Soil, Site, and Management Factors Affecting Cadmium Concentrations in Cacao-Growing Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling and Analysis
2.3. Site Factors, Agroecological Parameters and Land Management
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Cadmium Content in Soil of Cacao Plantations from Different Ecoregions
4.2. Factors Influencing Cadmium Concentrations in Cacao-Growing Soils
4.3. Implications for Land Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bateman, A.; Muñoz-Rojas, M. To whom the burden of soil degradation and management concerns. In Soil Degradation and Management in a Changing Environment; Pereira, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 4, pp. 1–22. [Google Scholar]
- Pereira, P.; Bogunovic, I.; Muñoz-Rojas, M.; Brevik, E.C. Soil ecosystem services, sustainability, valuation and management. Curr. Opin. Environ. Sci. Health 2018, 5, 7–13. [Google Scholar] [CrossRef]
- FAO/WHO, Food and Agriculture Organization/ World Health Organization. Evaluation of certain food additives and contaminants (Fifty-fifth report of the Joint FAO/WHO Expert Committee on Food Additives). In WHO Technical Report Series; No. 901; 2000, TRS 901-JECFA 55; WHO Press: Geneva, Switzerland, 2001. [Google Scholar]
- EFSA (European Food Safety Authority). Lead dietary exposure in the European population. Scientific report of EFSA. EFSA J. 2012, 10, 2831. [Google Scholar]
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapu, R.S.; Li, Y.C.; Baligar, V.C. Chemical speciation of cadmium: An approach to evaluate plant-available cadmium in Ecuadorian soils under cacao production. Chemosphere 2016, 150, 57–62. [Google Scholar] [CrossRef]
- Ramtahal, G.; Umaharan, P.; Hanuman, A.; Davis, C.; Ali, L. The effectiveness of soil amendments, biochar and lime, in mitigating cadmium bioaccumulation in Theobroma cacao L. Sci. Total Environ. 2019, 693, 133563. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation No 488/2014 amending regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs. Off. J. Eur. Union 2014, 138, 75. [Google Scholar]
- Netherlands Ministry of Foreign Affair. CBI Product Factsheet: Cocoa in Switzerland; CBI Market Intelligence: Amsterdam, The Netherlands, 2016. [Google Scholar]
- McLaughlin, M.J.; Maier, N.A.; Rayment, G.E.; Sparrow, L.A.; Berg, G.; McKay, A.; Milham, P.; Merry, R.H.; Smart, M.K. Cadmium in Australian Potato Tubers and Soils. J. Environ. Qual. 2010, 26, 1644. [Google Scholar] [CrossRef]
- Burt, R.; Wilson, M.A.; Mays, M.D.; Lee, C.W.; Burt, R.; Wilson, M.A.; Mays, M.D.; Lee, C.W. Major and trace elements of selected pedons in the USA. J. Environ. Qual. 2003, 32, 2109–2121. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor and Francis Group: London, UK, 2011; pp. 237–251. [Google Scholar]
- Chalmers, A.; Hounsome, B.; Rush, C. The British Survey of Fertiliser Practice: Fertiliser Use on Farm. Crops for Crop. Year 2000; The BSFP Authority: Peterborough, UK, 2001. [Google Scholar]
- Alloway, B.J. (Ed.) Heavy Metals in Soils; Blackie Academic & Professional: London, UK, 2013. [Google Scholar]
- Fauziah, C.I.; Zaharah, A.R.; Zauyah, S.; Anuar, A.R. Proposed soil heavy metal reference values to be used for site assessment. In Proc. Brownf. First National Conference on Contaminated Land; The Institution of Engineers: Petaling Jaya, Malaysia, 2001; pp. 1–20. [Google Scholar]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef]
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapu, R.S.; Li, Y.C.; Moyano, B.; Baligar, V.C. Concentration of cadmium in cacao beans and its relationship with soil cadmium in southern Ecuador. Sci. Total Environ. 2015, 533, 205–214. [Google Scholar] [CrossRef]
- Arévalo-Gardini, E.; Arévalo-Hernández, C.O.; Baligar, V.C.; He, Z.L. Heavy metal accumulation in leaves and beans of cacao (Theobroma cacao L.) in major cacao growing regions in Peru. Sci. Total Environ. 2017, 605–606, 792–800. [Google Scholar] [CrossRef]
- Barraza, F.; Schreck, E.; Lévêque, T.; Uzu, G.; López, F.; Ruales, J.; Prunier, J.; Marquet, A.; Maurice, L. Cadmium bioaccumulation and gastric bioaccessibility in cacao: A field study in areas impacted by oil activities in Ecuador. Environ. Pollut. 2017, 229, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Argüello, D.; Chavez, E.; Lauryssen, F.; Vanderschueren, R.; Smolders, E.; Montalvo, D. Soil properties and agronomic factors affecting cadmium concentrations in cacao beans: A nationwide survey in Ecuador. Sci. Total Environ. 2019, 649, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Manton, W.I. Nonnutritive constituents in chocolate and cocoa. In Chocolate in Health and Nutrition; Humana Press: Totowa, NJ, USA, 2013; pp. 73–87. [Google Scholar]
- Meter, A.; Atkinson, R.J.; Laliberte, B. Cadmium in Cacao From Latin America and The Caribbean. A Review of research and potential mitigation solutions 2019. Caracas: CAF. Available online: line://scioteca.caf.com/handle/123456789/1506 (accessed on 20 November 2019).
