Long-Term Fertilization with Potassium Modifies Soil Biological Quality in K-Rich Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Treatment Description
2.2. Soil Sampling
2.3. Soil Property Measurements
2.4. PLFA Analysis
2.5. Developing the SQI
2.6. Statistical Analysis
3. Results
3.1. Effect of Fertilization Regimes on Soil Characteristics
3.1.1. Soil Chemical Properties
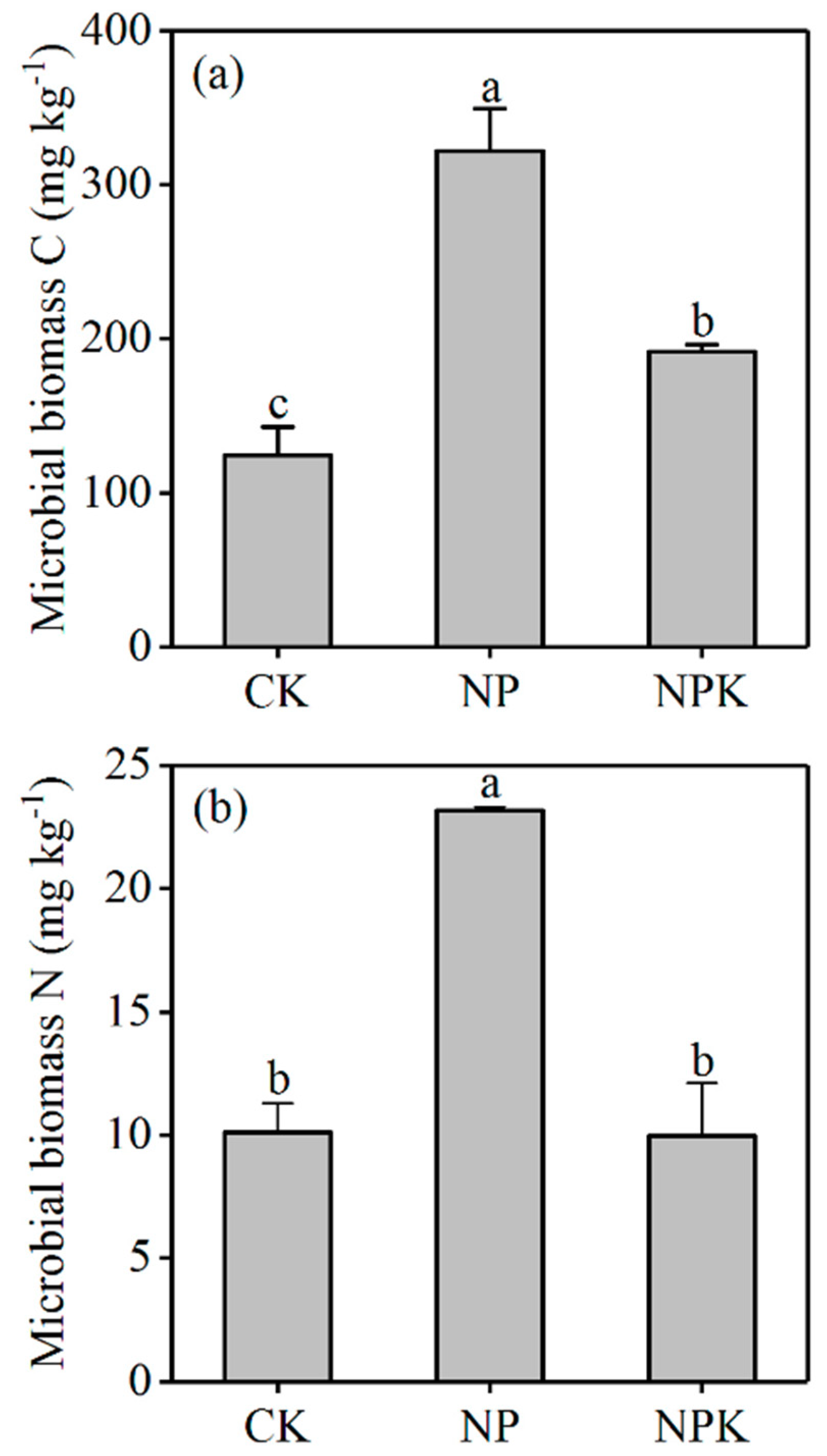
3.1.2. Microbial Biomass C and N
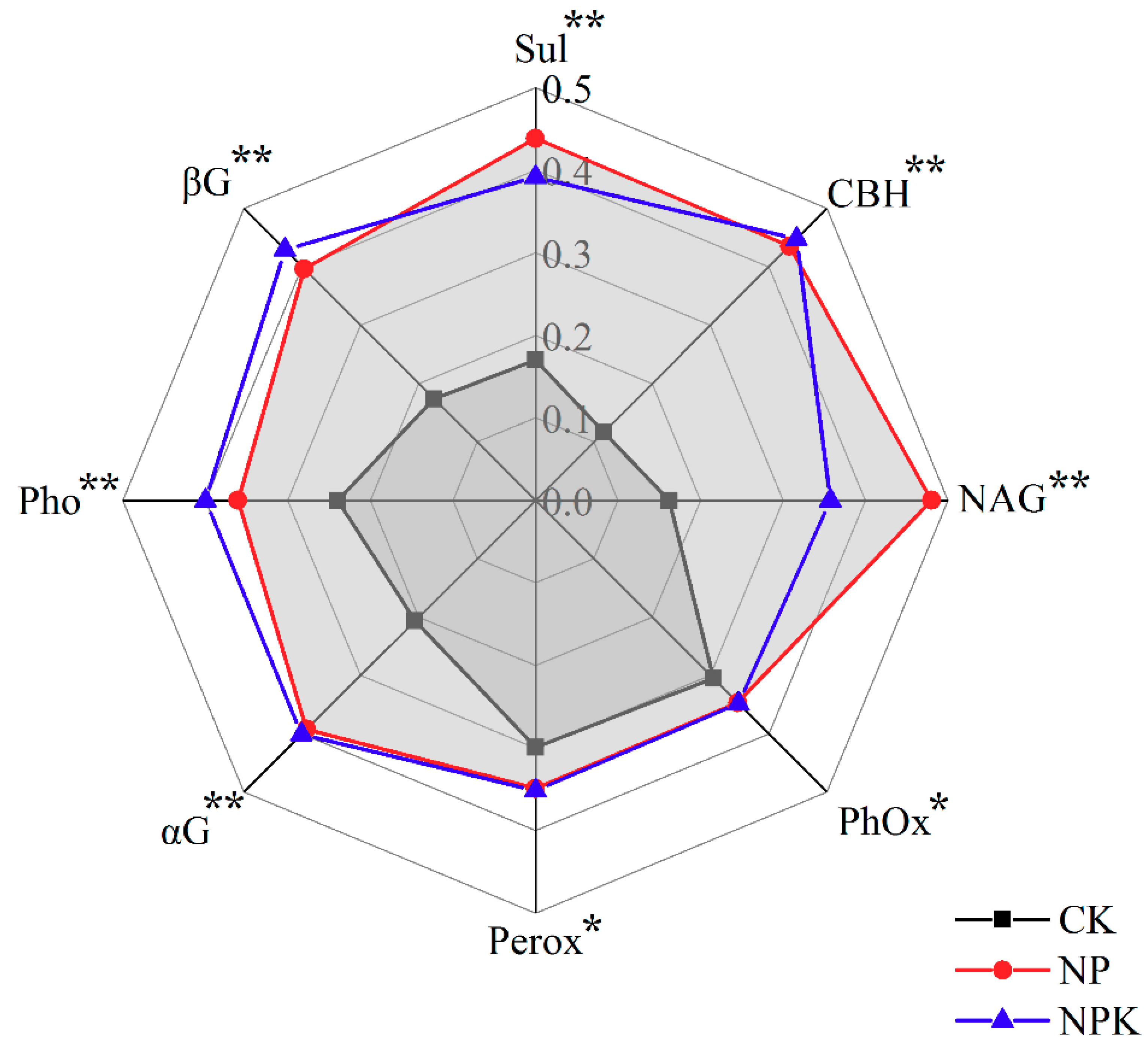
3.1.3. Enzymatic Activities
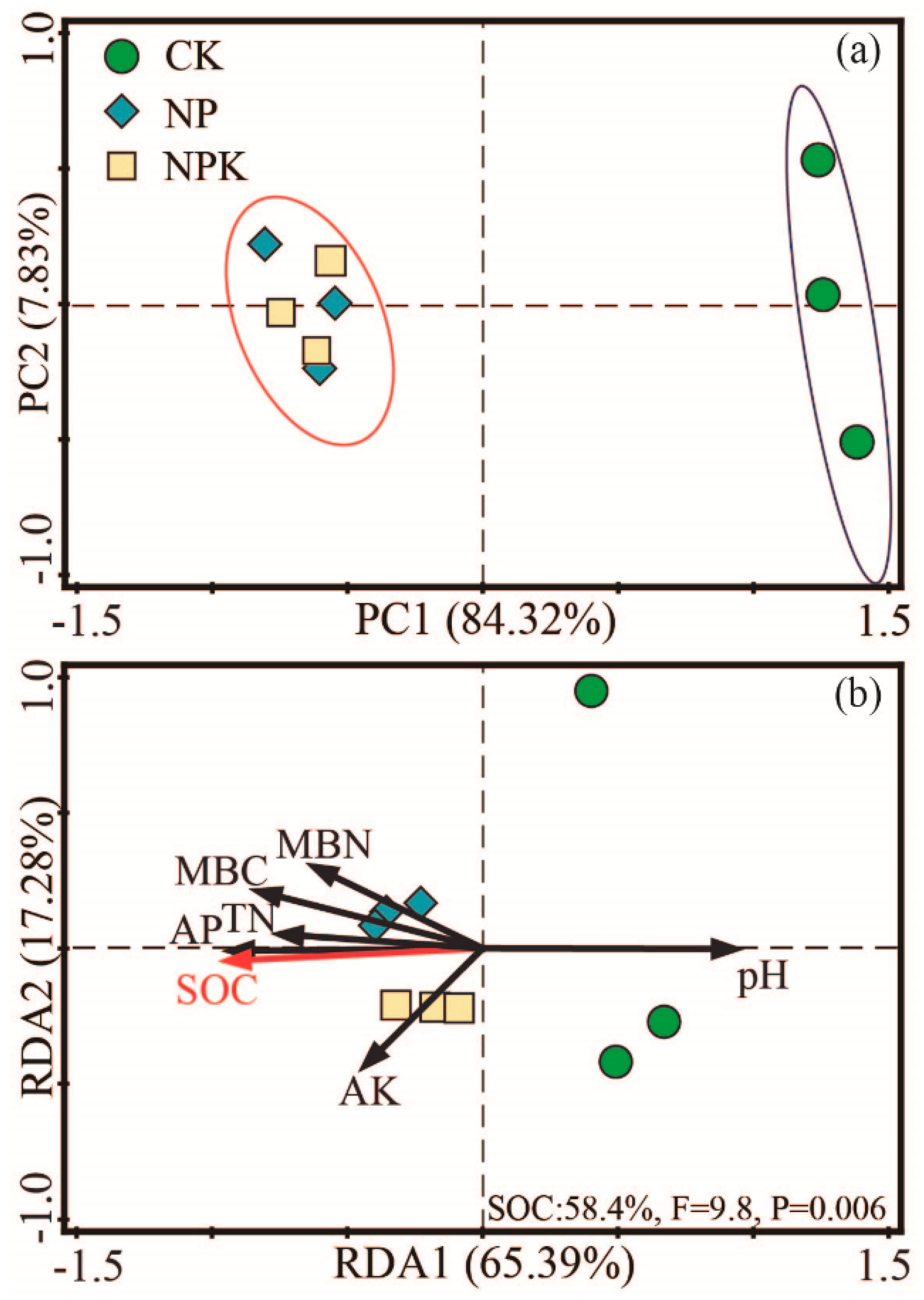
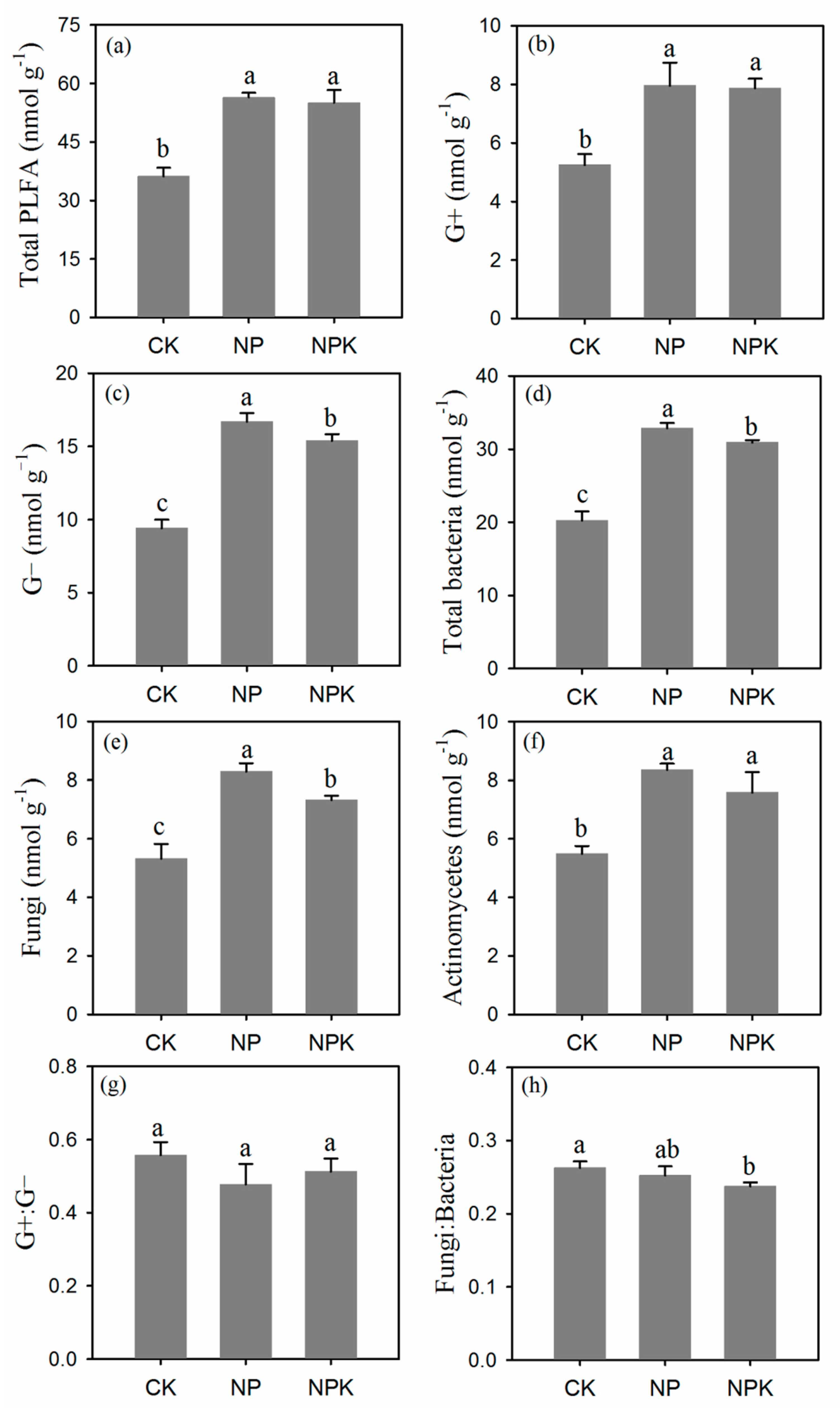
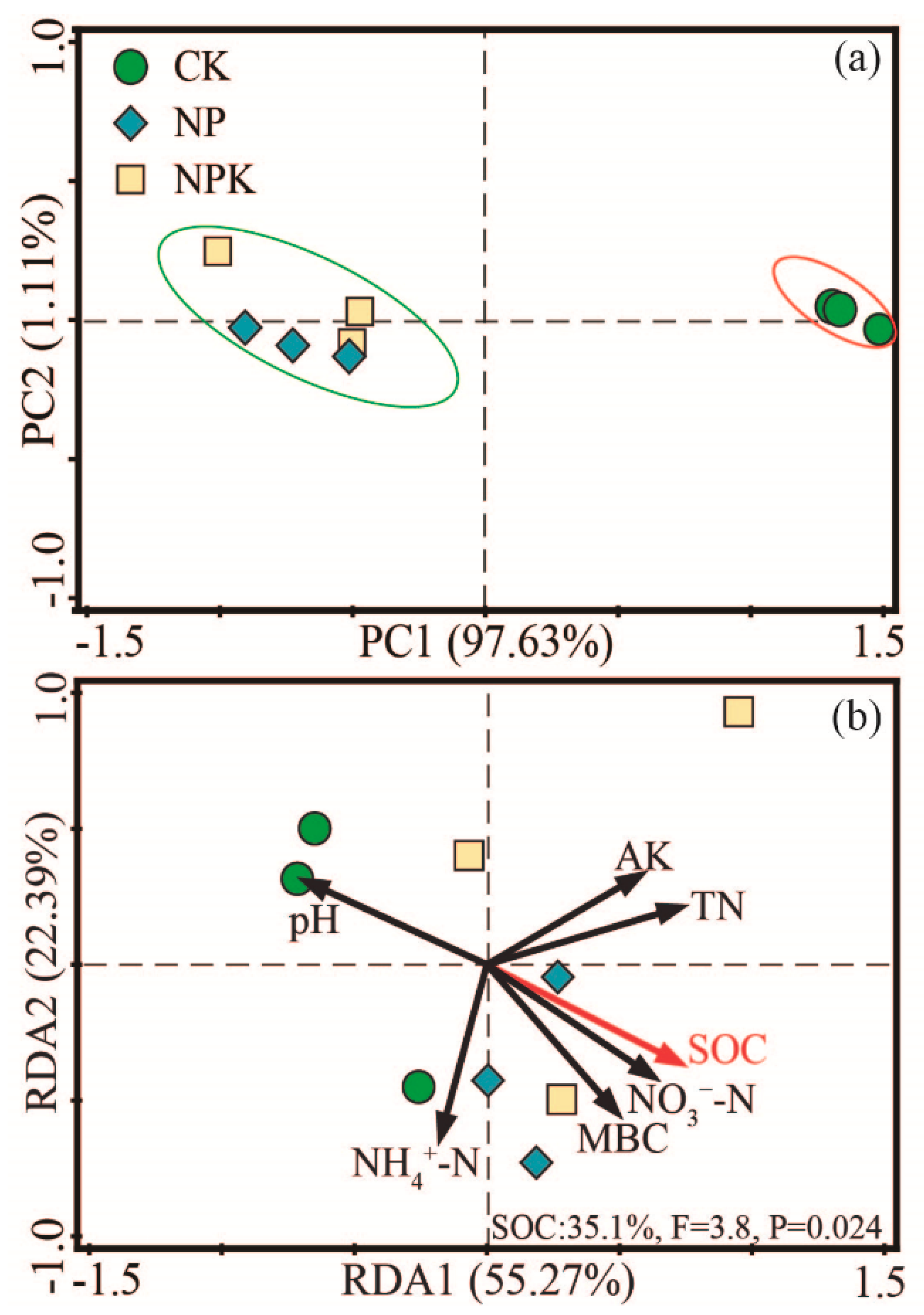
3.1.4. Microbial Community Composition
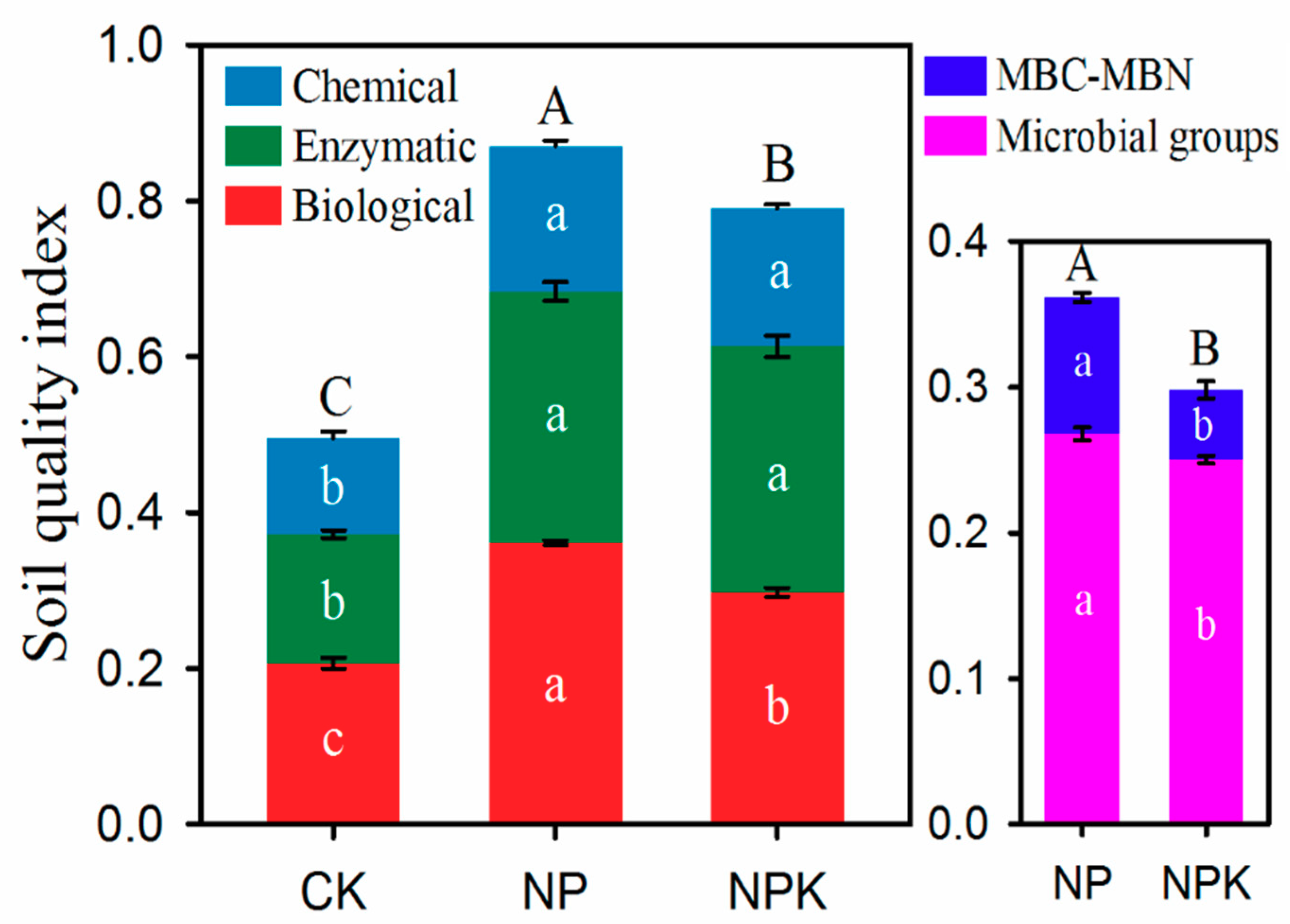
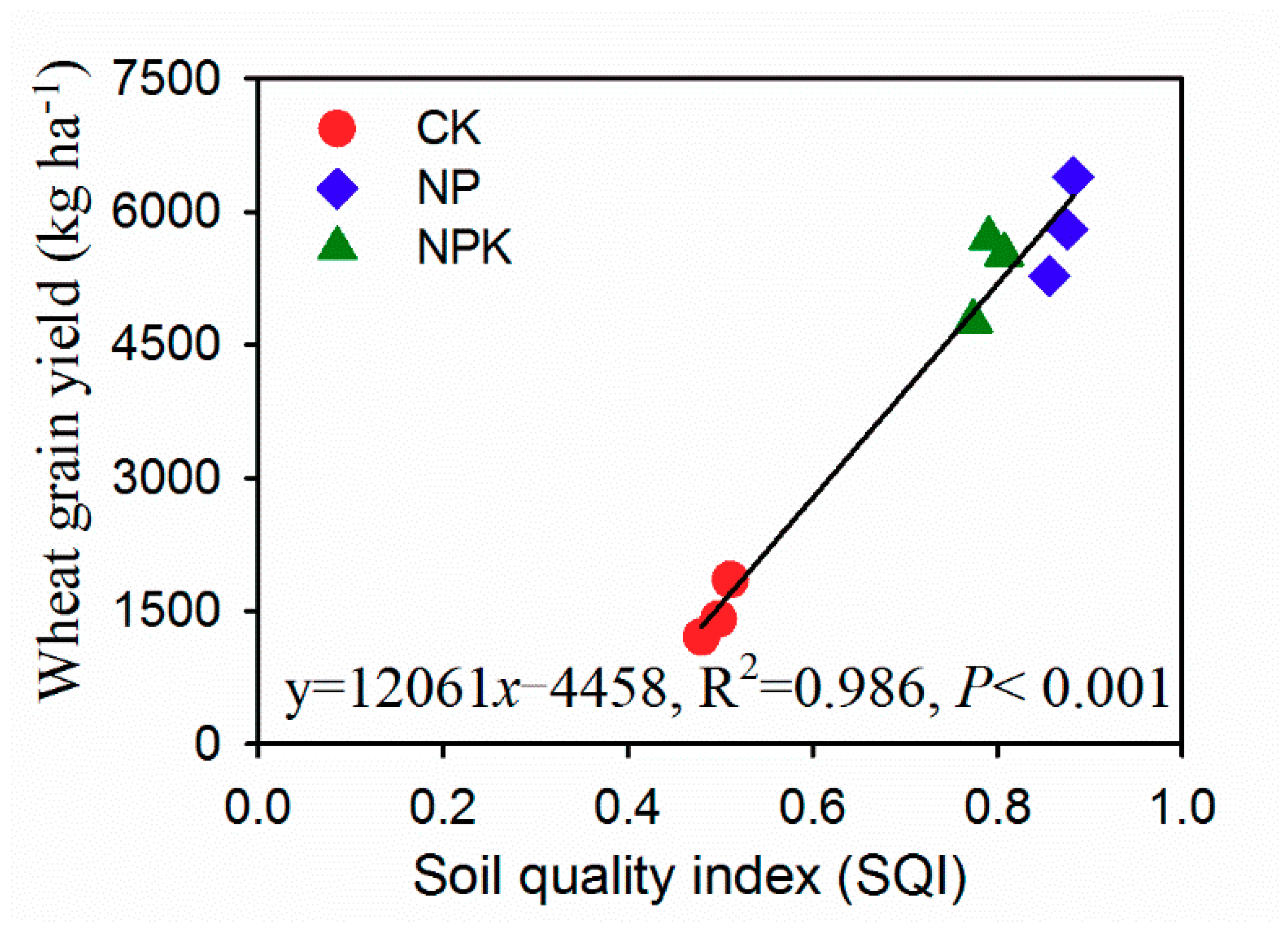
3.2. Soil Quality Index (SQI)
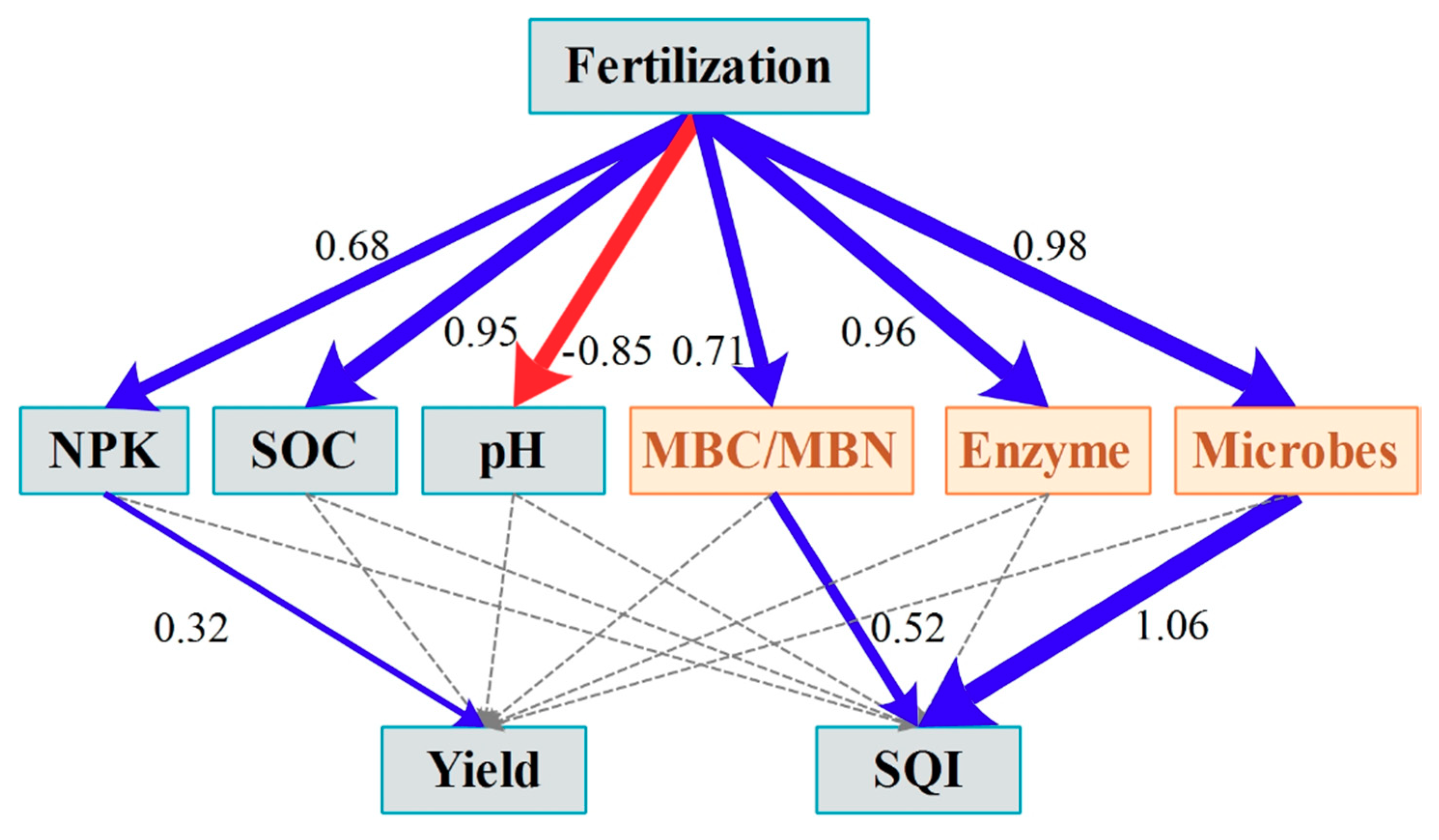
3.3. PLS-PM Analysis
4. Discussion
4.1. Soil Chemical Properties
4.2. Enzymatic Activities and Microbiological Properties
4.3. Soil Quality Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, F.S.; Cui, Z.L.; Chen, X.P.; Ju, X.T.; Shen, J.B.; Chen, Q.; Liu, X.J.; Zhang, W.F.; Mi, G.H.; Fan, M.S.; et al. Integrated Nutrient Management for Food Security and Environmental Quality in China. Adv. Agron. 2012, 116, 1–40. [Google Scholar] [CrossRef]
- Stewart, W.M.; Dibb, D.W.; Johnston, A.E.; Smyth, T.J. The Contribution of Commercial Fertilizer Nutrients to Food Production. Agron. J. 2005, 96, 1–6. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E.; Johnes, P.J.; Katzenberger, J.; Martinelli, L.A.; Matson, P.A.; et al. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Fatemi, A. Kinetics of Soil Potassium Release Under Long-Term Imbalanced Fertilization in Calcareous Soils. Pedosphere 2017, 27, 1105–1115. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M.; et al. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Das, D.; Dwivedi, B.S.; Datta, S.P.; Datta, S.C.; Meena, M.C.; Agarwal, B.K.; Shahi, D.K.; Singh, M.; Chakraborty, D.; Jaggi, S. Potassium supplying capacity of a red soil from eastern India after forty-two years of continuous cropping and fertilization. Geoderma 2019, 341, 76–92. [Google Scholar] [CrossRef]
