Volatile Profile of Wall Rocket Baby-Leaves (Diplotaxis erucoides) Grown under Greenhouse: Main Compounds and Genotype Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Cultivation and Preparation of Samples
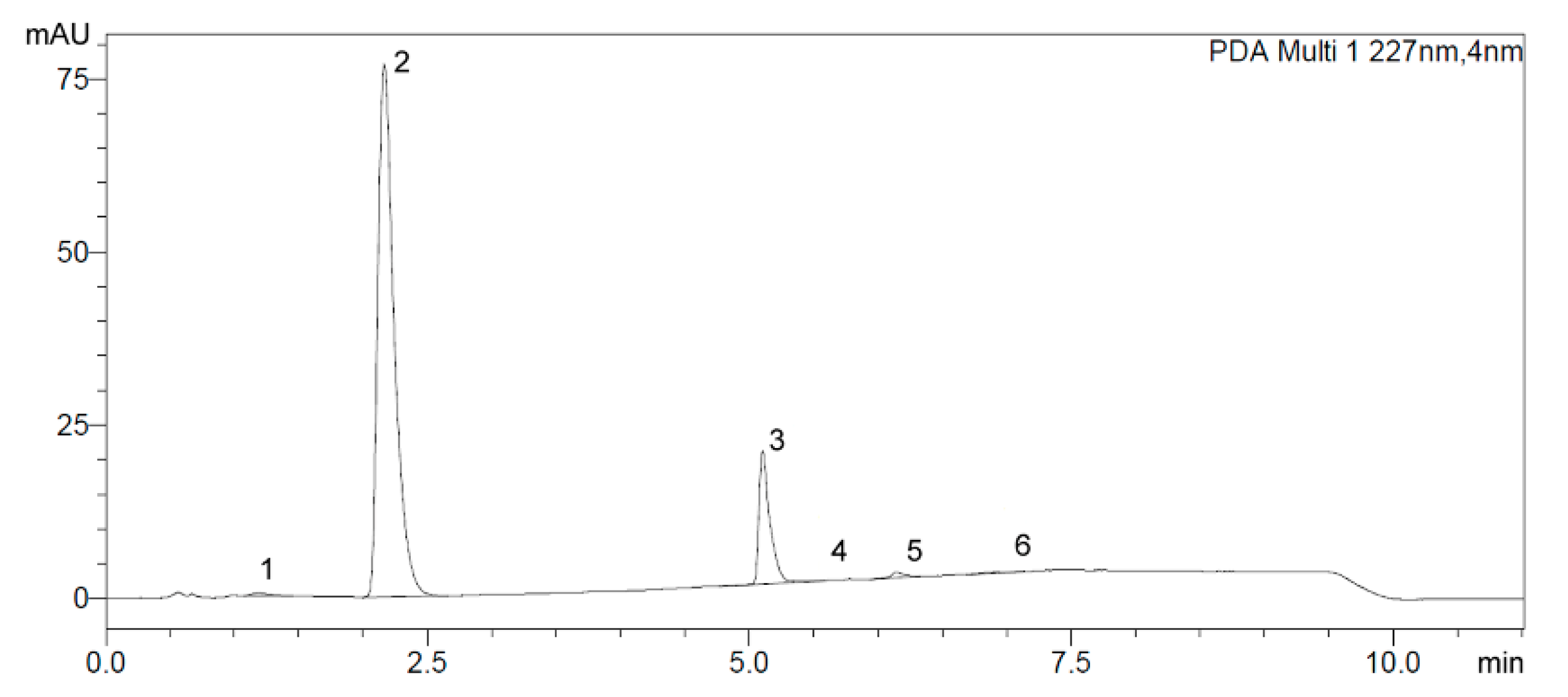
2.2. Extraction and Analysis of Volatile Organic Compounds
2.3. Extraction and Analysis of Sinigrin
2.4. Statistical Analysis
3. Results and Discussion
3.1. Profile in VOCs
3.1.1. Chemical Groups of Volatiles
3.1.2. Group of Isothiocyanates
3.1.3. Group of Esters and Other Compounds
3.2. Accumulation of Sinigrin
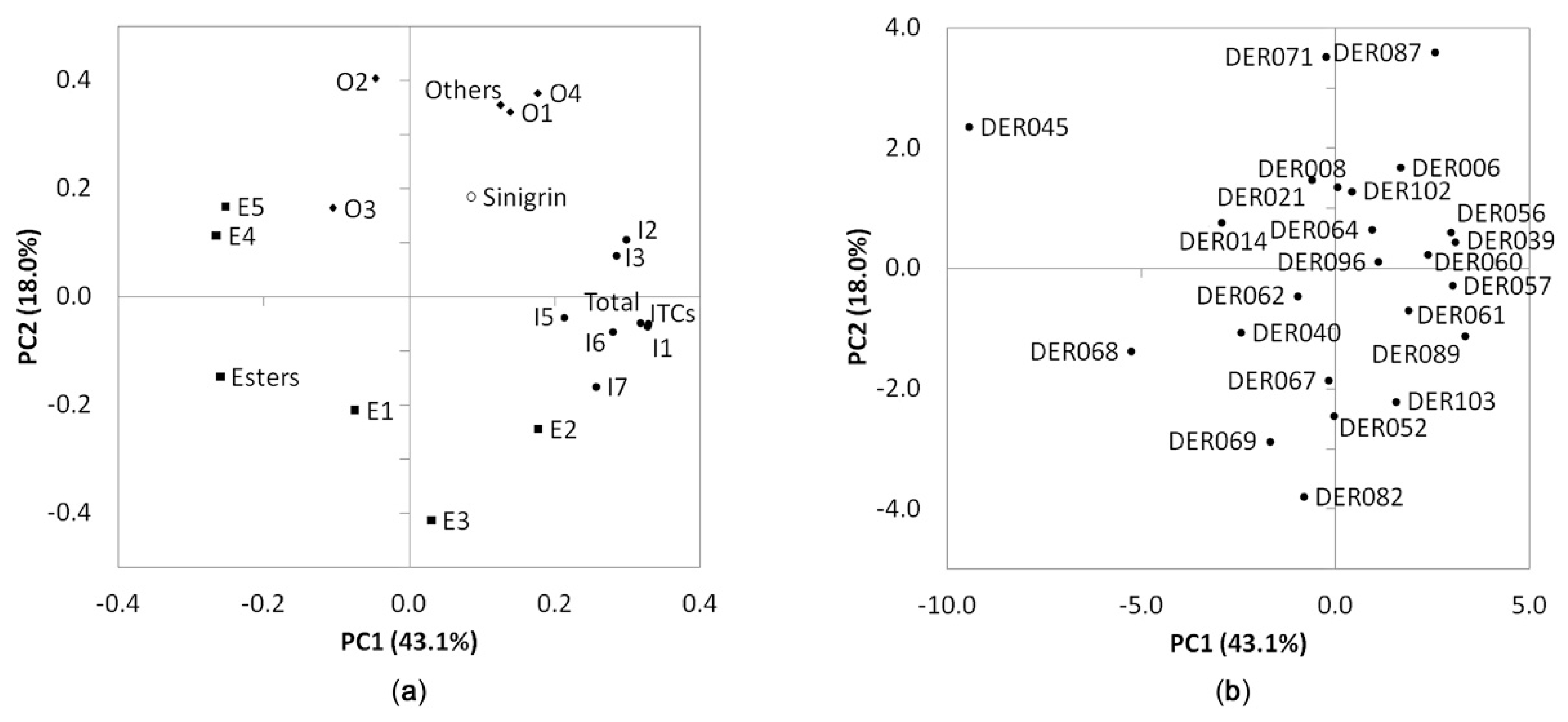
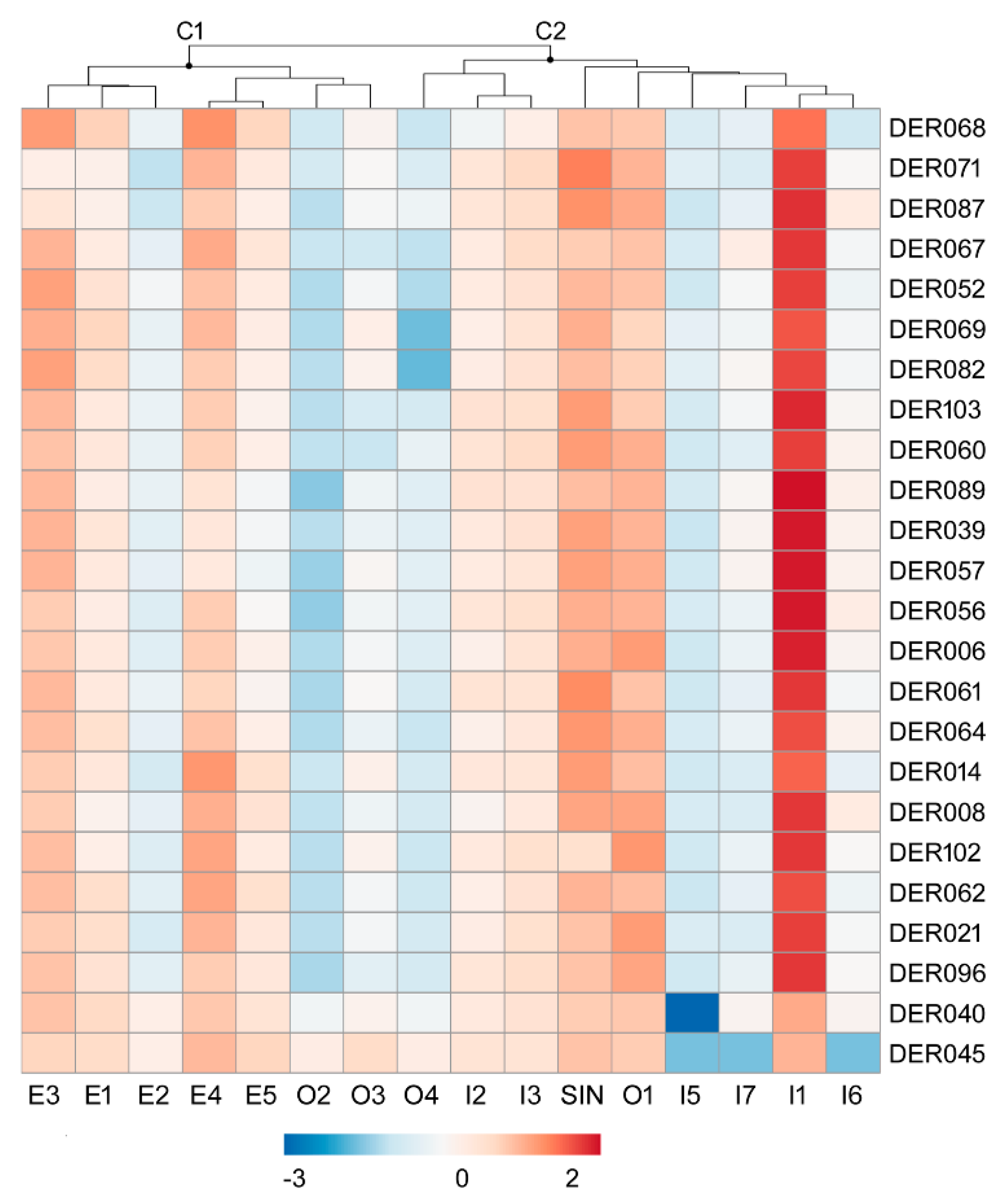
3.3. Principal Component Analysis and Hierarchical Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripodi, P.; Francese, G.; Mennella, G. Rocket salad: Crop description, bioactive compounds and breeding perspectives. Adv. Hortic. Sci. 2017, 31, 107–113. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Navarro, A.; Esposito, S.; Festa, G.; Macellaro, R.; Di Cesare, C.; Fita, A.; Rodríguez-Burruezo, A.; Cardi, T.; Prohens, J.; et al. Large scale phenotyping and molecular analysis in a germplasm collection of rocket salad (Eruca vesicaria) reveal a differentiation of the gene pool by geographical origin. Euphytica 2020, 216, 1–20. [Google Scholar] [CrossRef]
- D’Antuono, F.; Elementi, S.; Neri, R. Exploring new potential health-promoting vegetables: Glucosinolates and sensory attributes of rocket salads and related Diplotaxis and Eruca species. J. Sci. Food Agric. 2009, 89, 713–722. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Prohens, J.; Rodríguez-Burruezo, A.; Fita, A. Consumers acceptance and volatile profile of wall rocket (Diplotaxis erucoides). Food Res. Int. 2020, 132, 109008. [Google Scholar] [CrossRef]
- Pignone, D.; Martínez-Laborde, J.B. Diplotaxis. In Wild Crop Relatives: Genomic and Breeding Resources. Oilseeds; Kole, C., Ed.; Springer: Berlin, Germany, 2011; pp. 137–147. [Google Scholar]
- Couplan, F. Le Régal Végétal. Reconnaître et Cuisiner les Plantes Comestibles; Sang de la Terre: Paris, France, 2015. [Google Scholar]
- Guarrera, P.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Adalid-Martínez, A.M.; Aguirre, K.; Prohens, J.; Rodríguez-Burruezo, A.; Fita, A. Growing Conditions Affect the Phytochemical Composition of Edible Wall Rocket (Diplotaxis erucoides). Agronomy 2019, 9, 858. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Prohens, J.; Rodríguez-Burruezo, A.; Fita, A. Morphological Diversity and Bioactive Compounds in Wall Rocket (Diplotaxis erucoides (L.) DC.). Agronomy 2020, 10, 306. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Prohens, J.; Rodríguez-Burruezo, A.; Fita, A. Potential of wall rocket (Diplotaxis erucoides) as a new crop: Influence of the growing conditions on the visual quality of the final product. Sci. Hortic. 2019, 258, 108778. [Google Scholar] [CrossRef]