- Zug, K.L.M.; Huamaní Yupanqui, H.A.; Meyberg, F.; Cierjacks, J.S.; Cierjacks, A. Cadmium accumulation in Peruvian Cacao (Theobroma cacao L.) and opportunities for mitigation. Water. Air. Soil Pollut. 2019, 230, 72. [Google Scholar] [CrossRef]
- Kirkham, M.B. Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Wang, G.; Su, M.Y.; Chen, Y.H.; Lin, F.F.; Luo, D.; Gao, S.F. Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environ. Pollut. 2006, 144, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Hajabbasi, M.A.; Khademi, H.; Kazemian, H. Soil cadmium stabilization using an Iranian natural zeolite. Geoderma 2007, 137, 388–393. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Blommaert, H. The Uptake of Cadmium by Cacao Seedlings as Affected by the Root Distribution and Bioavailable Cadmium. Master’s Thesis, Ku Leuven, Leuven, Belgium, 2019. [Google Scholar]
- Sanaeiostovar, A.; Khoshgoftarmanesh, A.H.; Shariatmadari, H.; Afyuni, M.; Schulin, R. Combined effect of Zinc and Cadmium levels on root antioxidative responses in three different Zinc-efficient wheat genotypes. J. Agron. Crop. Sci. 2012, 198, 276–285. [Google Scholar] [CrossRef]
- Wickramasuriya, A.M.; Dunwell, J.M. Cacao biotechnology: Current status and future prospects. Plant Biotechnol. J. 2018, 16, 4–17. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.A.; Vaissière, B.E.; Cane, J.H.; Steffan, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T.; Klein, A.; Vaissi, B.E.; Cane, J.H.; et al. Importance of pollinators in changing lands for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D.; Crute, I.; Davies, B.; Dunwell, J.; Gale, M.; Jones, J.; Pretty, J.; Sutherland, W.; Toulmin, C. Reaping the Benefits: Science and the Sustainable Intensification of Global Agriculture; The Royal Society: London, UK, 2019. [Google Scholar]
- Perfecto, I.; Vandermeer, J. The agroecological matrix as alternative to the land-sparing/agriculture intensification model. Proc. Natl. Acad. Sci. USA 2010, 107, 5786–5791. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.J.; LaValle, L.A. Food security and biodiversity: Can we have both? An agroecological analysis. Agric. Human Values 2011, 28, 3–26. [Google Scholar] [CrossRef]
- Liebig, M.A.; Varvel, G.; Doran, J. A simple performance-based index for assessing multiple agroecosystem functions. Agron. J. 2001, 93, 313–318. [Google Scholar] [CrossRef]
- Wallace, A. Soil organic matter must be restored to near original levels. Commun. Soil Sci. Plant. Anal. 1994, 25, 29–35. [Google Scholar] [CrossRef]
- Zhang, W.; Ricketts, T.H.; Kremen, C.; Carney, K.; Swinton, S.M. Ecosystem services and dis-services to agriculture. Ecol. Econ. 2007, 64, 253–260. [Google Scholar] [CrossRef]
