R.O.; Espinoza-Trelles, J.A.; Bonierbale, M. UNICA: Variedad Peruana para mercado fresco y papa frita con tolerancia y resistencia para condiciones climáticas adversas. Rev. ALAP 2007, 14, 45–50. [Google Scholar]
- Twarakavi, N.K.; Sakai, M.; Šimůnek, J. An objective analysis of the dynamic nature of field capacity. Water Resour. Res. 2014, 45, 153–155. [Google Scholar] [CrossRef]
- Topp, G.C.; Parkin, G.W.; Ferré, T.P.; Carter, M.R.; Gregorich, E.G. Sample moisture content. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, New York, USA, 2008; pp. 41–44. [Google Scholar]
- Resco, V.; Ewers, B.E.; Sun, W.; Huxman, T.E.; Weltzin, J.F.; Williams, D.G. Drought-induced hydraulic limitations constrain leaf gas exchange recovery after precipitation pulses in the C3 woody legume, Prosopis velutina. New Phytol. 2009, 181, 672–682. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Soc. Agriic. 1937, 160, 268. [Google Scholar]
- Wolfinger, R.D.; Chang, M. Comparing the SAS GLM and MIXED procedures for repeated measures Cary. In NC: SUGI Proceedings; The SAS Institute: Cary, NC, USA, 1998. [Google Scholar]
- Ramírez, D.A.; Yactayo, W.; Gutiérrez, R.; Mares, V.; De Mendiburu, F.; Posadas, A.; Quiroz, R. Chlorophyll concentration in leaves is an indicator of potato tuber yield in water-shortage conditions. Sci. Hortic-Amst. 2014, 168, 202–209. [Google Scholar] [CrossRef]
- R Core Team. R Software. Version, 5.6.1. Available online: https://www.r-project.org/ (accessed on 15 May 2020).
- Silva, C.; Ramirez, D.; Rinza, J. Dataset for: The photosynthesis recovery, a key trait as indicator of an appropriate irrigation criteria in potato crop. Int. Potato Cent. 2019, V2. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Rolando, J.L.; Yactayo, W.; Monneveux, P.; Quiroz, R. Is discrimination of 13C in potato leaflets and tubers an appropriate trait to describe genotype responses to restrictive and well-watered conditions? J. Agron. Crop Sci. 2015, 201, 410–418. [Google Scholar] [CrossRef]
- Schapendonk, A.H.C.M.; Spitters, C.J.T.; Groot, P.J. Effects of water stress on photosynthesis and chlorophyll fluorescence of five potato cultivars. Potato Res. 1989, 32, 17–32. [Google Scholar] [CrossRef]
- Flexas, J.; Gallé, A.; Galmés, J.; Ribas-Carbo, M.; Medrano, H. The Response of Photosynthesis to Soil Water Stress. In Plant Resp. Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 32, pp. 129–144. [Google Scholar]
- Rivas, R.; Falcão, H.M.; Ribeiro, R.V.; Machado, E.C.; Pimentel, C.; Santos, M.G. Drought tolerance in cowpea species is driven by less sensitivity of leaf gas exchange to water deficit and rapid recovery of photosynthesis after rehydration. S. Afr. J. Bot. 2016, 103, 101–107. [Google Scholar] [CrossRef]
- Yi, X.P.; Zhang, Y.L.; Yao, H.S.; Luo, H.H.; Gou, L.; Chow, W.S.; Zhang, W.F. Rapid recovery of photosynthetic rate following soil water deficit and rewatering in cotton plants (Gossypium herbaceum L.) is related to the stability of the photosystems. J. Plant Physiol. 2016, 194, 23–34. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, L.; Ñústez, C.E.; Moreno, L.P. Drought stress affects physiological parameters but not tuber yield in three Andean potato (Solanum tuberosum L.) cultivars. Agron. Colomb. 2017, 35, 158–170. [Google Scholar] [CrossRef]