- Bell, L.; Yahya, H.N.; Oloyede, O.O.; Methven, L.; Wagstaff, C. Changes in rocket salad phytochemicals within the commercial supply chain: Glucosinolates, isothiocyanates, amino acids and bacterial load increase significantly after processing. Food Chem. 2017, 221, 521–534. [Google Scholar] [CrossRef]
- Baenas, N.; Cartea, M.E.; Moreno, D.A.; Tortosa, M.; Francisco, M. Processing and cooking effects on glucosinolates and their derivatives. In Glucosinolates: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier Inc., Academic Press: Cambridge, MA, USA, 2020; pp. 181–212. [Google Scholar]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and Flavor Perceptions of Glucosinolates, Isothiocyanates, and Related Compounds. Mol. Nutr. Food Res. 2018, 62, 1700990. [Google Scholar] [CrossRef]
- Sávio, A.L.V.; Da Silva, G.N.; Salvadori, D.M.F. Inhibition of bladder cancer cell proliferation by allyl isothiocyanate (mustard essential oil). Mutat. Res. Mol. Mech. Mutagen. 2015, 771, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sávio, A.L.V.; Da Silva, G.N.; De Camargo, E.A.; Salvadori, D.M.F. Cell cycle kinetics, apoptosis rates, DNA damage and TP53 gene expression in bladder cancer cells treated with allyl isothiocyanate (mustard essential oil). Mutat. Res. Mol. Mech. Mutagen. 2014, 762, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, T.; Pugalendhi, P.; Thilagavathi, S. Dose response chemopreventive potential of allyl isothiocyanate against 7,12-dimethylbenz(a)anthracene induced mammary carcinogenesis in female Sprague-Dawley rats. Chem. Interact. 2015, 231, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, F.; Avato, P.; Serio, F.; Argentieri, M. Glucosinolate profile of Eruca sativa, Diplotaxis tenuifolia and Diplotaxis erucoides grown in soil and soilless systems. J. Food Compos. Anal. 2018, 69, 197–204. [Google Scholar] [CrossRef]
- D’Antuono, F.; Elementi, S.; Neri, R. Glucosinolates in Diplotaxis and Eruca leaves: Diversity, taxonomic relations and applied aspects. Phytochemistry 2008, 69, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Real, C.; Adalid-Martínez, A.M.; Gregori-Montaner, A.; Prohens, J.; Rodríguez-Burruezo, A.; Fita, A. Factors affecting germination of Diplotaxis erucoides and their effect on selected quality properties of the germinated products. Sci. Hortic. 2020, 261, 109013. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Rodríguez-Burruezo, A.; Prohens, J.; Raigón, M.D.; Fita, A. HS-SPME analysis of the volatiles profile of water celery (Apium nodiflorum), a wild vegetable with increasing culinary interest. Food Res. Int. 2018, 121, 765–775. [Google Scholar] [CrossRef]
- Moreno, E.; Fita, A.; González-Mas, M.C.; Rodríguez-Burruezo, A. HS-SPME study of the volatile fraction of Capsicum accessions and hybrids in different parts of the fruit. Sci. Hortic. 2012, 135, 87–97. [Google Scholar] [CrossRef]
- Bell, L.; Spadafora, N.D.; Müller, C.T.; Wagstaff, C.; Rogers, H.J. Use of TD-GC–TOF-MS to assess volatile composition during post-harvest storage in seven accessions of rocket salad (Eruca sativa). Food Chem. 2016, 194, 626–636. [Google Scholar] [CrossRef]
