Steam Refining with Subsequent Alkaline Lignin Extraction as an Alternative Pretreatment Method to Enhance the Enzymatic Digestibility of Corn Stover
Abstract
1. Introduction
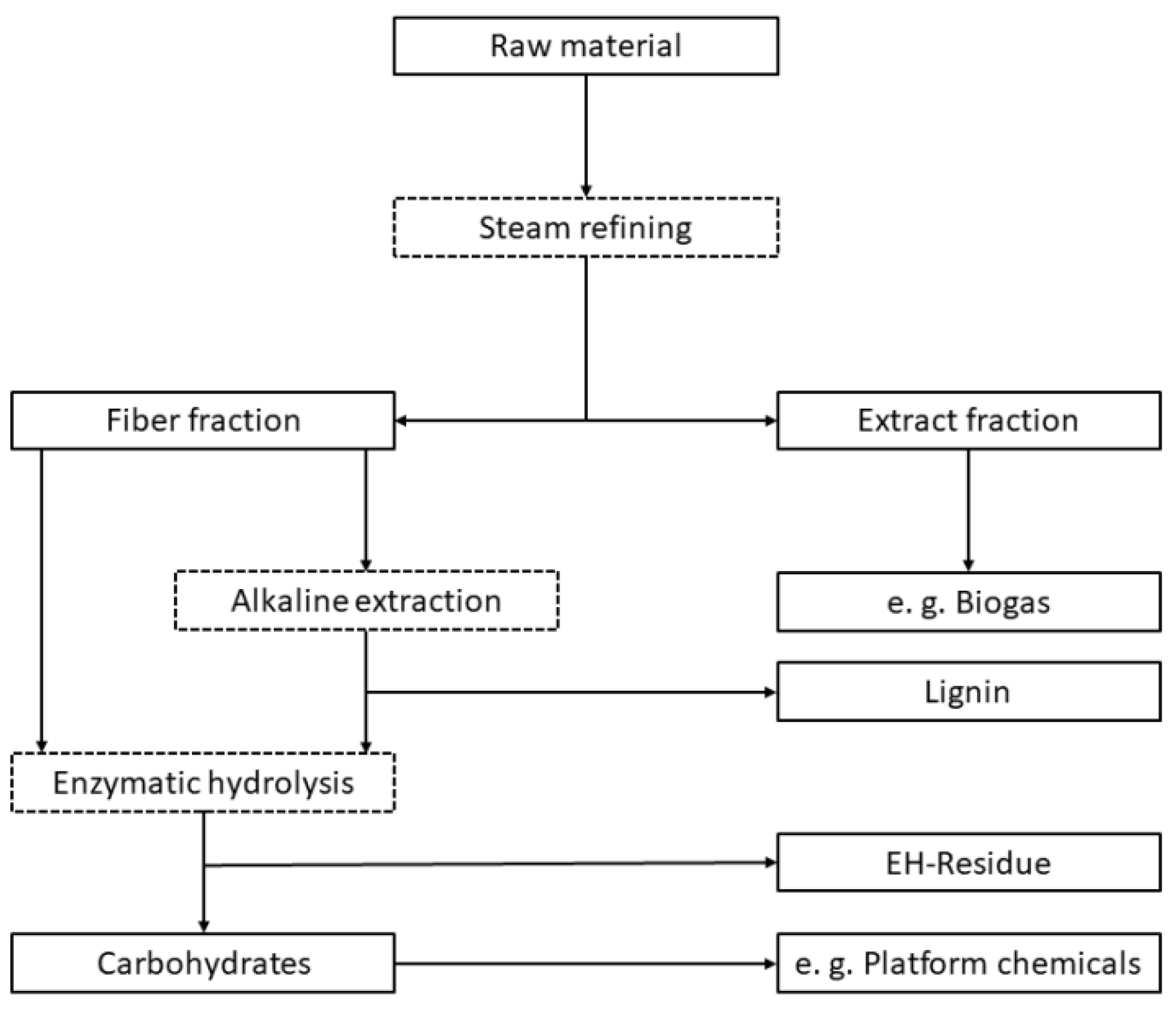
2. Materials and Methods
2.1. Raw Material
2.2. Steam Refining