- Prange, R.K. Chlorophyll fluorescence in vivo as an indicator of water stress in potato leaves. Am. Potato J. 1986, 63, 325–333. [Google Scholar] [CrossRef]
- Gucci, R.; Massai, R.; Casano, S.; Costagli, G. The effect of leaf age on CO2 assimilation and stomatal conductance of field-grown olive trees. Acta Hortic. 1991, 4, 325–333. [Google Scholar] [CrossRef]
- David, M.M.; Coelho, D.; Barrote, I.; Correia, M.J. The effect of leaf age on CO2 assimilation and stomatal conductance of field-grown olive trees. Funct. Plant Biol. 1998, 25, 299–306. [Google Scholar] [CrossRef]
- Erdem, T.; Erdem, Y.; Orta, H.; Okursoy, H. Water-yield relationships of potato under different irrigation methods and regimens. Sci. Agric. 2006, 63, 226–231. [Google Scholar] [CrossRef]
- Ati, A.S.; Iyada, A.D.; Najim, S.M. Water use efficiency of potato (Solanum tuberosum L.) under different irrigation methods and potassium fertilizer rates. Ann. Agric. Sci. 2012, 57, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.M.; Gallé, A.; Galmés, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought–adapted Vitis hybrid Richter–110 (V. berlandierixV. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot.-Lond. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Yactayo, W.; Ramírez, D.A.; Gutiérrez, R.; Mares, V.; Posadas, A.; Quiroz, R. Effect of partial root-zone drying irrigation timing on potato tuber yield and water use efficiency. Agric. Water Manag. 2013, 123, 65–70. [Google Scholar] [CrossRef]
- Clavel, D.; Diouf, O.; Khalfaoui, J.L.; Braconnier, S. Genotypes variations in fluorescence parameters among closely related groundnut (Arachis hypogaea L.) lines and their potential for drought screening programs. Field Crops Res. 2006, 96, 296–306. [Google Scholar] [CrossRef]
- Bürling, K.; Cerovic, Z.G.; Cornic, G.; Ducruet, J.M.; Noga, G.; Hunsche, M. Fluorescence-based sensing of drought-induced stress in the vegetative phase of four contrasting wheat genotypes. Environ. Exp. Bot. 2013, 89, 51–59. [Google Scholar] [CrossRef]
- Germ, M. The response of two potato cultivars on combined effects of selenium and drought. Acta Agric. Slov. 2008, 91, 121–137. [Google Scholar] [CrossRef]
- Hao, S.; Cao, H.; Wang, H.; Pan, X. A PRI-based water stress index combining structural and chlorophyll effects: Assessment using diurnal narrow-band airborne imagery and the CWSI thermal index. Sci. Hortic.-Amst. 2019, 249, 143–154. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of Photosynthesis in C3 Plants: Stomatal and Non stomatal Limitations Revisited. Ann. Bot.-Lond. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onder, S.; Caliskan, M.E.; Onder, D.; Caliskan, S. Different irrigation methods and water stress effects on potato yield and yield components. Agric. Water Manag. 2005, 73, 73–86. [Google Scholar] [CrossRef]
- Wang, F.-X.; Kang, Y.; Liu, S.-P. Effects of drip irrigation frequency on soil wetting pattern and potato growth in North China Plain. Agric. Water Manag. 2006, 79, 248–264. [Google Scholar] [CrossRef]
- Badr, M.A.; El-Tohamy, W.A.; Zaghloul, A.M. Yield and water use efficiency of potato grown under different irrigation and nitrogen levels in an arid region. Agric. Water Manag. 2012, 110, 9–15. [Google Scholar] [CrossRef]