- Pasini, F.; Verardo, V.; Caboni, M.F.; D’Antuono, L.F. Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD-MS: Evaluation of Eruca sativa Mill. and Diplotaxis tenuifolia L. genetic resources. Food Chem. 2012, 133, 1025–1033. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- López-Gresa, M.P.; Lisón, P.; Campos, L.; Rodrigo, I.; Rambla, J.; Granell, A.; Conejero, V.; Bellés, J.M. A Non-targeted Metabolomics Approach Unravels the VOCs Associated with the Tomato Immune Response against Pseudomonas syringae. Front. Plant Sci. 2017, 8, 1188. [Google Scholar] [CrossRef]
- Jirovetz, L.; Smith, D.; Buchbauer, G. Aroma Compound Analysis of Eruca sativa (Brassicaceae) SPME Headspace Leaf Samples Using GC, GC−MS, and Olfactometry. J. Agric. Food Chem. 2002, 50, 4643–4646. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, M.C.; Rambla, J.; Alamar, M.C.; Gutierrez, A.; Granell, A. Comparative Analysis of the Volatile Fraction of Fruit Juice from Different Citrus Species. PLoS ONE 2011, 6, e22016. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Burruezo, A.; Kollmannsberger, H.; González-MasM, C.; Nitz, S.; Fernando, N. HS-SPME Comparative Analysis of Genotypic Diversity in the Volatile Fraction and Aroma-Contributing Compounds of Capsicum Fruits from theannuum−chinense−frutescens Complex. J. Agric. Food Chem. 2010, 58, 4388–4400. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Mastelić, J. Free and bound volatiles of rocket (Eruca sativa Mill.). Flavour Fragr. J. 2008, 23, 278–285. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, Nitriles, and Epithionitriles from Glucosinolates Are Affected by Genotype and Developmental Stage in Brassica oleracea Varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef]
- Blažević, I.; Mastelic, J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem. 2009, 113, 96–102. [Google Scholar] [CrossRef]
- Miyazawa, M.; Nishiguchi, T.; Yamafuji, C. Volatile components of the leaves of Brassica rapa L. var. perviridis Bailey. Flavour Fragr. J. 2005, 20, 158–160. [Google Scholar] [CrossRef]
- Clemente-Villalba, J.; Ariza, D.; García-Garví, J.M.; Sánchez-Bravo, P.; Noguera-Artiaga, L.; Issa-Issa, H.; Hernández, F.; Carbonell-Barrachina, Á.A. Characterization and potential use of Diplotaxis erucoides as food ingredient for a sustainable modern cuisine and comparison with commercial mustards and wasabis. Eur. Food Res. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Raffo, A.; Masci, M.; Moneta, E.; Nicoli, S.; Del Pulgar, J.S.; Paoletti, F. Characterization of volatiles and identification of odor-active compounds of rocket leaves. Food Chem. 2017, 240, 1161–1170. [Google Scholar] [CrossRef]
- Mastrandrea, L.; Amodio, M.L.; Pati, S.; Colelli, G. Effect of modified atmosphere packaging and temperature abuse on flavor related volatile compounds of rocket leaves (Diplotaxis tenuifolia L.). J. Food Sci. Technol. 2017, 54, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Maehara, T.; Kurose, K. Composition of the essential oil from the leaves of Eruca sativa. Flavour Fragr. J. 2002, 17, 187–190. [Google Scholar] [CrossRef]