- Costanza, R.; D’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Arévalo-Gardini, E.; Canto, M.; Alegre, J.; Loli, O.; Julca, A.; Baligar, V. Changes in soil physical and chemical properties in long term improved natural and traditional agroforestry management systems of cacao genotypes in Peruvian Amazon. PLoS ONE 2015, 10, e0132147. [Google Scholar] [CrossRef]
- Gramlich, A.; Tandy, S.; Andres, C.; Chincheros Paniagua, J.; Armengot, L.; Schneider, M.; Schulin, R. Cadmium uptake by cocoa trees in agroforestry and monoculture systems under conventional and organic management. Sci. Total Environ. 2017, 580, 677–686. [Google Scholar] [CrossRef]
- Arévalo-Gardini, E. Identificación Del Cacao Criollo Como Producto Nativo de la Biodiversidad de San Martín y Evaluación de Su Potencialidad Regional; Perù Biodiverso: Lima, Peru, 2011. [Google Scholar]
- Hoorn, C.; Wesselingh, F.P.; ter Steege, H.; Bermudez, M.A.; Mora, A.; Sevink, J.; Sanmartín, I.; Sanchez-Meseguer, A.; Anderson, C.L.; Figueiredo, J.P.; et al. Amazonia Through Time: Andean. Science 2010, 330, 927–931. [Google Scholar] [CrossRef]
- Antonelli, A.; Sanmartín, I. Why are there so many plant species in the Neotropics? Taxon 2011, 60, 403–414. [Google Scholar] [CrossRef]
- ONERN (Oficina Nacional de Evaluación de Recursos Naturales). Mapa Ecologico del Peru. Guía Esplicativa; ONERN: Lima, Peru, 1976; Available online: http://repositorio.ana.gob.pe/handle/20.500.12543/1052 (accessed on 15 March 2020).
- Pidwirny, M. Köppen Climate Classification System, The Encyclopedia of the Earth. Available online: https://editors.eol.org/eoearth/wiki/K%C3%B6ppen_Climate_Classification_System (accessed on 12 January 2011).
- Climate-Data Peru. AM Online Projects—Alexander Merkel. 2012. Available online: https://en.climate-data.org/ (accessed on 15 March 2020).
- Olson, D.M.; Dinerstein, E. The global 200: A representation approach to conserving the Earth’s most biologically valuable ecoregions. Conserv. Biol. 1998, 12, 502–515. [Google Scholar] [CrossRef]
- Link, D.D.; Walter, P.J.; Kingston, H.M. Development and validation of the new EPA microwave-assisted leach method 3051A. Environ. Sci. Technol. 1998, 32, 3628–3632. [Google Scholar] [CrossRef]
- US EPA Environmental Protection Agency, Office of Research and Development. Environmental Monitoring and Support Laboratory. Flame atomic absorption spectrophotometry (EPA method 7000B) 2007. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/7000b.pdf (accessed on 15 February 2007).
- Davey, B.G.; Conyers, M.K. Determining the pH of acid soils. Soil Sci. 1988, 146, 141–150. [Google Scholar] [CrossRef]
- Ingemmet. Instituto Geólogico Minero y Metalúrgico. Mapa Geólogico del Perú 1: 1 000 000. Available online: https://www.ingemmet.gob.pe/mapa-geologico-nacional (accessed on 15 March 2020).
- Ministero de Agricoltura y Riego, Peru. Associaciones de Suelos. Available online: https://www.minagri.gob.pe/portal/43-sector-agrario/suelo (accessed on 15 March 2020).