- Zhao, H.B.; Wang, Z.H.; Gao, Y.J.; Zhang, W.F. Investigation and evaluation of household wheat fertilizer application in Shaanxi Province. Plant Nutr. Fertil. Sci. 2016, 22, 245–253. [Google Scholar] [CrossRef]
- Zhang, D.B.; Yao, P.W.; Na, Z.; Cao, W.D.; Zhang, S.Q.; Li, Y.Y.; Gao, Y.J. Soil Water Balance and Water Use Efficiency of Dryland Wheat in Different Precipitation Years in Response to Green Manure Approach. Sci. Rep. 2016, 6, 26856. [Google Scholar] [CrossRef]
- Chen, W.; Sun, J.H.; Zhao, J.H. Analysis and evaluation of fertilization situation for wheat in Gansu Province. Agric. Res. Arid Areas 2013, 31, 23–27. [Google Scholar]
- Tan, D.S.; Jin, J.Y.; Huang, S.W. Effect of long-term K fertilizer application and returning wheat straw to soil on crop yield and soil K under different planting systems in northwestern China. Plant Nutr. Fertil. Sci. 2008, 14, 886–893. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.L.; Yang, X.Y.; Sun, B.H. Effect of long-term fertilization on wheat yield, nutrient use efficiency and nutrient balance in rainfed Lou soil. Chin. J. Soil Sci. 2017, 48, 1162–1168. [Google Scholar] [CrossRef]
- Ge, W.J.; Chang, Y.L.; Liu, J.M.; Zhang, S.L.; Sun, B.H.; Yang, X.Y. Potassium balance and pool as influenced by long-term fertilization under continuous winter wheat-summer maize cropping system. Plant Nutr. Fertil. Sci. 2012, 18, 629–636. [Google Scholar] [CrossRef]
- Ma, J.C.; He, P.; Xu, X.P.; He, W.T.; Liu, Y.X.; Yang, F.Q.; Chen, F.; Li, S.T.; Tu, S.H.; Jin, J.Y.; et al. Temporal and spatial changes in soil available phosphorus in China (1990–2012). Field Crops Res. 2016, 192, 13–20. [Google Scholar] [CrossRef]
- Zhang, C.; Ju, X.T.; Powlson, D.; Oenema, O.; Smith, P. Nitrogen Surplus Benchmarks for Controlling N Pollution in the Main Cropping Systems of China. Environ. Sci. Technol. 2019, 53, 6678–6687. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.Q.; Sun, J.W.; Wang, X.B.; He, P.; Zhou, W.; He, X.H. Reduced dependence of rhizosphere microbiome on plant-derived carbon in 32-year long-term inorganic and organic fertilized soils. Soil Biol. Biochem. 2015, 80, 70–78. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, B.Z.; Guo, Z.B.; Wang, D.Z.; Li, D.D.; Xu, J.S.; Li, Z.Q.; Zhang, J.B. Divergent responses of bacterial activity, structure, and co-occurrence patterns to long-term unbalanced fertilization without nitrogen, phosphorus, or potassium in a cultivated vertisol. Environ. Sci. Pollut. Res. 2019, 26, 12741–12754. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, B.Z.; Zhang, J.B.; Shen, L.L.; Wang, M.N.; Qin, S.W. Effect of long-term fertilization on microbial biomass and activity in fluvo-aquic soil during maize growth period. Acta Pedol. Sin. 2010, 47, 122–130. [Google Scholar] [CrossRef]
- Deng, C.; Bi, L.D.; Qin, J.T.; Zhang, T.L.; Yu, X.C. Effects of long-term fertilization on soil property changes and soil microbial biomass. Soils 2013, 45, 888–893. [Google Scholar] [CrossRef]
- Ma, X.X.; Wang, L.L.; Li, Q.H.; Li, H.; Zhang, S.L.; Sun, B.H.; Yang, X.Y. Effects of long-term fertilization on soil microbial biomass carbon and nitrogen and enzyme activities during maize growing season. Acta Ecol. Sin. 2012, 32, 5502–5511. [Google Scholar] [CrossRef]
- Liu, L.P. Effects of Potassium Application Rate and Matter on Soil Fertility and Potassium Use Efficiency in Cotton. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2014. [Google Scholar]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Ed.; Soil Science Society of America Special Publication: Madison, WI, USA, 1994; pp. 3–21. [Google Scholar]
- Andrews, S.S.; Mitchell, J.P.; Mancinelli, R.; Karlen, K.L.; Hartz, T.K.; Horwath, W.R.; Prttygrove, F.S.; Scow, K.M.; Munk, D.S. On-farm assessment of soil quality in California’s central valley. Agron. J. 2002, 94, 12–23. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhou, W.; Shen, J.B.; Li, S.T.; Ai, C. Soil quality assessment of yellow clayey paddy soils with different productivity. Biol. Fertil. Soils. 2014, 50, 537–548. [Google Scholar] [CrossRef]
- Qi, Y.B.; Darilek, J.L.; Huang, B.; Zhao, Y.C.; Sun, W.X.; Gu, Z.Q. Evaluating soil quality indices in an agricultural regional of Jiangsu Province, China. Geoderma 2009, 149, 325–334. [Google Scholar] [CrossRef]
- Ling, N.; Chen, D.M.; Guo, H.; Wei, J.X.; Bai, Y.F.; Shen, Q.R.; Hu, S.J. Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 2017, 292, 25–33. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.W.; Zhou, B.K.; Zhao, B.S.; Ma, M.C.; Qin, J.; Jiang, X.; Chen, S.F.; Cao, F.M.; Shen, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Eo, J.; Park, K. Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agric. Ecosyst. Environ. 2016, 231, 176–182. [Google Scholar] [CrossRef]