- Petretto, G.L.; Urgeghe, P.P.; Massa, D.; Melito, S. Effect of salinity (NaCl) on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant Physiol. Biochem. 2019, 141, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, N.D.; Amaro, A.L.; Pereira, M.J.V.; Müller, C.T.; Pintado, M.; Rogers, H.J.; Pintado, M.M. Multi-trait analysis of post-harvest storage in rocket salad (Diplotaxis tenuifolia) links sensorial, volatile and nutritional data. Food Chem. 2016, 211, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, N.D.; Cocetta, G.; Ferrante, A.; Herbert, R.J.; Dimitrova, S.; Davoli, D.; Fernández, M.; Patterson, V.; Vozel, T.; Amarysti, C.; et al. Short-Term Post-Harvest Stress that Affects Profiles of Volatile Organic Compounds and Gene Expression in Rocket Salad during Early Post-Harvest Senescence. Plants 2019, 9, 4. [Google Scholar] [CrossRef]
- Villatoro, M.; Priego-Capote, F.; Álvarez-Sánchez, B.; Saha, S.; Philo, M.; Cano, S.O.; De Haro-Bailón, A.; Font, R.; Del Río-Celestino, M. An approach to the phytochemical profiling of rocket [Eruca sativa (Mill.) Thell]. J. Sci. Food Agric. 2013, 93, 3809–3819. [Google Scholar] [CrossRef]
- Bending, G.; Lincoln, S.D. Characterisation of volatile sulphur-containing compounds produced during decomposition of Brassica juncea tissues in soil. Soil Boil. Biochem. 1999, 31, 695–703. [Google Scholar] [CrossRef]
- Kroener, E.-M.; Buettner, A. Unravelling important odorants in horseradish (Armoracia rusticana). Food Chem. 2017, 232, 455–465. [Google Scholar] [CrossRef]
- Sultana, T.; Porter, N.G.; Savage, G.; McNeil, D.L. Comparison of Isothiocyanate Yield from Wasabi Rhizome Tissues Grown in Soil or Water. J. Agric. Food Chem. 2003, 51, 3586–3591. [Google Scholar] [CrossRef]
- DePree, J.A.; Howard, T.M.; Savage, G.P. Flavour and pharmaceutical properties of the volatile sulphur compounds of Wasabi (Wasabia japonica). Food Res. Int. 1998, 31, 329–337. [Google Scholar] [CrossRef]
- Sultana, T.; Savage, G.P.; McNeil, D.L.; Porter, N.G.; Clark, B. Comparison of flavour compounds in wasabi and horseradish. J. Food Agric. Environ. 2003, 1, 117–121. [Google Scholar]
- Pasini, F.; Verardo, V.; Cerretani, L.; Caboni, M.; D’Antuono, L.F. Rocket salad (Diplotaxis and Eruca spp.) sensory analysis and relation with glucosinolate and phenolic content. J. Sci. Food Agric. 2011, 91, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Ruther, J. Retention index database for identification of general green leaf volatiles in plants by coupled capillary gas chromatography-mass spectrometry. J. Chromatogr. A 2000, 890, 313–319. [Google Scholar] [CrossRef]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2006, 49, 194–207. [Google Scholar] [CrossRef]
- The Good Scents Company. Available online: http://www.thegoodscentscompany.com/ (accessed on 5 February 2020).