- WRB, World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Report 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Pfiffner, O.A.; Gonzalez, L. Mesozoic-Cenozoic evolution of the western margin of South America: Case study of the peruvian andes. Geoscience 2013, 3, 262–310. [Google Scholar] [CrossRef]
- Norris, K. Agriculture and biodiversity conservation: Opportunity knocks. Conserv. Lett. 2008, 1, 2–11. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Otero, J.T.; Sandino, J.C. Capture rates of male Euglossine bees across a human intervention gradient, chocó region, Colombia1. Biotropica 2003, 35, 520–529. [Google Scholar]
- Hedström, I.; Harris, J.; Fergus, K. Euglossine bees as potential bio-indicators of coffee farms: Does forest access, on a seasonal basis, affect abundance? Rev. Biol. Trop. 2006, 54, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Briggs, H.M.; Perfecto, I.; Brosi, B.J. The role of the agricultural matrix: Coffee management and Euglossine Bee (Hymenoptera: Apidae: Euglossini) communities in Southern Mexico. Environ. Entomol. 2013, 42, 1210–1217. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; RC Team: Vienna, Austria, 2018. [Google Scholar]
- Arévalo-Gardini, E.; Obando-Cerpa, M.E.; Zúñiga-Cernades, L.B.; Arévalo-Hernández, C.O.; Baligar, V.; He, Z. Metales pesados en suelos de plantaciones de Cacao (Theobroma cacao L.) en tres regiones del Perù. Ecol. Apl. 2016, 15, 81. [Google Scholar] [CrossRef]
- EU Directive 86/278 EEC. Soil protection when sewage sludge is used in agriculture. In Official Journal L 181, 04/07/1986 pp. 0006–0012; The Council of European Communities: Bruxelles, Belgium, 1986. [Google Scholar]
- McGrath, S.P.; Chang, A.C.; Page, A.L.; Witter, E. Land application of sewage sludge: Scientific perspectives of heavy metal loading limits in Europe and the United States. Environ. Rev. 1994, 2, 108–118. [Google Scholar] [CrossRef]
- US EPA, 2002. United States Environmental Protection Agency. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites. Available online: //www.epa.gov/superfund/health/conmedia/soil/index.htm (accessed on 6 February 2014).
- D.Lgs 152/2006. Decreto legislativo 3 aprile 2006, n. 152. Norme in materia ambientale; Gazzetta Ufficiale n. 88 del 14-4-200, Suppl. Ordinario n. 96; Government of Italian Republic: Rome, Italy, 2006.
- Carlon, C.; D’Alessandro, M.; Swartjes, F. Derivation methods of soil screening values in Europe. In A Review and Evaluation of National Procedures towards Harmonization; Carlon, C., Ed.; European Commission, Joint Research Centre: Ispra, Varese, Italy, 2007. [Google Scholar]
- Finnish Ministry of the Environment. Government Decree on the Assessment of Soil Contamination and Remediation Needs, 214/2007; Finnish Government: Helsinki, Finland, 2007.
- Chinese Ministry of Environmental Protection & Ministry of Land and Resources. Report on the National Soil Contamination Survey. Available online: http://www.mep.gov.cn/gkml/hbb/qt/201404/t20140417_270670.htm (accessed on 27 August 2014).
- Fadigas, F.D.S.; Do Amaral Sobrinho, N.M.B.; Mazur, N.; Cunha Dos Anjos, L.H. Estimation of reference values for cadmium, cobalt, chromium, copper, nickel, lead, and zinc in Brazilian soils. Commun. Soil Sci. Plant. Anal. 2006, 37, 945–959. [Google Scholar] [CrossRef]
- Chen, W.; Chang, A.C.; Wu, L. Assessing long-term environmental risks of trace elements in phosphate fertilizers. Ecotoxicol. Environ. Saf. 2007, 67, 48–58. [Google Scholar] [CrossRef]
- Andersen, M.K.; Refsgaard, A.; Raulund-Rasmussen, K.; Strobel, B.W.; Hansen, H.C.B. Content, distribution, and solubility of Cadmium in arable and forest soils. Soil Sci. Soc. Am. J. 2002, 66, 1829. [Google Scholar] [CrossRef]
- Winder, J.A. Cocoa flower diptera; their identity, pollinating activity and breeding sites. Pans 1978, 24, 5–18. [Google Scholar] [CrossRef]
- Gramlich, A.; Tandy, S.; Gauggel, C.; López, M.; Perla, D.; Gonzalez, V.; Schulin, R. Soil cadmium uptake by cocoa in Honduras. Sci. Total Environ. 2018, 612, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; García-Lorenzo, M.L.; Molina, J.; Tudela, M.L. Geochemical background levels of zinc, cadmium and mercury in anthropically influenced soils located in a semi-arid zone (SE, Spain). Geoderma 2009, 148, 307–317. [Google Scholar] [CrossRef]
- Noble, D.C.; Silberman, M.L.; Megard, F.; Bowman, H.R. Comendite (peralkaline rhyolite) and basalt in the Mitu Group, Peru: Evidence for Permian-Triassic lithospheric extension in the central Andes. US Geol. Surv. J. Res. 1978, 6, 453–457. [Google Scholar]
- Bertoldi, D.; Barbero, A.; Camin, F.; Caligiani, A.; Larcher, R. Multielemental fingerprinting and geographic traceability of Theobroma cacao beans and cocoa products. Food Control 2016, 65, 46–53. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Riego. Análisis de la Catena Productive del Cacao; Minagri-Deeia: Lima, Peru, 2018. [Google Scholar]
- Ministerio de Agricultura y Riego. Observatorio de Commodities: Cacao. In Boletin de Publicatiòn Trimestral; Minagri-Deeia: Lima, Peru, 2019. [Google Scholar]
- AgrodataPeru. Exportaciones Agropecuarias Perú. Periodo 2017–2019. Available online: https://www.agrodataperu.com/exportaciones (accessed on 15 January 2020).