- Li, B.Z.; Wang, G.F.; Qin, X.F.; Zhang, L.S.; Han, M.Y.; Zhang, L. Effect of application of biogas slurry with potassium on orchard soil properties and soil microorganism and fruit quality. Sci. Agric. Sin. 2010, 43, 4671–4677. [Google Scholar] [CrossRef]
- Jose, T.A.S.; Lourival, F.C.; Jarisson, C.N.; Francisco, T.C.B.; Juliete, A.d.S.N.; Aldeir, R.S.; Denizard, O.; Adailza, G.C. Effect of saline water, bovine biofertilizer and potassium on yellow passion fruit growth after planting and on soil salinity. Afr. J. Agric. Res. 2016, 11, 2994–3003. [Google Scholar] [CrossRef]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Setia, R.; Marschner, P.; Baldock, J.; Chittleborough, D.; Verma, V. Relationships between carbon dioxide emission and soil properties in salt-affected landscapes. Soil Biol. Biochem. 2011, 43, 667–674. [Google Scholar] [CrossRef]
- Farahani, E.; Emami, H.; Keller, T.; Fotovat, A.; Khorassani, R. Impact of monovalent cations on soil structure. Part I. Results of an Iranian soil. Int. Agrophys. 2018, 32, 57–67. [Google Scholar] [CrossRef]
- Rengasamy, P.; Tavakkoli, E.; Mcdonald, G.K. Exchangeable cations and clay dispersion: Net dispersive charge, a new concept for dispersive soil. Eur. J. Soil Sci. 2016, 67, 659–665. [Google Scholar] [CrossRef]
- Mori, T.; Wang, S.H.; Wang, Z.H.; Wang, C.; Mo, H.; Mo, J.M.; Lu, X.K. Testing potassium limitation on soil microbial activity in a sub-tropical forest. J. For. Res. 2018, 30, 2341–2347. [Google Scholar] [CrossRef]
- Mann, C.; Lynch, D.; Fillmore, S.; Mills, A. Relationships between field management, soil health, and microbial community composition. Appl. Soil Ecol. 2019, 144, 12–21. [Google Scholar] [CrossRef]
- Orwin, K.H.; Dickie, I.A.; Holdaway, R.; Wood, J.R. A comparison of the ability of PLFA and 16S rRNA gene metabarcoding to resolve soil community change and predict ecosystem functions. Soil Biol. Biochem. 2018, 117, 27–35. [Google Scholar] [CrossRef]