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A.; Viguera, G. Influence of Cooking Methods on Glucosinolates and Isothiocyanates Content in Novel Cruciferous Foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef]
- Agneta, R.; Lelario, F.; De Maria, S.; Möllers, C.; Bufo, S.A.; Rivelli, A.R. Glucosinolate profile and distribution among plant tissues and phenological stages of field-grown horseradish. Phytochemistry 2014, 106, 178–187. [Google Scholar] [CrossRef]
- Cools, K.; Terry, L.A. The effect of processing on the glucosinolate profile in mustard seed. Food Chem. 2018, 252, 343–348. [Google Scholar] [CrossRef]
- Bell, L.; Concha, M.J.O.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC-MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2014, 172, 852–861. [Google Scholar] [CrossRef]
- Taranto, F.; Francese, G.; Di Dato, F.; D’Alessandro, A.; Greco, B.; Sanajà, V.O.; Pentangelo, A.; Mennella, G.; Tripodi, P. Leaf Metabolic, Genetic, and Morphophysiological Profiles of Cultivated and Wild Rocket Salad (Eruca and Diplotaxis Spp.). J. Agric. Food Chem. 2016, 64, 5824–5836. [Google Scholar] [CrossRef]
- Borges, C.V.; Seabra, S.; Ponce, F.S.; Lima, G. Agronomic factors influencing Brassica productivity and phytochemical quality. In Brassica Germplasm—Characterization, Breeding and Utilization; El-Esawi, M.A., Ed.; IntechOpen: London, UK, 2018; pp. 57–74. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Bell, L.; Methven, L.; Signore, A.; Concha, M.J.O.; Wagstaff, C. Analysis of seven salad rocket (Eruca sativa) accessions: The relationships between sensory attributes and volatile and non-volatile compounds. Food Chem. 2017, 218, 181–191. [Google Scholar] [CrossRef] [PubMed]
| Code | Location | Province | Coordinates | |
|---|---|---|---|---|
| Latitude | Longitude | |||
| DER089 | Cabanes | Castellón | 40°11′06″ N | 0°10′17″ E |
| DER096 | Puebla-Tornesa | Castellón | 40°05′26″ N | 0°00′19″ W |
| DER087 | Benicásim | Castellón | 40°02′49″ N | 0°03′19″ E |
| DER069 | Aras de los Olmos | Valencia | 39°55′12″ N | 1°07′04″ W |
| DER082 | Chilches | Castellón | 39°46′48″ N | 0°11′06″ W |
| DER068 | Tuéjar | Valencia | 39°46′31″ N | 1°02′23″ W |
| DER071 | Albalat dels Tarongers | Valencia | 39°41′60″ N | 0°20′01″ W |
| DER064 | Casinos | Valencia | 39°41′49″ N | 0°42′49″ W |
| DER067 | Losa del Obispo | Valencia | 39°41′48″ N | 0°53′18″ W |
| DER062 | Liria | Valencia | 39°39′06″ N | 0°34′27″ W |
| DER102 | Puzol | Valencia | 39°36′38″ N | 0°17′53″ W |
| DER061 | La Eliana | Valencia | 39°32′59″ N | 0°31′18″ W |
| DER060 | Paterna | Valencia | 39°32′13″ N | 0°26′54″ W |
| DER103 | Pueblo Nuevo | Valencia | 39°31′19″ N | 0°23′17″ W |
| DER056 | Pueblo Nuevo | Valencia | 39°30′39″ N | 0°22′57″ W |
| DER057 | Valencia | Valencia | 39°29′04″ N | 0°24′19″ W |
| DER052 | Sollana | Valencia | 39°16′18’’ N | 0°22′55″ W |
| DER039 | Játiva | Valencia | 38°58′41″ N | 0°30′53″ W |
| DER040 | Bellús | Valencia | 38°56′38″ N | 0°29′23″ W |
| DER006 | Oliva | Valencia | 38°54′42″ N | 0°06′49″ W |
| DER008 | Javea | Alicante | 38°47′35″ N | 0°06′41″ E |
| DER045 | Jijona | Alicante | 38°38′30″ N | 0°28′37″ W |
| DER021 | Aspe | Alicante | 38°18′33″ N | 0°47′35″ W |
| DER014 | Santa Pola | Alicante | 38°14′36″ N | 0°32′17″ W |
| Isothiocyanates | Esters | Others | Total | |
|---|---|---|---|---|