2.3. Acid Hydrolysis of the Extract and Fibers
2.4. Alkaline Lignin Extraction
2.5. Enzymatic Hydrolysis (EH)
2.6. Analytical Methods
3. Results and Discussion
3.1. Raw Material Characterization
3.2. Fractions after Steam Refining and Characterization
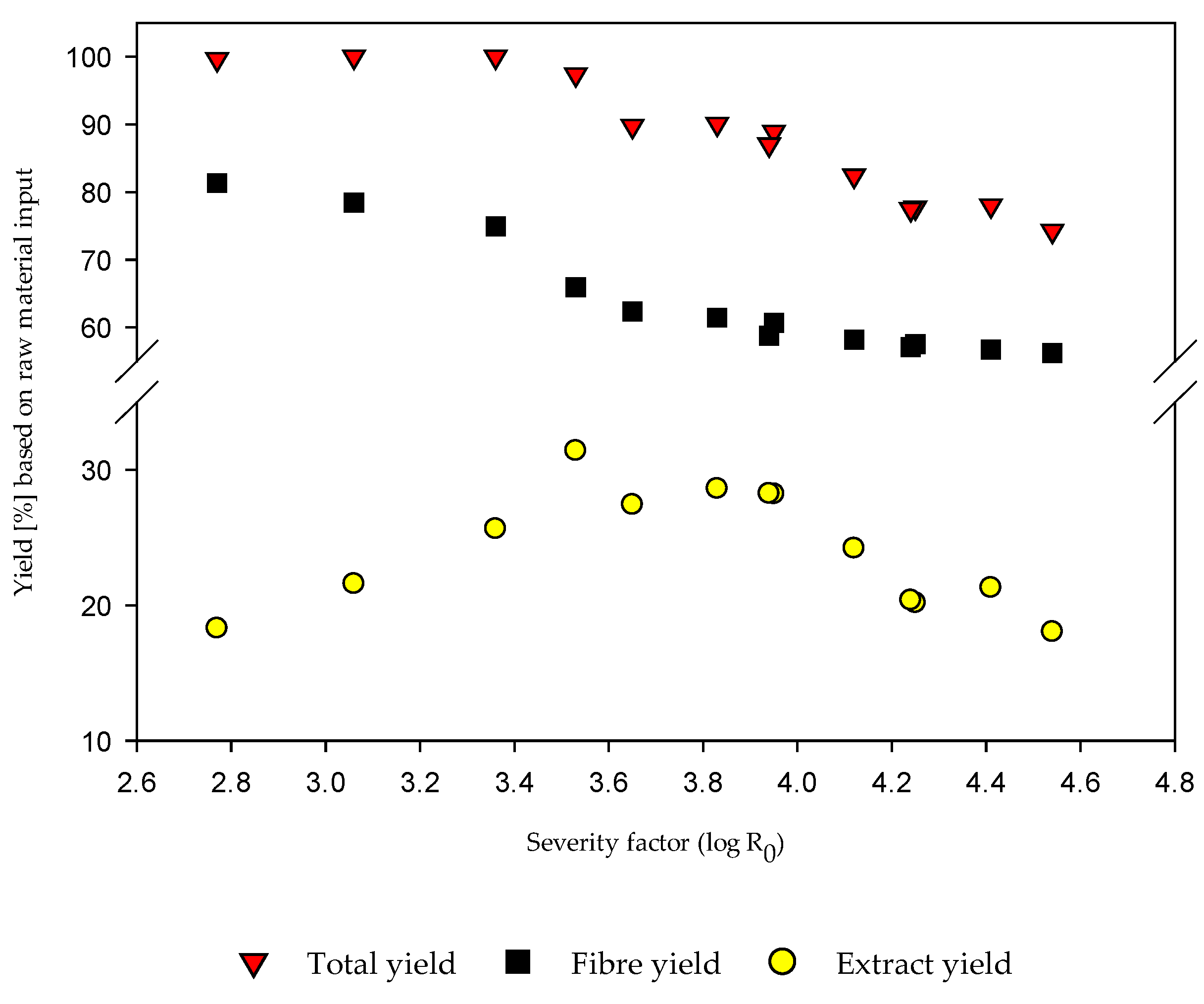
3.2.1. Fiber and Extract Yields
3.2.2. Composition of Fibers and Extracts
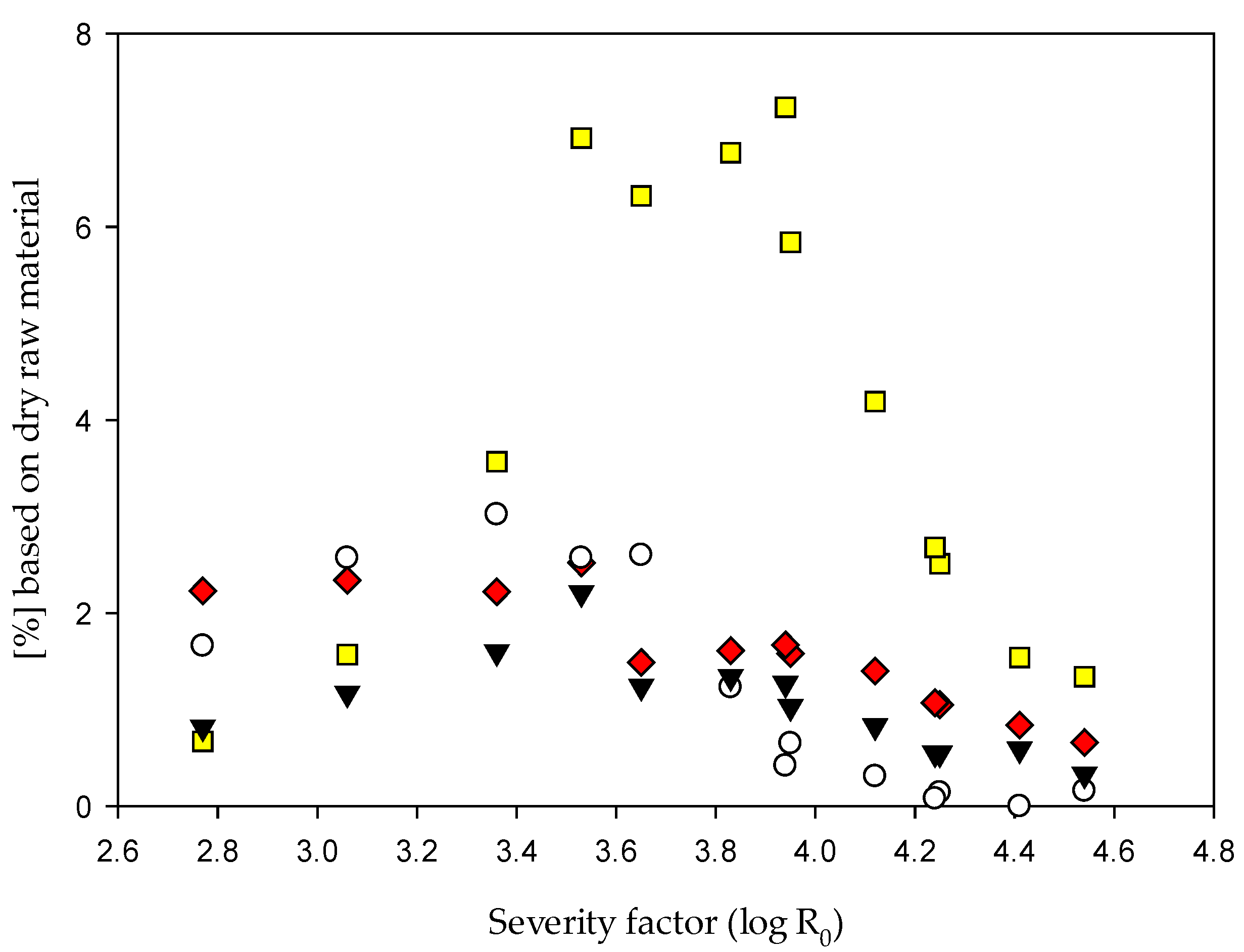
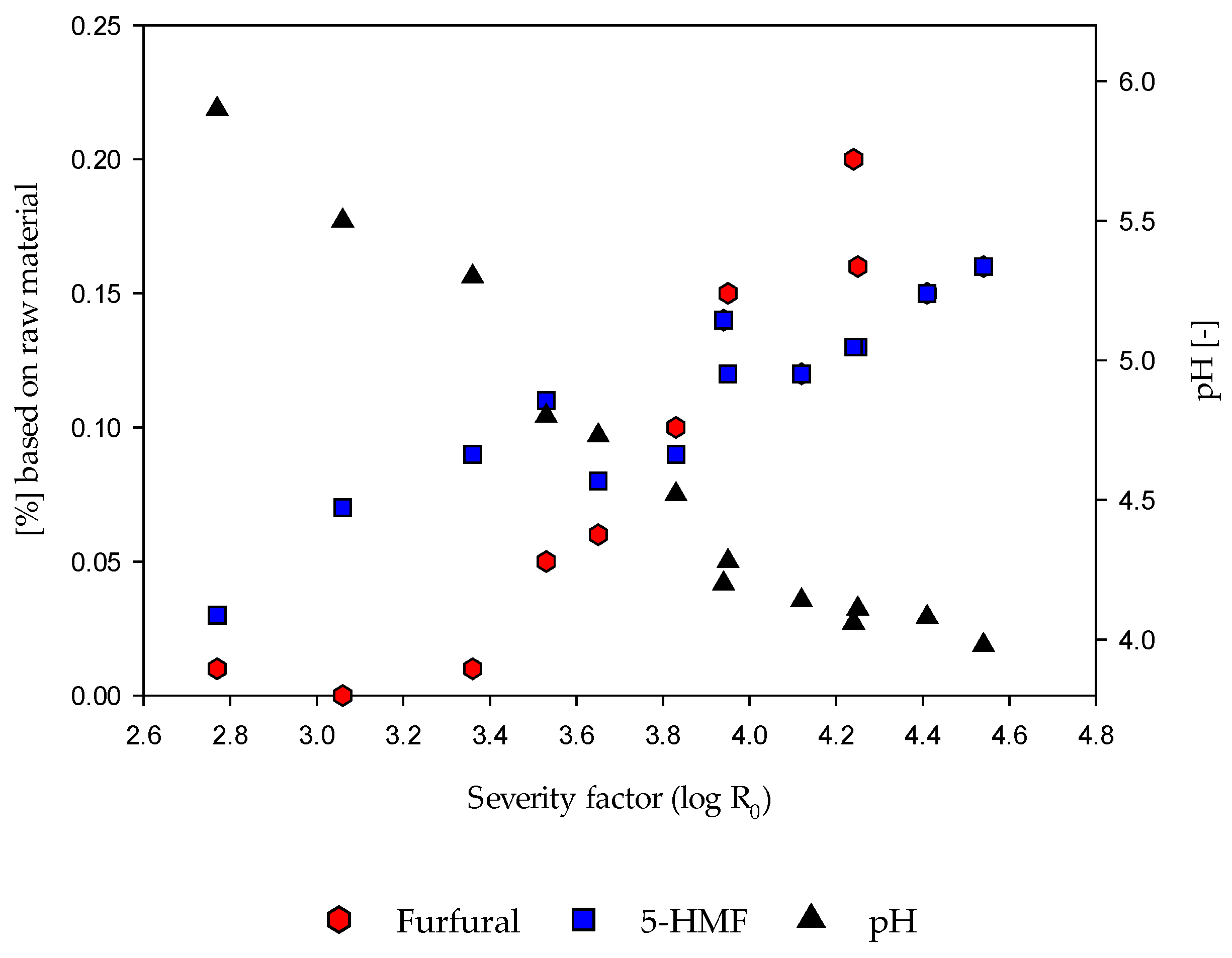
3.2.3. Detection of 5-HMF, Furfural and pH Value
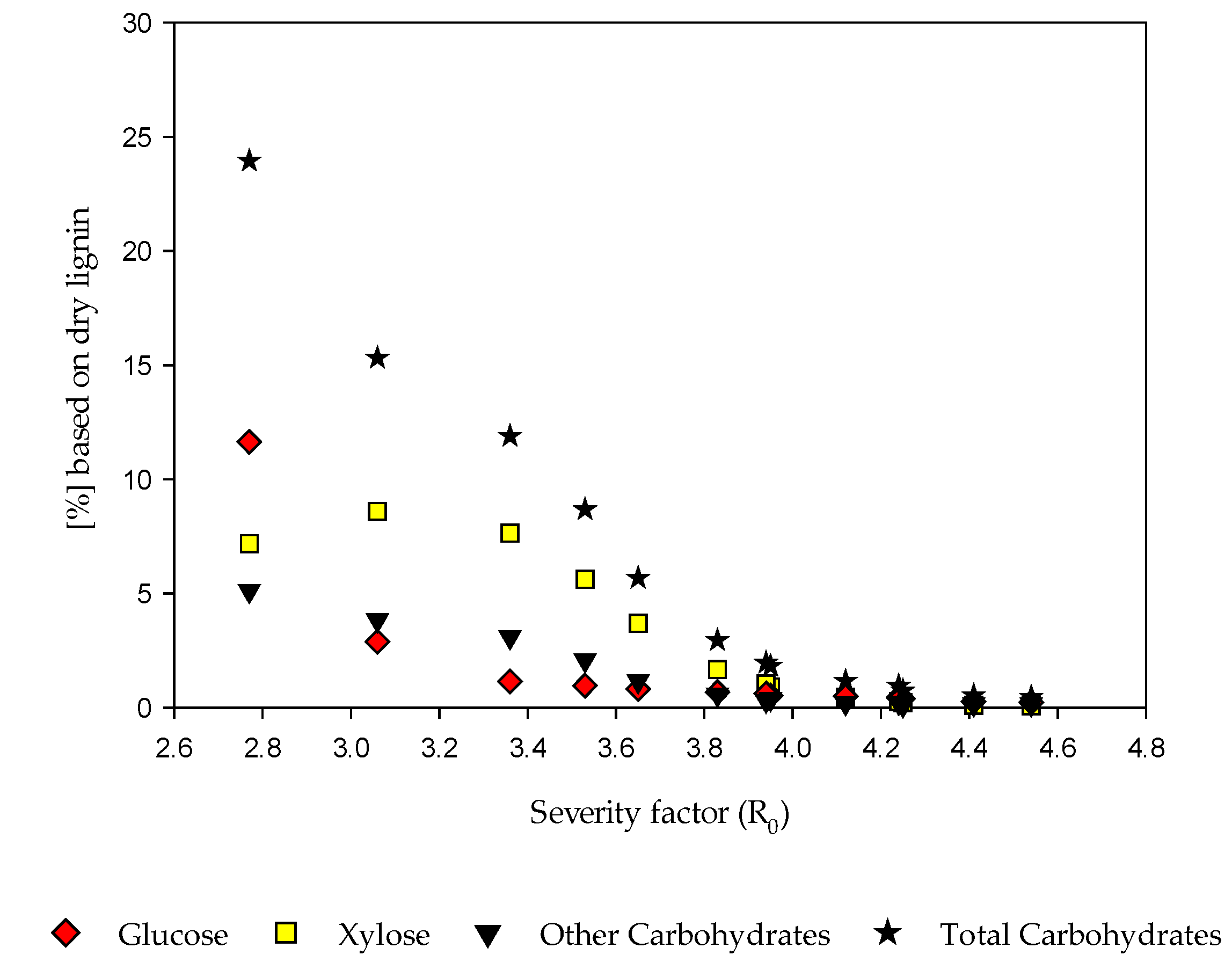