- Dong, W.Y.; Zhang, X.Y.; Dai, X.Q.; Fu, X.L.; Yang, F.T.; Liu, X.Y.; Sun, X.M.; Wen, X.F.; Schaeffer, S. Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- Yang, X.Y.; Ren, W.D.; Sun, B.H.; Zhang, S.L. Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma 2012, 177–178, 49–56. [Google Scholar] [CrossRef]
- Liu, Z.J.; Chen, Z.J.; Ma, P.Y.; Meng, Y.; Zhou, J.B. Effects of tillage, mulching and N management on yield, water productivity, N uptake and residual soil nitrate in a long-term wheat-summer maize cropping system. Field Crops Res. 2017, 213, 154–164. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil and Agro-Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Moeskops, B.; Sukristiyonubowo; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Korschens, M.; Schulz, E. Long-term management impacts on soil C, N and physical fertility. Soil Tillage Res. 2006, 91, 39–47. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R. The importance of long-term experiments in agriculture: Their management to ensure continued crop production and soil fertility; the Rothamsted experience. Eur. J. Soil Sci. 2018, 69, 113125. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Tong, Y.A.; Liu, X.J.; Xu, W.; Luo, X.S.; Christie, P. High nitrogen deposition in an agricultural ecosystem of Shaanxi, China. Environ. Sci. Pollut. Res. 2016, 23, 13210–13221. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.K.; Vitousek, P.M.; Mao, Q.G.; Gilliam, F.S.; Luo, Y.Q.; Zhou, G.Y.; Zou, X.M.; Bai, E.; Scanlon, T.M.; Hou, E.Q.; et al. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef]
- Meng, H.Q. Characteristics and Mechanisms for Soil Acidification under Long-Term Fertilization in Chinese Croplands. Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2009. [Google Scholar]
- Ji, C.J.; Yang, Y.H.; Han, W.X.; He, Y.F.; Smith, J.; Smith, P. Climate and edaphic controls on soil pH in Alpine grassland on the Tibetan Plateau, China: A quantitative analysis. Pedosphere 2014, 24, 39–44. [Google Scholar] [CrossRef]
- Guo, S.L.; Zhu, H.H.; Dang, T.H.; Wu, J.S.; Liu, W.Z.; Hao, M.D.; Li, Y.; Syers, J.K. Winter wheat grain yield associated with precipitation distribution under long-term nitrogen fertilization in the semiarid Loess Plateau in China. Geoderma 2012, 189–190, 442–450. [Google Scholar] [CrossRef]