| DER006 | 819.9c (81.1) | 87.5a (8.6) | 103.6d (10.3) | 1011.0bc |
| DER008 | 741.2c (79.1) | 112.4a (12.0) | 83.1a-d (8.9) | 936.7bc |
| DER014 | 340.7bc (64.4) | 148.9a (28.2) | 39.2a-d (7.4) | 528.8ab |
| DER021 | 456.9bc (71.3) | 96.0a (15.0) | 87.9bcd (13.7) | 640.7abc |
| DER039 | 1041.2c (86.8) | 88.8a (7.4) | 68.9a-d (5.8) | 1198.9c |
| DER040 | 413.0bc (59.7) | 206.5a (29.9) | 72.1a-d (10.4) | 691.5bc |
| DER045 | 186.2a (45.9) | 182.2a (44.9) | 37.1a (9.2) | 405.6a |
| DER052 | 715.4c (79.7) | 145.2a (16.2) | 37.0a-d (4.1) | 897.7bc |
| DER056 | 1102.5c (88.9) | 75.7a (6.1) | 62.2a-d (5.0) | 1240.4c |
| DER057 | 1006.0c (87.8) | 74.9a (6.5) | 65.6a-d (5.7) | 1146.5c |
| DER060 | 882.9c (85.5) | 81.5a (7.9) | 68.6a-d (6.6) | 1033.0bc |
| DER061 | 906.2c (87.4) | 88.9a (8.6) | 41.4a-d (4.0) | 1036.6bc |
| DER062 | 630.1bc (74.6) | 166.6a (19.7) | 48.4a-d (5.7) | 845.0bc |
| DER064 | 729.9c (75.1) | 143.7a (14.8) | 98.7cd (10.1) | 972.3bc |
| DER067 | 722.5c (83.5) | 112.4a (13.0) | 30.3a-d (3.5) | 865.2bc |
| DER068 | 230.8b (45.3) | 248.2a (48.7) | 30.7a-d (6.0) | 509.7ab |
| DER069 | 500.8bc (75.3) | 139.4a (20.9) | 25.3abc (3.8) | 665.5bc |
| DER071 | 498.5bc (82.3) | 60.3a (9.9) | 47.0a-d (7.8) | 605.8abc |
| DER082 | 647.4c (81.7) | 122.1a (15.4) | 23.0abc (2.9) | 792.5bc |
| DER087 | 909.1c (85.2) | 62.2a (5.8) | 96.1bcd (9.0) | 1067.4bc |
| DER089 | 1097.6c (91.1) | 58.8a (4.9) | 48.6a-d (4.0) | 1205.1c |
| DER096 | 696.8c (81.5) | 84.5a (9.9) | 74.0a-d (8.6) | 855.4bc |
| DER102 | 674.4c (72.5) | 138.1a (14.9) | 117.3d (12.6) | 929.8bc |
| DER103 | 938.0c (91.4) | 66.4a (6.5) | 21.6ab (2.1) | 1026.0bc |
| Mean | 703.7 (80.0) | 116.3 (13.2) | 59.5 (6.8) | 879.5 |
| Code | RIa | VOC | Groupb | GSL |
|---|---|---|---|---|
| I1 | 846 | allyl isothiocyanate | Itc | 2-propenyl GSL (sinigrin) |
| I2 | 951 | 3-butenyl isothiocyanate | Itc | 3-butenyl GSL (gluconapin) |
| I3 | 1041 | 3-methylbutyl isothiocyanate | Itc | 3-methylbutyl GSL |
| I4 | 1077 | pentyl isothiocyanate | Itc | pentyl GSL |
| I5 | 1185 | hexyl isothiocyanate | Itc | hexyl GSL |
| I6 | 1318 | benzyl isothiocyanate | Itc | benzyl GSL (glucotropaeolin) |
| I7 | 1429 | phenylethyl isothiocyanate | Itc | phenylethyl GSL (gluconasturtiin) |
| E1 | 1091 | cis-3-hexenyl propionate | Est | |
| E2 | 1183 | hexyl butyrate | Est | |
| E3 | 1191 | cis-3-hexenyl butyrate | Est | |
| E4 | 1226 | cis-3-hexenyl isovalerate | Est | |
| E5 | 1290 | cis-3-hexenyl valerate | Est | |
| O1 | 868 | cis-3-hexen-1-ol | Alc | |
| O2 | 1204 | Decanal | Ald | |
| O3 | 1413 | Tetradecane | Hyd | |
| O4 | 1457 | β-ionone | Norc |
| I1 | I2 | I3 | I4 | I5 | I6 | I7 | E1 | E2 | E3 | E4 | E5 | O1 | O2 | O3 | O4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DER006 | 793.8c (78.5) | 5.7b (0.6) | 12.9bc (1.3) | tr | 0.6a (0.1) | 4.7b (0.5) | 2.1b (0.2) | 8.8a (0.9) | 1.3a (0.1) | 37.7ab (3.7) | 34.0c-f (3.4) | 5.6a-e (0.6) | 99.1bc (9.8) | 0.4b (0.0) | 2.9b (0.3) | 1.2b (0.1) |
| DER008 | 722.8c (77.2) | 3.4b (0.4) | 7.3bc (0.8) | tr | 0.6a (0.1) | 6.6b (0.7) | 0.7b (0.1) | 3.9a (0.4) | 1.1a (0.1) | 29.8ab (3.2) | 65.4d-g (7.0) | 12.1d-f (1.3) | 80.5bc (8.6) | 0.3b (0.0) | 1.7b (0.2) | 0.5b (0.1) |
| DER014 | 326.1bc (61.7) | 6.1b (1.2) | 7.3bc (1.4) | - | 0.3a (0.0) | 0.6ab (0.1) | 0.4ab (0.1) | 6.4a (1.2) | 0.3a (0.1) | 23.5ab (4.4) | 108.4e-g (20.5) | 10.3c-f (1.9) | 35.9abc (6.8) | 0.2b (0.0) | 2.8b (0.5) | 0.3b (0.1) |