- Lima, L.J.R.; Almeida, M.H.; Rob Nout, M.J.; Zwietering, M.H. Theobroma cacao L., “the food of the gods”: Quality determinants of commercial cocoa beans, with particular reference to the impact of fermentation. Crit. Rev. Food Sci. Nutr. 2011, 51, 731–761. [Google Scholar] [CrossRef] [PubMed]
- Saltini, R.; Akkerman, R.; Frosch, S. Optimizing chocolate production through traceability: A review of the influence of farming practices on cocoa bean quality. Food Control 2013, 29, 167–187. [Google Scholar] [CrossRef]
- Loor, R.G.; Risterucci, A.M.; Courtois, B.; Fouet, O.; Jeanneau, M.; Rosenquist, E.; Amores, F.; Vasco, A.; Medina, M.; Lanaud, C. Tracing the native ancestors of the modern Theobroma cacao L. population in Ecuador. Tree Genet. Genomes 2009, 5, 421–433. [Google Scholar] [CrossRef]
- Alvarez, A.; Polti, M.A.P. Bioremediation and Biotransformation of Carbon Nanostructures through Enzymatic and Microbial Systems; Springer: Cham, Germany, 2014; pp. 123–134. [Google Scholar]
- Engbersen, N.; Gramlich, A.; Lopez, M.; Schwarz, G.; Hattendorf, B.; Gutierrez, O.; Schulin, R. Cadmium accumulation and allocation in different cacao cultivars. Sci. Total Environ. 2019, 678, 660–670. [Google Scholar] [CrossRef] [PubMed]
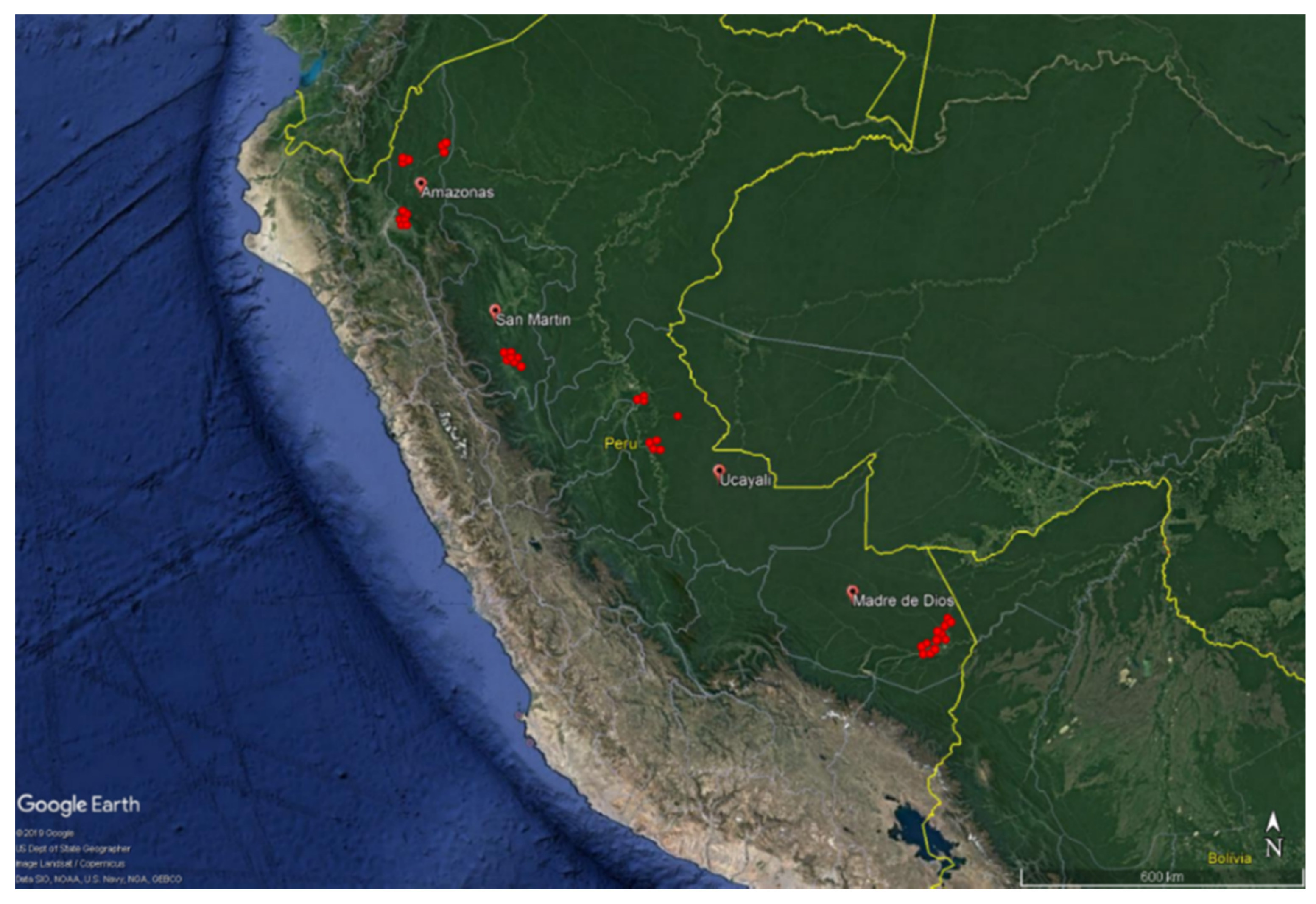

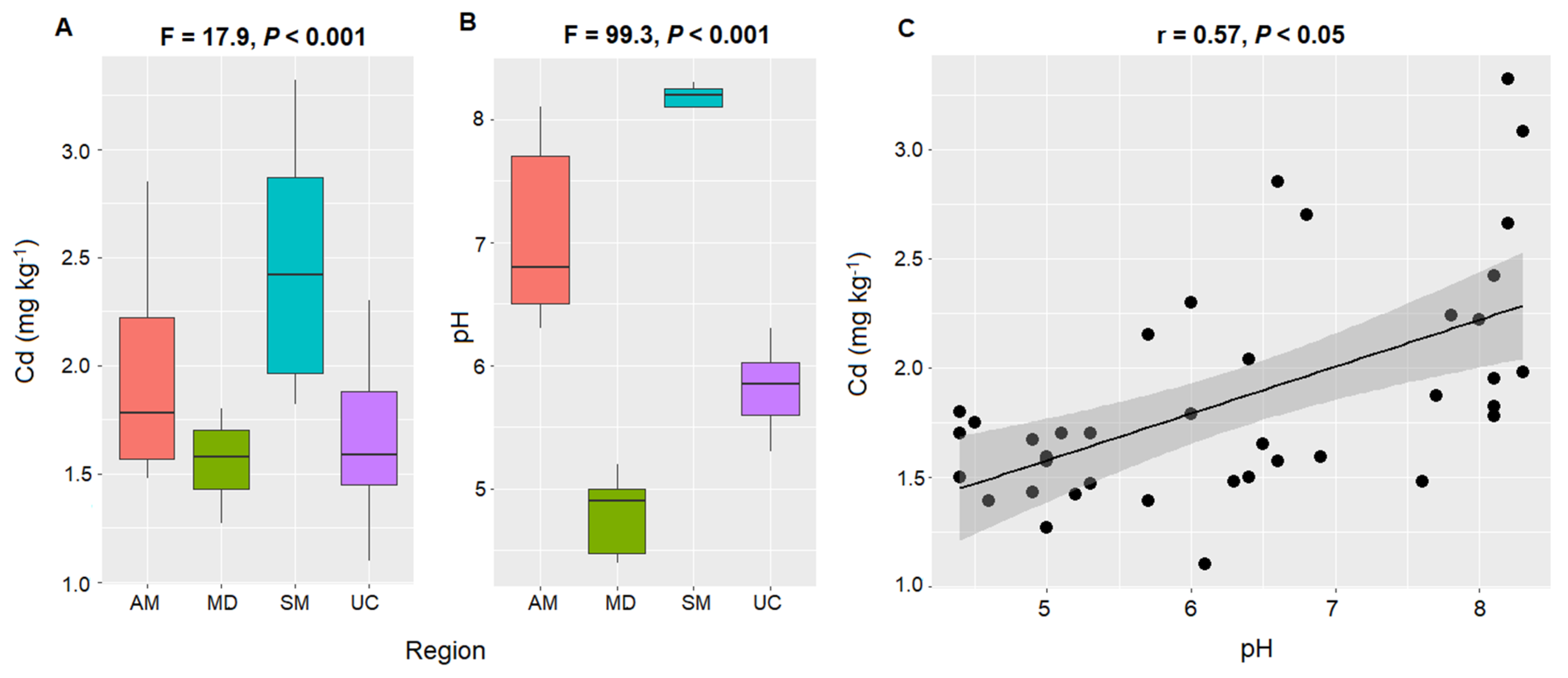

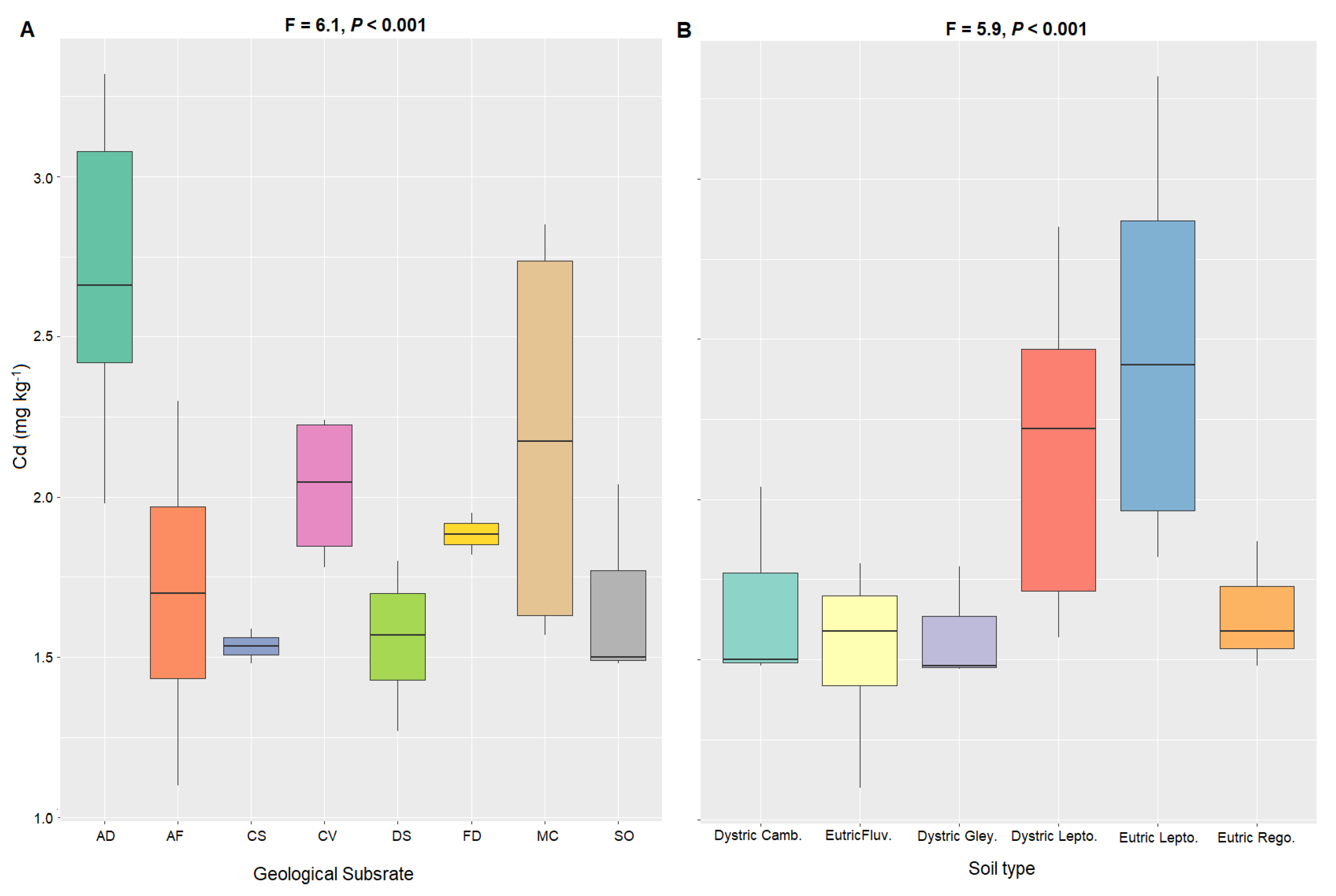
| Region | Sites (n) | Climate 1 | 2 TAP (mm) | 3 MAT (°C) | 4 Ecoregion |
|---|---|---|---|---|---|
| AM | 13 | Temperate, warm summer (Cfb) | 995.0 | 24.1 | Napo moist forest/Iquitos varzea |
| MD | 12 | Tropical rainforest (Af) | 1938.5 | 26.0 | Southwest Amazon moist forests/Peruvian Yungas |