- Wright, S.J.; Yavitt, J.B.; Wurzburger, N.; Turner, B.L.; Tanner, E.V.; Sayer, E.J.; Santiago, L.S.; Kaspari, M.; Hedin, L.O.; Harms, K.E.; et al. Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 2011, 92, 1616–1625. [Google Scholar] [CrossRef]
- Liu, L. Biomass Estimation and Spatial Distribution of Maize Root in China. Ph.D. Thesis, China Agricultural University, Beijing, China, 2016. [Google Scholar]
- Khan, A.; Lu, G.Y.; Ayaz, M.; Zhang, H.T.; Wang, R.J.; Lv, F.L.; Yang, X.Y.; Sun, B.H.; Zhang, S.L. Phosphorus efficiency, soil phosphorus dynamics and critical phosphorus level under long-term fertilization for single and double cropping systems. Agrics. Ecosyst. Environ. 2018, 256, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.L.; Yang, X.Y. Effects of long-term fertilization on winter wheat yield and soil nutrients in rainfed Loess soil. Chin. J. Soil Sci. 2018, 49, 402–408. [Google Scholar] [CrossRef]
- Wang, W.; Lai, D.Y.F.; Wang, C.; Pan, T.; Zeng, C. Effects of rice straw incorporation on active soil organic carbon pools in a subtropical paddy field. Soil Tillage Res. 2015, 152, 8–16. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils. 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Das, S.; Varma, A. Role of enzymes in maintaining soil health. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Heidelberg, Germany, 2011; pp. 25–42. [Google Scholar]
- Bragazza, L.; Robroek, B.J.M.; Jassey, V.E.J.; Arif, M.S.; Marchesini, R.; Guglielmin, M.; Cannone, N. Soil microbial community structure and enzymatic activity along a plant cover gradient in Victoria Land (continental Antarctica). Geoderma 2019, 353, 144–151. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.Q.; Sun, J.W.; Wang, X.B.; Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 2012, 173–174, 330–338. [Google Scholar] [CrossRef]
- Li, J.; Cooper, J.M.; Lin, Z.A.; Li, Y.T.; Yang, X.D.; Zhao, B.Q. Soil microbial community structure and function are significantly affected by long-term organic and mineral fertilization regimes in the North China Plain. Appl. Soil Ecol. 2015, 96, 75–87. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, W.; Liang, G.Q.; Sun, J.W.; Wang, X.B.; He, P. Distribution of soil nutrients, extracellular enzyme activities and microbial communities across particle-size fractions in a long-term fertilizer experiment. Appl. Soil Ecol. 2015, 94, 59–71. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Mikha, M.M.; Vigil, M.F. Microbial communities and enzyme activities in soils under alternative crop rotations compared to wheat–fallow for the Central Great Plains. Appl. Soil Ecol. 2007, 37, 41–52. [Google Scholar] [CrossRef]
- Li, J.; Tong, X.G.; Awasthi, M.K.; Wu, F.Y.; Ha, S.; Ma, J.Y.; Sun, X.H.; He, C. Dynamics of soil microbial biomass and enzyme activities along a chronosequence of desertified land revegetation. Ecol. Eng. 2018, 111, 22–30. [Google Scholar] [CrossRef]
- Gao, J.T.; Wang, E.X.; Ren, W.L.; Liu, X.F.; Chen, Y.; Shi, Y.W.; Yang, Y.S. Effects of simulated climate change on soil microbial biomass and enzyme activities in young Chinese fir (Cunninghamia lanceolata) in subtropical China. Acta Ecol. Sin. 2017, 37, 272–278. [Google Scholar] [CrossRef]
- Liu, Z.J.; Rong, Q.L.; Zhou, W.; Liang, G.Q. Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS ONE 2017, 12, e0172767. [Google Scholar] [CrossRef]
- Pabst, H.; Kühnel, A.; Kuzyakov, Y. Effect of land-use and elevation on microbial biomass and water extractable carbon in soils of Mt. Kilimanjaro ecosystems. Appl. Soil Ecol. 2013, 67, 10–19. [Google Scholar] [CrossRef]
- Zhao, S.C.; Qiu, S.J.; Xu, X.P.; Ciampitti, I.A.; Zhang, S.Q.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Shi, P.; Wang, S.P.; Jia, S.G.; Gao, Q. Effect of 25-year fertilization on soil microbial biomass and community structure in a continuous corn cropping system. Arch. Agron. Soil Sci. 2015, 61, 1303–1317. [Google Scholar] [CrossRef]
- Guo, A.N.; Zhao, Z.Q.; Zhang, P.F.; Yang, Q.; Li, Y.F.; Wang, G.Y. Linkage between soil nutrient and microbial characteristic in an opencast mine, China. Sci. Total Environ. 2019, 671, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Kramer, C.; Gleixner, G. Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biol. Biochem. 2008, 40, 425–433. [Google Scholar] [CrossRef]