| DER021 | 435.2bc (67.9) | 5.7b (0.9) | 12.2bc (1.9) | tr | 0.8a (0.1) | 2.3b (0.4) | 0.7b (0.1) | 13.7a (2.1) | 0.6a (0.1) | 27.5ab (4.3) | 46.0d-g (7.2) | 8.0b-f (1.3) | 84.8bc (13.2) | 0.3b (0.0) | 2.2b (0.3) | 0.6b (0.1) |
| DER039 | 1007.3c (84.0) | 9.2b (0.8) | 14.5bc (1.2) | tr | 0.5a (0.0) | 5.6b (0.4) | 4.5b (0.4) | 12.0a (1.0) | 1.4a (0.1) | 61.2ab (5.1) | 11.1a-c (0.9) | 3.0ab (0.2) | 65.4bc (5.5) | 0.4b (0.0) | 1.9b (0.2) | 1.3b (0.1) |
| DER040 | 398.6bc (57.6) | 3.2b (0.5) | 9.8bc (1.4) | - | - | 0.7b (0.1) | 0.7b (0.1) | 26.2a (3.8) | 1.7a (0.2) | 98.2b (14.2) | 72.5d-g (10.5) | 8.0b-f (1.2) | 70.6bc (10.2) | 0.2b (0.0) | 1.1ab (0.2) | 0.2b (0.0) |
| DER045 | 182.8a (45.1) | 1.5a (0.4) | 1.8a (0.5) | - | tr | - | - | 5.9a (1.4) | 0.2a (0.0) | 13.4ab (3.3) | 146.6g (36.1) | 16.2ef (4.0) | 30.4a (7.5) | 0.2b (0.1) | 6.3b (1.5) | 0.2ab (0.1) |
| DER052 | 696.6c (77.6) | 5.1b (0.6) | 10.4bc (1.2) | tr | 0.3a (0.0) | 1.3b (0.1) | 1.8b (0.2) | 10.3a (1.1) | 1.5a (0.2) | 89.4b (10.0) | 38.7c-g (4.3) | 5.3ª-e (0.6) | 35.0abc (3.9) | 0.1b (0.0) | 1.7b (0.2) | 0.1b (0.0) |
| DER056 | 1065.2c (85.9) | 10.9b (0.9) | 16.8bc (1.4) | tr | 0.8a (0.1) | 6.8b (0.5) | 1.9b (0.2) | 7.0a (0.6) | 1.0a (0.1) | 32.5ab (2.6) | 31.8b-e (2.6) | 3.3a-c (0.3) | 58.2abc (4.7) | 0.2b (0.0) | 2.4b (0.2) | 1.4b (0.1) |
| DER057 | 980.5c (85.5) | 7.1b (0.6) | 10.3bc (0.9) | - | 0.5a (0.0) | 4.1b (0.4) | 3.6b (0.3) | 8.3a (0.7) | 1.3a (0.1) | 55.6ab (4.8) | 7.8a (0.7) | 1.9a (0.2) | 60.8abc (5.3) | 0.2b (0.0) | 3.5b (0.3) | 1.1b (0.1) |
| DER060 | 852.9c (82.6) | 9.2b (0.9) | 16.5bc (1.6) | tr | 0.3a (0.0) | 3.2b (0.3) | 0.6b (0.1) | 7.7a (0.7) | 0.9a (0.1) | 43.0ab (4.2) | 25.3b-d (2.5) | 4.6ª−d (0.4) | 67.3bc (6.5) | 0.2b (0.0) | 0.2a (0.0) | 0.9b (0.1) |
| DER061 | 881.1c (85.0) | 10.5b (1.0) | 11.3bc (1.1) | tr | 0.3a (0.0) | 2.0ab (0.2) | 1.0b (0.1) | 7.4a (0.7) | 1.4a (0.1) | 51.9ab (5.0) | 25.1b-d (2.4) | 3.3ª−c (0.3) | 38.1abc (3.7) | 0.2b (0.0) | 2.7b (0.3) | 0.5b (0.0) |
| DER062 | 609.8bc (72.2) | 5.0b (0.6) | 12.7bc (1.5) | - | 0.3a (0.0) | 1.4b (0.2) | 0.9b (0.1) | 15.0a (1.8) | 0.8a (0.1) | 44.8ab (5.3) | 92.6d-g (11.0) | 13.3d-f (1.6) | 46.1abc (5.5) | 0.2b (0.0) | 1.8b (0.2) | 0.3b (0.0) |
| DER064 | 702.9c (72.3) | 6.4b (0.7) | 12.5bc (1.3) | tr | 0.8a (0.1) | 5.4b (0.6) | 1.9b (0.2) | 19.0a (2.0) | 1.6a (0.2) | 60.5ab (6.2) | 55.2d-g (5.7) | 7.4b-f (0.8) | 95.9bc (9.9) | 0.3b (0.0) | 2.0b (0.2) | 0.4b (0.0) |
| DER067 | 700.2c (80.9) | 4.4b (0.5) | 12.8bc (1.5) | tr | 0.3a (0.0) | 1.2b (0.1) | 3.6b (0.4) | 4.3a (0.5) | 0.6a (0.1) | 42.4ab (4.9) | 58.7d-g (6.8) | 6.4b-f (0.7) | 29.8abc (3.4) | 0.1b (0.0) | 0.2a (0.0) | 0.1ab (0.0) |
| DER068 | 223.8b (43.9) | 1.4b (0.3) | 3.9b (0.8) | - | 0.5a (0.1) | 0.3a (0.1) | 0.8a (0.2) | 21.4a (4.2) | 1.1a (0.2) | 88.5b (17.4) | 117.6fg (23.1) | 19.7f (3.9) | 27.4abc (5.4) | 0.4b (0.1) | 2.7b (0.5) | 0.2b (0.0) |
| DER069 | 483.3bc (72.6) | 4.1b (0.6) | 9.6bc (1.4) | tr | 0.8a (0.1) | 1.6b (0.2) | 1.5b (0.2) | 22.1a (3.3) | 0.9a (0.1) | 65.5ab (9.8) | 46.0d-g (6.9) | 4.8ª−e (0.7) | 20.9ab (3.1) | 0.1b (0.0) | 4.1b (0.6) | 0.1a (0.0) |
| DER071 | 470.4bc (77.7) | 7.8b (1.3) | 16.8bc (2.8) | tr | 0.7a (0.1) | 2.3b (0.4) | 0.6b (0.1) | 3.4a (0.6) | 0.2a (0.0) | 4.2a (0.7) | 46.5c-g (7.7) | 6.0b-e (1.0) | 43.8abc (7.2) | 0.4b (0.1) | 2.2b (0.4) | 0.5b (0.1) |