| SM | 7 | Temperate, cold summer (Cfc) | 1188.0 | 25.0 | Peruvian Yungas |
| UC | 8 | Tropical monsoon (Am) | 1667.1 | 26.4 | Southwest Amazon moist forests |
| Variable | Tree | Herb | Year | pH | Cd | AEF | Elevation |
|---|---|---|---|---|---|---|---|
| TC | 1.00 | 0.37 * | −0.13 | −0.36 * | 0.17 | 0.60 ** | −0.10 |
| HC | 1.00 | −0.34 * | −0.49 * | −0.24 | 0.40 * | −0.31 * | |
| Year | 1.00 | 0.56 ** | 0.15 | −0.18 | 0.54 ** | ||
| pH | 1.00 | 0.57 ** | −0.40 * | 0.76 ** | |||
| Cd | 1.00 | −0.1 | 0.40 * | ||||
| AEF | 1.00 | −0.26 | |||||
| Elevation | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaccabarozzi, D.; Castillo, L.; Aromatisi, A.; Milne, L.; Búllon Castillo, A.; Muñoz-Rojas, M. Soil, Site, and Management Factors Affecting Cadmium Concentrations in Cacao-Growing Soils. Agronomy 2020, 10, 806. https://doi.org/10.3390/agronomy10060806
Scaccabarozzi D, Castillo L, Aromatisi A, Milne L, Búllon Castillo A, Muñoz-Rojas M. Soil, Site, and Management Factors Affecting Cadmium Concentrations in Cacao-Growing Soils. Agronomy. 2020; 10(6):806. https://doi.org/10.3390/agronomy10060806
Chicago/Turabian StyleScaccabarozzi, Daniela, Luis Castillo, Andrea Aromatisi, Lynne Milne, Adolfo Búllon Castillo, and Miriam Muñoz-Rojas. 2020. "Soil, Site, and Management Factors Affecting Cadmium Concentrations in Cacao-Growing Soils" Agronomy 10, no. 6: 806. https://doi.org/10.3390/agronomy10060806
APA StyleScaccabarozzi, D., Castillo, L., Aromatisi, A., Milne, L., Búllon Castillo, A., & Muñoz-Rojas, M. (2020). Soil, Site, and Management Factors Affecting Cadmium Concentrations in Cacao-Growing Soils. Agronomy, 10(6), 806. https://doi.org/10.3390/agronomy10060806