- Luo, P.Y.; Han, X.R.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H.Z. Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Gonzaga, M.I.S.; da Silva, T.O.; da Silva, T.L.; da Silva Dias, N.; Matias, M.I.S. Soil organic matter pools and carbon fractions in soil under different land uses. Soil Tillage Res. 2013, 126, 177–182. [Google Scholar] [CrossRef]
- Ananbeh, H.; Stojanovic, M.; Pompeiano, A.; Voberkova, S.; Trasar-Cepeda, C. Use of soil enzyme activities to assess the recovery of soil functions in abandoned coppice forest systems. Sci. Total Environ. 2019, 694, 133692. [Google Scholar] [CrossRef]
| Enzyme | Abbreviation | Substrate | EC |
|---|---|---|---|
| Phosphatase | Pho | 4-MUB-phosphate | 3.1.3.1 |
| Sulfatase | Sul | 4-MUB-sulfate | 3.1.6.1 |
| β-Glucosidase | βG | 4-MUB-β-d-glucoside | 3.2.1.21 |
| Cellobiohydrolase | CBH | 4-MUB-β-d-cellobioside | 3.2.1.91 |
| N-Acetyl-glucosaminidase | NAG | 4-MUB-N-acetyl-β-d-glucosaminide | 3.2.1.30 |
| α-Glucosidase | αG | 4-MUB-α-d-glucoside | 3.2.1.20 |
| Phenol oxidase | PhOx | L-DOPA | 1.10.3.2 |
| Peroxidase | Perox | L-DOPA | 1.11.1.7 |
| Treatment | pH | Soil Organic C (g kg−1) | Total N (g kg−1) | Available N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) |
|---|---|---|---|---|---|---|
| CK | 8.34 ± 0.02a | 8.18 ± 0.35c | 1.05 ± 0.18b | 3.87 ± 0.10c | 4.13 ± 0.67c | 170.27 ± 26.57b |
| NP | 8.10 ± 0.04c | 10.94 ± 0.32a | 1.30 ± 0.16a | 6.92 ± 0.41a | 47.50 ± 2.86a | 178.73 ± 29.56b |
| NPK | 8.20 ± 0.04b | 10.34 ± 0.12b | 1.44 ± 0.19a | 5.40 ± 0.10b | 32.80 ± 3.40b | 306.93 ± 6.35a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Xin, Y.; Liu, Z. Long-Term Fertilization with Potassium Modifies Soil Biological Quality in K-Rich Soils. Agronomy 2020, 10, 771. https://doi.org/10.3390/agronomy10060771
Chen Q, Xin Y, Liu Z. Long-Term Fertilization with Potassium Modifies Soil Biological Quality in K-Rich Soils. Agronomy. 2020; 10(6):771. https://doi.org/10.3390/agronomy10060771
Chicago/Turabian StyleChen, Qiuyu, Ying Xin, and Zhanjun Liu. 2020. "Long-Term Fertilization with Potassium Modifies Soil Biological Quality in K-Rich Soils" Agronomy 10, no. 6: 771. https://doi.org/10.3390/agronomy10060771
APA StyleChen, Q., Xin, Y., & Liu, Z. (2020). Long-Term Fertilization with Potassium Modifies Soil Biological Quality in K-Rich Soils. Agronomy, 10(6), 771. https://doi.org/10.3390/agronomy10060771