| DER082 | 630.8c (79.6) | 4.1b (0.5) | 8.8bc (1.1) | tr | 0.5a (0.1) | 1.2b (0.1) | 2.0b (0.3) | 12.9a (1.6) | 0.8a (0.1) | 81.5b (10.3) | 23.7b-d (3.0) | 3.2ª−c (0.4) | 20.4abc (2.6) | 0.1b (0.0) | 2.4b (0.3) | 0.0a (0.0) |
| DER087 | 866.3c (81.2) | 11.7b (1.1) | 20.8c (2.0) | tr | 0.5a (0.0) | 8.2b (0.8) | 1.4b (0.1) | 5.8a (0.5) | 0.5a (0.0) | 11.9ab (1.1) | 37.6c-f (3.5) | 6.4b-e (0.6) | 90.4bc (8.5) | 0.4b (0.0) | 3.0b (0.3) | 2.3b (0.2) |
| DER089 | 1069.8c (88.8) | 10.5b (0.9) | 10.5bc (0.9) | tr | 0.5a (0.0) | 3.7b (0.3) | 2.7b (0.2) | 5.4a (0.4) | 1.1a (0.1) | 42.0ab (3.5) | 8.9ab (0.7) | 1.8a (0.1) | 46.2abc (3.8) | 0.1b (0.0) | 1.5b (0.1) | 0.8b (0.1) |
| DER096 | 668.9c (78.2) | 9.0b (1.1) | 15.1bc (1.8) | tr | 0.4a (0.0) | 2.3b (0.3) | 1.1b (0.1) | 11.1a (1.3) | 0.9a (0.1) | 37.0ab (4.3) | 28.2b-d (3.3) | 7.4b-f (0.9) | 72.5bc (8.5) | 0.2a (0.0) | 0.8ab (0.1) | 0.5b (0.1) |
| DER102 | 646.5c (69.5) | 7.9b (0.8) | 15.2bc (1.6) | tr | 0.5a (0.1) | 2.7b (0.3) | 1.5b (0.2) | 5.3a (0.6) | 0.8a (0.1) | 41.6ab (4.5) | 83.1d-g (8.9) | 7.3b-f (0.8) | 112.6c (12.1) | 0.3b (0.0) | 4.1b (0.4) | 0.4b (0.0) |
| DER103 | 918.7c (89.5) | 7.9b (0.8) | 8.5bc (0.8) | tr | 0.2a (0.0) | 1.6b (0.2) | 1.1b (0.1) | 4.4a (0.4) | 0.7a (0.1) | 36.9ab (3.6) | 22.0b-d (2.1) | 2.4ab (0.2) | 21.0abc (2.0) | 0.1b (0.0) | 0.3a (0.0) | 0.2b (0.0) |
| Mean | 680.6 | 6.6 | 11.6 | tr | 0.5 | 2.9 | 1.5 | 10.3 | 0.9 | 46.7 | 51.4 | 7.0 | 56.4 | 0.2 | 2.3 | 0.6 |
| Sinigrin | CV (%) | |
|---|---|---|
| DER006 | 6.60de | 1.7 |
| DER008 | 8.07ef | 7.3 |
| DER014 | 9.52f | 10.2 |
| DER021 | 3.31c | 4.9 |
| DER039 | 10.13f | 27.6 |
| DER040 | 5.97cd | 7.3 |
| DER045 | 5.82cd | 6.6 |
| DER052 | 4.65c | 18.0 |
| DER056 | 6.88de | 13.3 |
| DER057 | 9.04f | 10.1 |
| DER060 | 12.12g | 6.5 |
| DER061 | 16.87h | 1.1 |
| DER062 | 5.79cd | 11.4 |
| DER064 | 18.18h | 4.5 |
| DER067 | 2.28b | 24.8 |
| DER068 | 3.06c | 11.5 |
| DER069 | 6.04cd | 14.4 |
| DER071 | 15.79h | 6.4 |
| DER082 | 3.39c | 10.3 |
| DER087 | 15.48h | 15.5 |
| DER089 | 3.60c | 7.6 |
| DER096 | 3.26c | 16.7 |
| DER102 | 1.35a | 8.1 |
| DER103 | 8.98f | 8.3 |
| Mean | 7.76 | 62.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guijarro-Real, C.; Rodríguez-Burruezo, A.; Fita, A. Volatile Profile of Wall Rocket Baby-Leaves (Diplotaxis erucoides) Grown under Greenhouse: Main Compounds and Genotype Diversity. Agronomy 2020, 10, 802. https://doi.org/10.3390/agronomy10060802
Guijarro-Real C, Rodríguez-Burruezo A, Fita A. Volatile Profile of Wall Rocket Baby-Leaves (Diplotaxis erucoides) Grown under Greenhouse: Main Compounds and Genotype Diversity. Agronomy. 2020; 10(6):802. https://doi.org/10.3390/agronomy10060802
Chicago/Turabian StyleGuijarro-Real, Carla, Adrián Rodríguez-Burruezo, and Ana Fita. 2020. "Volatile Profile of Wall Rocket Baby-Leaves (Diplotaxis erucoides) Grown under Greenhouse: Main Compounds and Genotype Diversity" Agronomy 10, no. 6: 802. https://doi.org/10.3390/agronomy10060802
APA StyleGuijarro-Real, C., Rodríguez-Burruezo, A., & Fita, A. (2020). Volatile Profile of Wall Rocket Baby-Leaves (Diplotaxis erucoides) Grown under Greenhouse: Main Compounds and Genotype Diversity. Agronomy, 10(6), 802. https://doi.org/10.3390/agronomy10060802