- Stikic, R.; Savíc, S.; Jovanovic, Z.; Jacobsen, S.E.; Liu, F.; Jensen, C.R. Deficit irrigation strategies: Use of stress physiology knowledge to increase water use efficiency in tomato and potato. In Horticulture in the 21st Century; Sampson, A.N., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2010; pp. 161–178. [Google Scholar]
- Medici, L.O.; Reinert, F.; Carvalho, D.F.; Kozak, M.; Azevedo, R.A. What about keeping plants well watered? Environ. Exp. Bot. 2014, 99, 38–42. [Google Scholar] [CrossRef]
- Shahnazari, A.; Liu, F.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Effects of partial root-zone drying on yield, tuber size and water use efficiency in potato under field conditions. Field Crops Res. 2007, 100, 117–124. [Google Scholar] [CrossRef]
- Xie, K.; Wang, X.X.; Zhang, R.; Gong, X.; Zhang, S.; Mares, V.; Gavilán, C.; Posadas, A.; Quiroz, R. Partial root-zone drying irrigation and water utilization efficiency by the potato crop in semi-arid regions in China. Sci. Hortic. 2012, 134, 20–25. [Google Scholar] [CrossRef]


| July | August | September | October | |
|---|---|---|---|---|
| Average temperature (C) | 16.8 ± 0.10 | 15.8 ± 0.10 | 15.6 ± 0.12 | 16.8 ± 0.15 |
| Maximum temperature (C) | 19.8 ± 0.29 | 19.2 ± 0.27 | 19.0 ± 0.38 | 21.5 ± 0.30 |
| Minimum temperature (C) | 15.3 ± 0.14 | 14.3 ± 0.10 | 14.0 ± 0.08 | 14.6 ± 0.08 |
| Average relative humidity (%) | 85.4 ± 0.61 | 88.7 ± 0.57 | 91.2 ± 0.59 | 89.6 ± 0.46 |
| Average VPD (kPa) | 0.24 ± 0.01 | 0.18 ± 0.01 | 0.14 ± 0.01 | 0.17 ± 0.01 |
| Maximum VPD (kPa) | 0.68 ± 0.03 | 0.60 ± 0.03 | 0.56 ± 0.05 | 0.84 ± 0.03 |
| Solar radiation (MJ m day) | 8.5 ± 0.77 | 8.8 ± 0.69 | 9.4 ± 0.96 | 16.8 ± 0.68 |
| Maximum PAR density (mol m s) | 1084.0 ± 62.2 | 1057.2 ± 75.0 | 1006.0 ± 89.1 | 1692.3 ± 30.5 |
| Comp.1 | Comp.2 | |
|---|---|---|
| IP | 0.35 | 0.80 |
| TT | 0.67 | −0.59 |
| D | 0.83 | 0.37 |
| PMA (−1) | −0.92 | −0.27 |
| PMA+ | −0.84 | 0.51 |
| % TCV | 56.5 | 85.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Díaz, C.; Ramírez, D.A.; Rodríguez-Delfín, A.; de Mendiburu, F.; Rinza, J.; Ninanya, J.; Loayza, H.; Quiroz, R. Unraveling Ecophysiological Mechanisms in Potatoes under Different Irrigation Methods: A Preliminary Field Evaluation. Agronomy 2020, 10, 827. https://doi.org/10.3390/agronomy10060827
Silva-Díaz C, Ramírez DA, Rodríguez-Delfín A, de Mendiburu F, Rinza J, Ninanya J, Loayza H, Quiroz R. Unraveling Ecophysiological Mechanisms in Potatoes under Different Irrigation Methods: A Preliminary Field Evaluation. Agronomy. 2020; 10(6):827. https://doi.org/10.3390/agronomy10060827
Chicago/Turabian StyleSilva-Díaz, Cecilia, David A. Ramírez, Alfredo Rodríguez-Delfín, Felipe de Mendiburu, Javier Rinza, Johan Ninanya, Hildo Loayza, and Roberto Quiroz. 2020. "Unraveling Ecophysiological Mechanisms in Potatoes under Different Irrigation Methods: A Preliminary Field Evaluation" Agronomy 10, no. 6: 827. https://doi.org/10.3390/agronomy10060827
APA StyleSilva-Díaz, C., Ramírez, D. A., Rodríguez-Delfín, A., de Mendiburu, F., Rinza, J., Ninanya, J., Loayza, H., & Quiroz, R. (2020). Unraveling Ecophysiological Mechanisms in Potatoes under Different Irrigation Methods: A Preliminary Field Evaluation. Agronomy, 10(6), 827. https://doi.org/10.3390/agronomy10060827