3.3. Alkaline Lignin Extraction
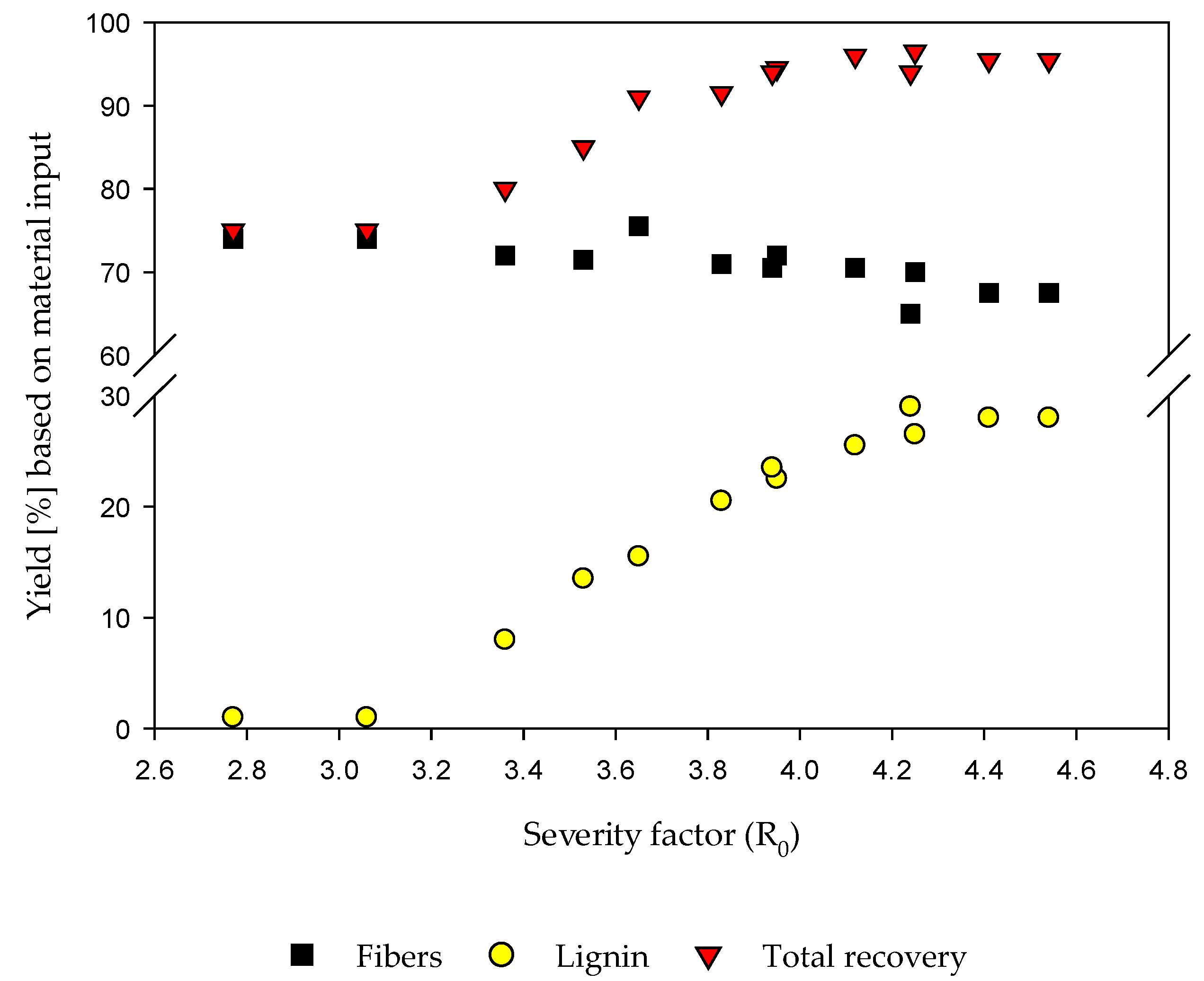
3.3.1. Fiber and Lignin Yields
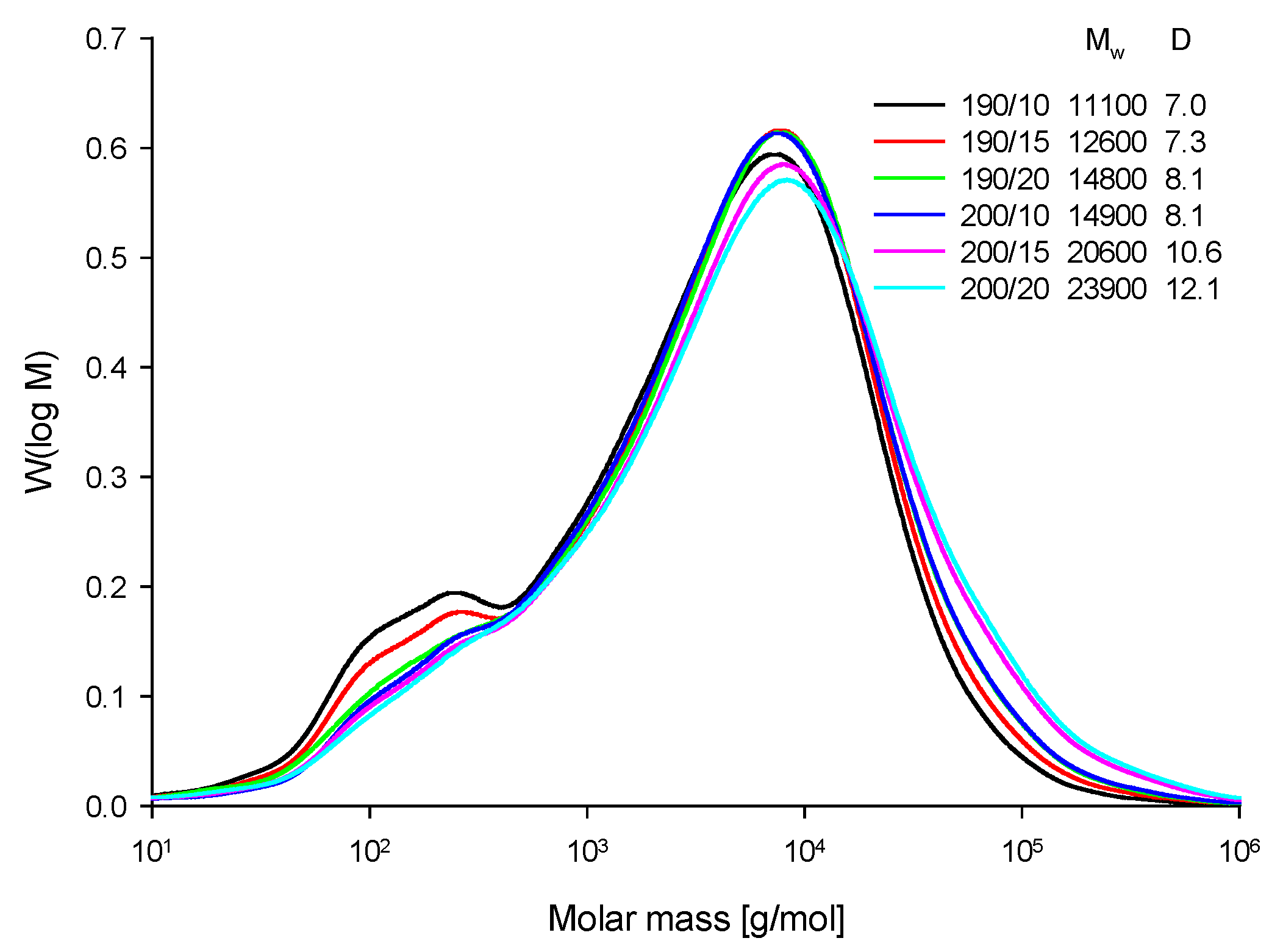
3.3.2. Analysis of the Lignins
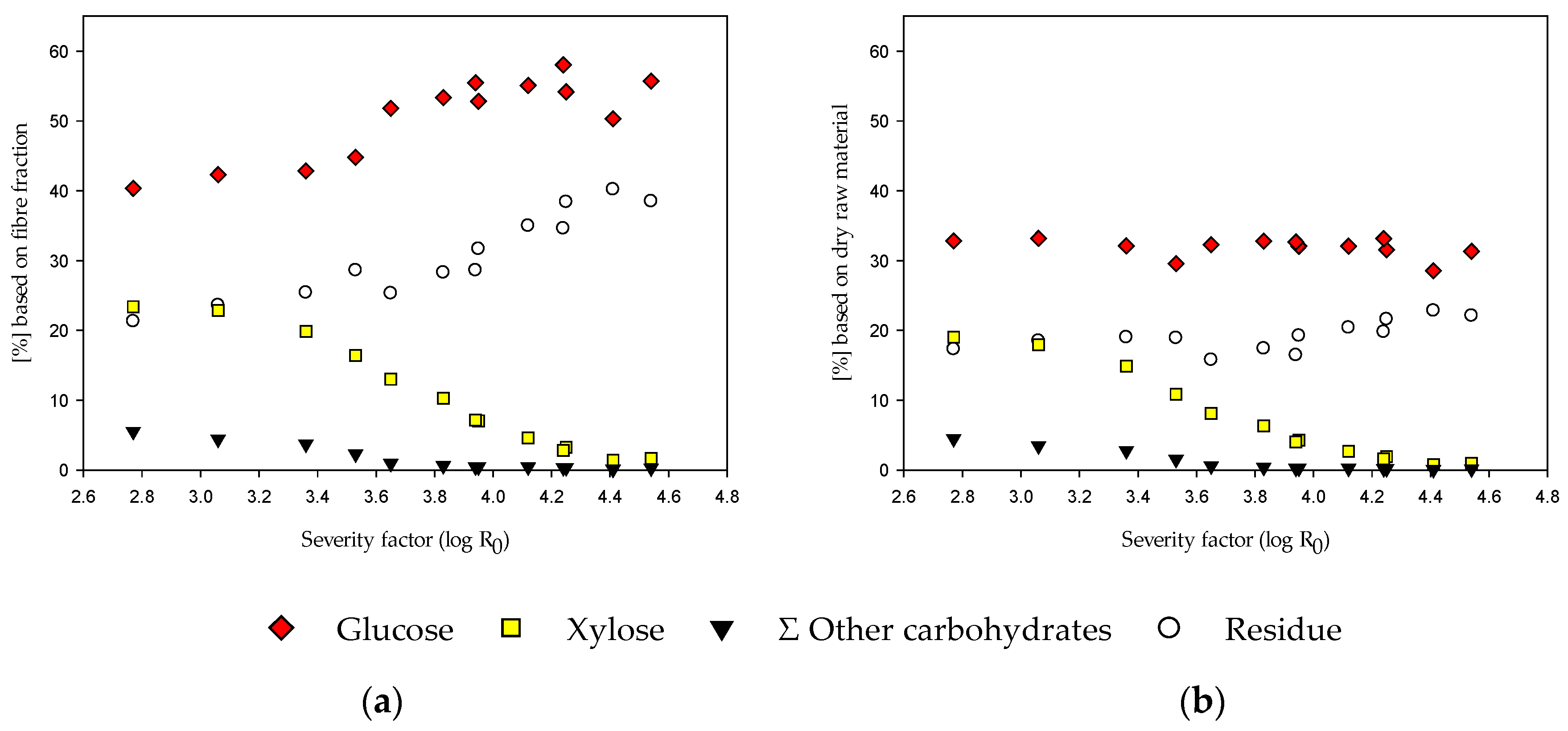
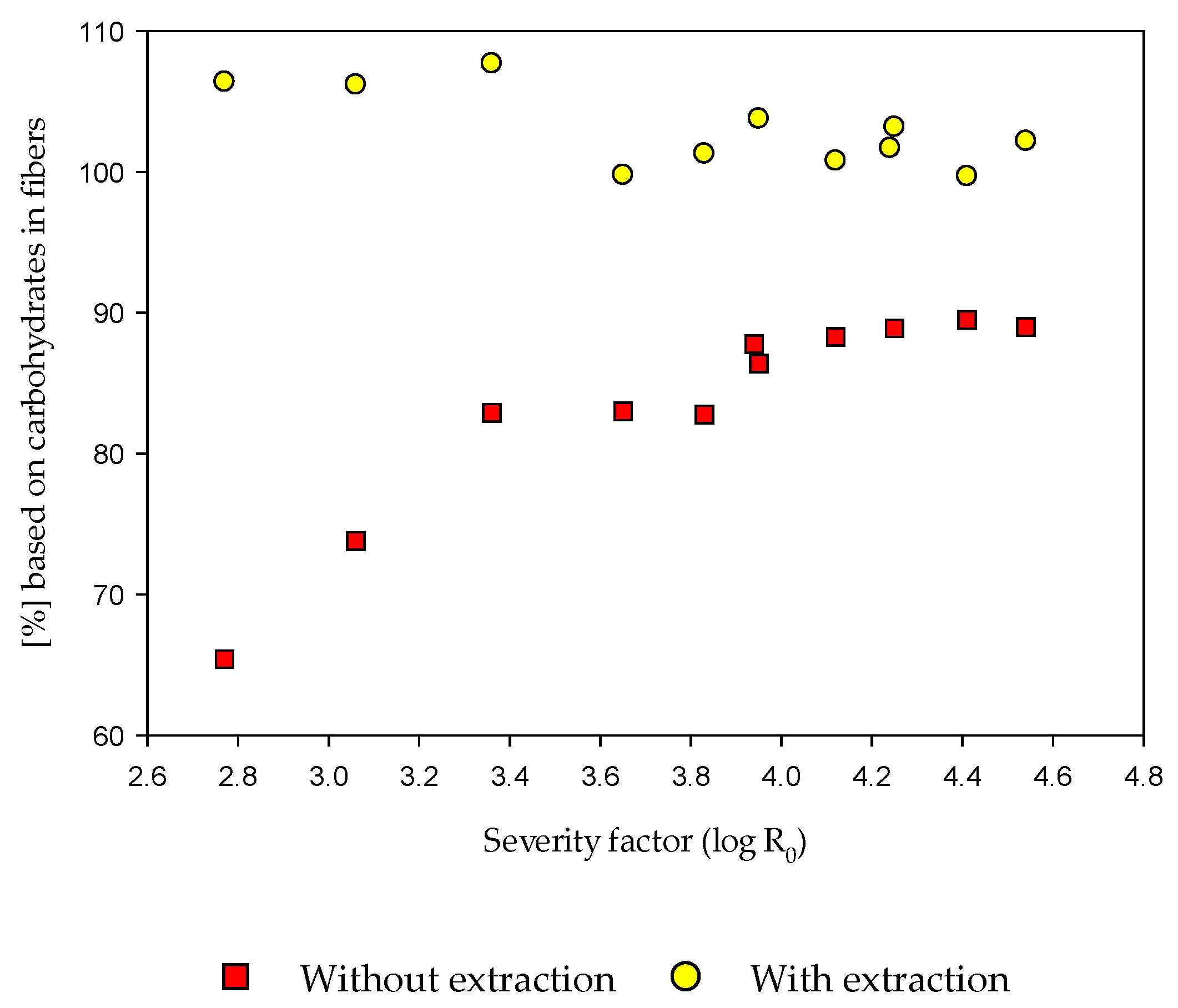
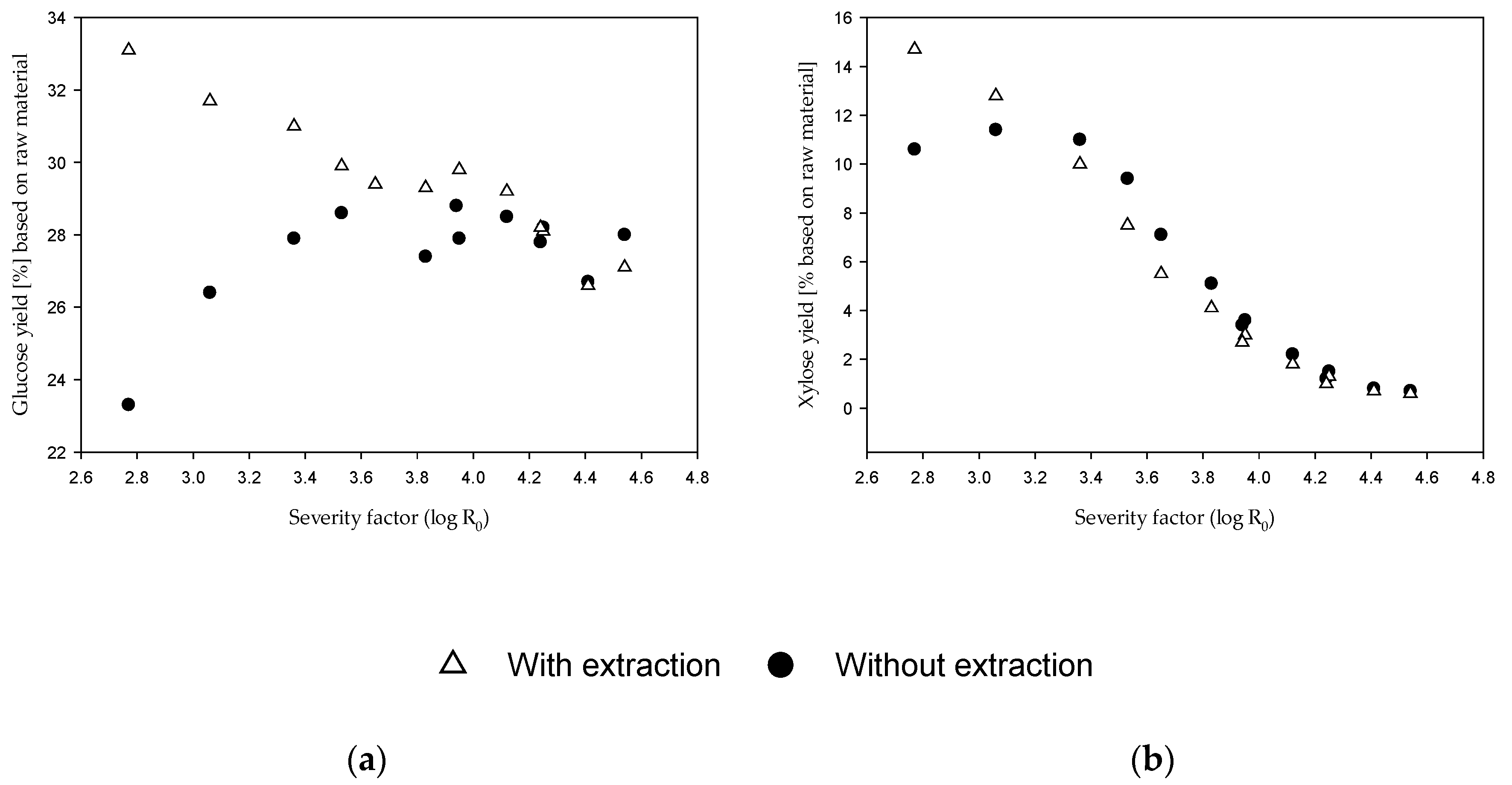
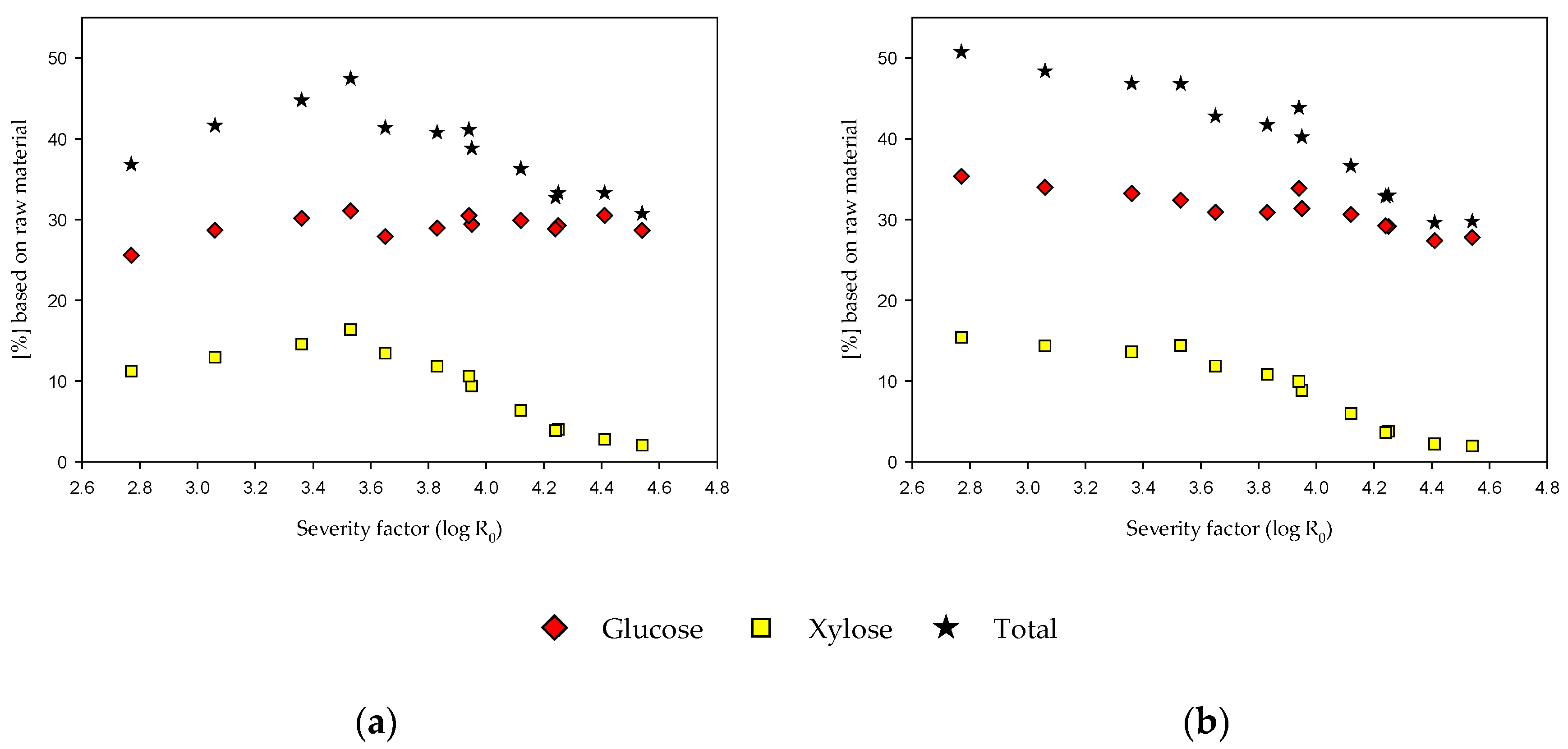
3.4. EH of the Fiber Fraction with and without Alkaline Extraction
3.5. Overall Process Balances
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Damgaard, A.; Christensen, T.H. Bioethanol from corn stover—A review and technical assessment of alternative biotechnologies. Prog. Energy Combust. Sci. 2018, 67, 275–291. [Google Scholar] [CrossRef]
- U.S. Department of Energy. U.S. Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry; U.S. Department of Energy: Washington, DC, USA, 2011.
- Garlock, R.J.; Chundawat, S.P.S.; Balan, V.; Dale, B. Optimizing harvest of corn stover fractions based on overall sugar yields following ammonia fiber expansion pretreatment and enzymatic hydrolysis. Biotechnol. Biofuels 2009, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Kadam, K.L.; McMillan, J.D. Availability of corn stover as a sustainable feedstock for bioethanol production. Bioresour. Technol. 2003, 88, 17–25. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 2002, 59, 618–628. [Google Scholar] [CrossRef]
- Klupsch, R. Untersuchungen zur Herstellung von Chemiezellstoff aus Aspen-und Buchenholz nach dem Dampfdruck-Extraktionsverfahren. Dissertation Thesis, University of Hamburg, Hamburg, Germany, 2000. [Google Scholar]
- Klupsch, R.; Kordsachia, O.; Puls, J.; Karstens, T. Herstellung von Chemiezellstoffen nach dem Dampfdruck-Extraktionsverfahren. Ipw Int. Pap. Das Pap. 2001, 55, 73–79. [Google Scholar]
- Schütt, F.; Puls, J.; Saake, B. Optimization of steam pretreatment conditions for enzymatic hydrolysis of poplar wood. Holzforschung 2011, 65, 453–459. [Google Scholar] [CrossRef]
- Schütt, F.; Westereng, B.; Horn, S.J.; Puls, J.; Saake, B. Steam refining as an alternative to steam explosion. Bioresour. Technol. 2012, 111, 476–481. [Google Scholar] [CrossRef]
- Stücker, A.; Schütt, F.; Saake, B.; Lehnen, R. Lignins from enzymatic hydrolysis and alkaline extraction of steam refined poplar wood: Utilization in lignin-phenol-formaldehyde resins. Ind. Crop. Prod. 2016, 85, 300–308. [Google Scholar] [CrossRef]
- Poutanen, K.; Viikari, L. Biotechnical utilization of wood carbohydrates after steaming pretreatment. Appl. Microbiol. Biotechnol. 1985, 22, 416–423. [Google Scholar] [CrossRef]
- Brownell, H.H.; Yu, E.K.C.; Saddler, J.N. Steam-explosion pretreatment of wood: Effect of chip size, acid, moisture content and pressure drop. Biotechnol. Bioeng. 1986, 28, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb. Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef]
- Chinnappan, A.; Baskar, C.; Kim, H. Biomass into chemicals: Green chemical conversion of carbohydrates into 5-hydroxymethylfurfural in ionic liquids. RSC Adv. 2016, 6, 63991–64002. [Google Scholar] [CrossRef]
- Mamman, A.S.; Lee, J.-M.; Kim, Y.-C.; Hwang, I.T.; Park, N.-J.; Hwang, Y.K.; Chang, J.-S.; Hwang, J.-S. Furfural: Hemicellulose/xylosederived biochemical. Biofuels Bioprod. Biorefining 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Himmel, M.E.; Adney, W.S.; Baker, J.O.; Nieves, R.A.; Thomas, S.R. Cellulases: Structure, Function, and Applications. In Handbook on Bioethanol: Production and Utilization; Wyman, C.E., Ed.; Routledge: Boca Raton, FL, USA, 1996; pp. 143–161. ISBN 1-56032-553-4. [Google Scholar]
- Becker, J.; Lange, A.; Fabarius, J.; Wittmann, C. Top value platform chemicals: Bio-based production of organic acids. Curr. Opin. Biotechnol. 2015, 36, 168–175. [Google Scholar] [CrossRef]
- Choi, S.; Song, C.W.; Shin, J.; Lee, S.Y. Biorefineries for the production of top building block chemicals and their derivatives. Metab. Eng. 2015, 28, 223–239. [Google Scholar] [CrossRef]
- Rosenau, T. Advances in biorefinery research. Holzforschung 2018, 73, 1–2. [Google Scholar] [CrossRef]
- Chang, V.S.; Holtzapple, M.T. Fundamental factors affecting biomass enzymatic reactivity. Appl. Biochem. Biotechnol. 2000, 84, 5–37. [Google Scholar] [CrossRef]
- Pareek, N.; Gillgren, T.; Jönsson, L.J. Adsorption of proteins involved in hydrolysis of lignocellulose on lignins and hemicelluloses. Bioresour. Technol. 2013, 148, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, C.I.; Jeoh, T.; Adney, W.S.; Himmel, M.; Johnson, D.K.; Davis, M. Can delignification decrease cellulose digestibility in acid pretreated corn stover? Cellulose 2009, 16, 677–686. [Google Scholar] [CrossRef]
- Chen, Y.; Stevens, M.A.; Zhu, Y.; Holmes, J.; Xu, H. Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnol. Biofuels 2013, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.; Erasmy, N.; Akil, Y.; Saake, B. A new method for the quantification of monosaccharides, uronic acids and oligosaccharides in partially hydrolyzed xylans by HPAEC-UV/VIS. Carbohydr. Polym. 2016, 140, 181–187. [Google Scholar] [CrossRef]
- Bendler, M. Dampfdruck-Refiner-Aufschluss und Enzymatische Hydrolyse von Maisstroh. Bachelor’s Thesis, Hamburg University of Applied Sciences, Hamburg, Germany, 2018. [Google Scholar]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Podschun, J.; Saake, B.; Lehnen, R. Reactivity enhancement of organosolv lignin by phenolation for improved bio-based thermosets. Eur. Polym. J. 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Pordesimo, L.O.; Edens, W.C.; Sokhansanj, S. Distribution of Above Ground Biomass in Corn Stover. In Proceedings of the 2002 ASAE Annual International Meeting/CIGR XVth World Congress, Chicago, IL, USA, 28–31 July 2002; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2002. [Google Scholar]
- Templeton, D.; Sluiter, A.D.; Hayward, T.K.; Hames, B.R.; Thomas, S.R. Assessing corn stover composition and sources of variability via NIRS. Cellulose 2009, 16, 621–639. [Google Scholar] [CrossRef]
- Deutschle, A. Charakterisierung und Anwendung von Kationischen Arabinoxylanen. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2015. [Google Scholar]
- Baar, J.; Paschová, Z.; Hofmann, T.; Kolář, T.; Koch, G.; Saake, B.; Rademacher, P. Natural durability of subfossil oak: Wood chemical composition changes through the ages. Holzforschung 2019, 74, 47–59. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Soybean, S. Corn Stover and Sunflower Stalk as Possible Substrates for Biogas Production in Croatia: A Review. Chem. Biochem. Eng. Q. 2017, 31, 187–198. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.J.; Ding, S.-Y.; Mielenz, J.R.; Cui, J.-B.; Elander, R.T.; Laser, M.; Himmel, M.E.; McMillan, J.R.; Lynd, L.R. Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 2007, 97, 214–223. [Google Scholar] [CrossRef]
- Saha, B.C.; Yoshida, T.; Cotta, M.; Sonomoto, K. Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Ind. Crop. Prod. 2013, 44, 367–372. [Google Scholar] [CrossRef]
- Takada, M.; Chandra, R.P.; Saddler, J.N. The influence of lignin migration and relocation during steam pretreatment on the enzymatic hydrolysis of softwood and corn stover biomass substrates. Biotechnol. Bioeng. 2019, 116, 2864–2873. [Google Scholar] [CrossRef] [PubMed]
- Schütt, F. Dampfdruckaufschluss und Enzymatische Hydrolyse von Pappelholz. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2012. [Google Scholar]
- Bura, R.; Chandra, R.; Saddler, J.N. Influence of xylan on the enzymatic hydrolysis of steam-pretreated corn stover and hybrid poplar. Biotechnol. Prog. 2009, 25, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B.; Galbe, M.; Zacchi, G. The effect of water-soluble inhibitors from steam-pretreated willow on enzymatic hydrolysis and ethanol fermentation. Enzym. Microb. Technol. 1996, 19, 470–476. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Martín, C.; Galbe, M.; Nilvebrant, N.-O.; Jönsson, L.J. Comparison of the fermentability of enzymatic hydrolyzates of sugarcane bagasse pretreated by steam explosion using different impregnating agents. Appl. Biochem. Biotechnol. 2002, 98, 699–716. [Google Scholar] [CrossRef]
- Gurram, R.N.; Datta, S.; Lin, Y.J.; Snyder, S.; Menkhaus, T.J. Removal of enzymatic and fermentation inhibitory compounds from biomass slurries for enhanced biorefinery process efficiencies. Bioresour. Technol. 2011, 102, 7850–7859. [Google Scholar] [CrossRef]
- Kaar, W.; Gutierrez, C.; Kinoshita, C. Steam explosion of sugarcane bagasse as a pretreatment for conversion to ethanol. Biomass Bioenergy 1998, 14, 277–287. [Google Scholar] [CrossRef]
- Li, H.; Chen, H. Detoxification of steam-exploded corn straw produced by an industrial-scale reactor. Process. Biochem. 2008, 43, 1447–1451. [Google Scholar] [CrossRef]
- Jacquet, N.; Quiévy, N.; Vanderghem, C.; Janas, S.; Blecker, C.; Wathelet, B.; Devaux, J.; Paquot, M. Influence of steam explosion on the thermal stability of cellulose fibres. Polym. Degrad. Stab. 2011, 96, 1582–1588. [Google Scholar] [CrossRef]
- Han, G.; Deng, J.; Zhang, S.; Bicho, P.; Wu, Q. Effect of steam explosion treatment on characteristics of wheat straw. Ind. Crop. Prod. 2010, 31, 28–33. [Google Scholar] [CrossRef]
- Ruiz, E.; Cara, C.; Manzanares, P.; Ballesteros, M.; Castro, E.; Castro, E. Evaluation of steam explosion pre-treatment for enzymatic hydrolysis of sunflower stalks. Enzym. Microb. Technol. 2008, 42, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Um, B.-H.; Van Walsum, G.P. Effect of Pretreatment Severity on Accumulation of Major Degradation Products from Dilute Acid Pretreated Corn Stover and Subsequent Inhibition of Enzymatic Hydrolysis of Cellulose. Appl. Biochem. Biotechnol. 2012, 168, 406–420. [Google Scholar] [CrossRef]
- Li, J.; Gellerstedt, G.; Toven, K. Steam explosion lignins; their extraction, structure and potential as feedstock for biodiesel and chemicals. Bioresour. Technol. 2009, 100, 2556–2561. [Google Scholar] [CrossRef]
- Hendriks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Qin, L.; Jin, M.; Pang, F.; Li, B.-Z.; Kang, Y.; Dale, B.; Yuan, Y.-J. Evaluation of storage methods for the conversion of corn stover biomass to sugars based on steam explosion pretreatment. Bioresour. Technol. 2013, 132, 5–15. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Wong, K.K.Y.; Mackie, K.L. Ultrastructure of steam-exploded wood. Wood Sci. Technol. 1988, 22, 103–114. [Google Scholar] [CrossRef]
- Ramos, L.P.; Breuil, C.; Saddler, J.N. Comparison of steam pretreatment of eucalyptus, aspen, and spruce wood chips and their enzymatic hydrolysis. Appl. Biochem. Biotechnol. 1992, 34, 37–48. [Google Scholar] [CrossRef]
- Schell, D.; Nguyen, Q.; Tucker, M.; Boynton, B. Pretreatment of softwood by acid-catalyzed steam explosion followed by alkali extraction. Appl. Biochem. Biotechnol. 1998, 70, 17–24. [Google Scholar] [CrossRef]
- Schwald, W.; Breuil, C.; Brownell, H.H.; Chan, M.; Saddler, J.M. Assessment of pretreatment conditions to obtain fast complete hydrolysis on high substrate concentrations. Appl. Biochem. Biotechnol. 1989, 20, 29–44. [Google Scholar] [CrossRef]
- Toussaint, B.; Vignon, M.R. Saccharification of steam-exploded poplar wood. Biotechnol. Bioeng. 1991, 38, 1308–1317. [Google Scholar] [CrossRef]
| Temperature (°C) | Time (min) | Severity Factor (log R0) | |
|---|---|---|---|
| 1 | 160 | 10 | 2.77 |
| 2 | 170 | 10 | 3.06 |
| 3 | 180 | 10 | 3.36 |
| 4 | 180 | 15 | 3.53 |
| 5 | 190 | 10 | 3.65 |
| 6 | 190 | 15 | 3.83 |
| 7 | 190 | 20 | 3.95 |
| 8 | 200 | 10 | 3.94 |
| 9 | 200 | 15 | 4.12 |
| 10 | 200 | 20 | 4.25 |
| 11 | 210 | 10 | 4.24 |
| 12 | 210 | 15 | 4.41 |
| 13 | 210 | 20 | 4.54 |
| Time (min) | c (Eluent A) % | c (Eluent B) % |
|---|---|---|
| 0 | 97.5 | 2.5 |
| 20 | 85 | 15 |
| 50 | 68 | 32 |
| 56 | 62 | 38 |
| 60 | 59 | 41 |
| 63 | 58 | 42 |
| 70 | 58 | 42 |
| 80 | 0 | 100 |
| Raw Material | ||
|---|---|---|
| Extractives | Petrol ether | 0.8 |
| Acetone/Water (9:1) | 8.1 | |
| Water | 7.3 | |
| Σ | 16.2 | |
| Carbohydrates | Glucose | 35.6 |
| Xylose | 19.5 | |
| Arabinose | 2.9 | |
| Galactose | 0.9 | |
| Mannose | 0.3 | |
| Rhamnose | 0.1 | |
| Σ | 59.3 | |
| Lignin | acid-insoluble 1 | 17.1 2 |
| acid-soluble | 2.2 | |
| Σ | 19.3 2 | |
| Ash | 6.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krafft, M.J.; Bendler, M.; Schreiber, A.; Saake, B. Steam Refining with Subsequent Alkaline Lignin Extraction as an Alternative Pretreatment Method to Enhance the Enzymatic Digestibility of Corn Stover. Agronomy 2020, 10, 811. https://doi.org/10.3390/agronomy10060811
Krafft MJ, Bendler M, Schreiber A, Saake B. Steam Refining with Subsequent Alkaline Lignin Extraction as an Alternative Pretreatment Method to Enhance the Enzymatic Digestibility of Corn Stover. Agronomy. 2020; 10(6):811. https://doi.org/10.3390/agronomy10060811
Chicago/Turabian StyleKrafft, Malte Jörn, Marie Bendler, Andreas Schreiber, and Bodo Saake. 2020. "Steam Refining with Subsequent Alkaline Lignin Extraction as an Alternative Pretreatment Method to Enhance the Enzymatic Digestibility of Corn Stover" Agronomy 10, no. 6: 811. https://doi.org/10.3390/agronomy10060811
APA StyleKrafft, M. J., Bendler, M., Schreiber, A., & Saake, B. (2020). Steam Refining with Subsequent Alkaline Lignin Extraction as an Alternative Pretreatment Method to Enhance the Enzymatic Digestibility of Corn Stover. Agronomy, 10(6), 811. https://doi.org/10.3390/agronomy10060811






