Potential Use of Rice Husk Biochar and Compost to Improve P Availability and Reduce GHG Emissions in Acid Sulfate Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Incubation Experiment
2.3. Gas Sampling and Measurement
2.4. Soil Sampling for Chemical Analysis
2.5. Chemical Analysis
2.6. Adsorption Ability
2.7. Data Analysis
3. Results
3.1. Change in Soil Chemical Properties with Biochar and Compost Amendment
3.1.1. Soil pH and EC
3.1.2. Phosphorus Factions
3.1.3. Ammonium and Nitrate in Soil
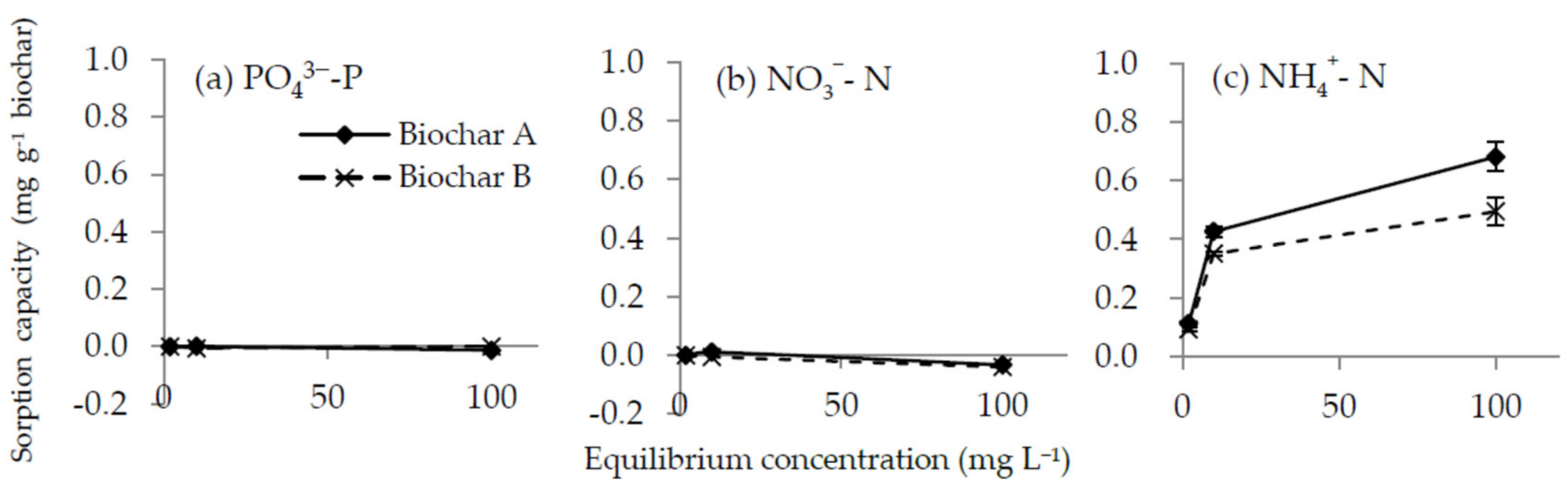
3.2. Biochar Adsorption Capacity
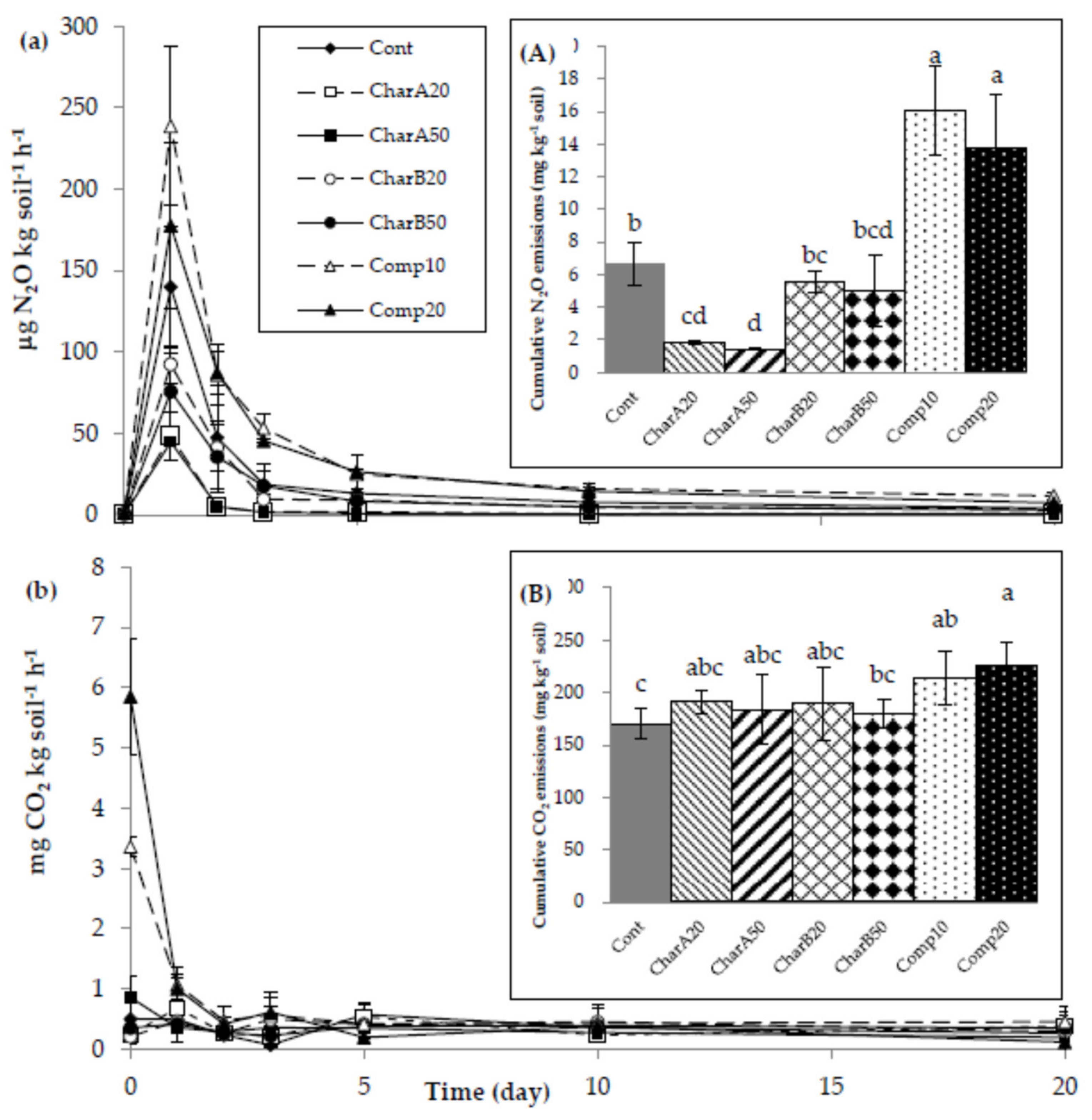
3.3. Gas Emissions
4. Discussion
4.1. Phosphorus Fractions of Soil
4.2. Nitrous Oxide Emissions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torell, M.; Salamanca, A.M. Wetlands management in Vietnam’s Mekong Delta: An overview of the pressures and responses. In Wetlands Management in Vietnam: Issues and Perspectives; Torell, M., Salamanca, A.M., Ratner, B.D., Eds.; WorldFish: Penang, Malaysia, 2003. [Google Scholar]
- Hedley, M.J.; Kirk, G.J.R.; Santos, M.B. Phosphorus efficiency and the forms of soil phosphorus utilized by upland rice cultivars. Plant Soil 1994, 158, 53–62. [Google Scholar] [CrossRef]
- Linquist, B.; Singleton, P.; Yost, R.; Cassman, K. Aggregate size effects on the sorption and release of phosphorus in an ultisol. Soil Sci. Soc. Am. J. 1997, 61, 160–166. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Guppy, C.N.; Menzies, N.; Moody, P.W.; Blamey, F. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Viễn, D.M.; Gương, V.T.; Đông, N.M.; Phượng, N.T.K. Application of compost from sugarcane filter cake to alleviate al toxicity and to improve P availability on acid sulfate soil. Can Tho Uni. J. Sci. 2006, 6, 118–125. [Google Scholar]
- Maurice, P.A.; Hochella, M.F.; Parks, G.A.; Sposito, G.; Schwertmann, U. Evolution of hematite surface microtopography upon dissolution by simple organic acids. Clay. Clay Miner. 1995, 43, 29–38. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Agriculture. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- CGIAR. The Drought and Salinity Intrusion in the Mekong River Delta of Vietnam-Assessment Report; Research Program on Climate Change Agriculture and Food Security-Southeast Asia (CCAFS-SEA): Ha Noi, Vietnam, 2016. [Google Scholar]
- Denmead, O.T.; Macdonald, B.; Bryant, G.; Wang, W.; White, I. Nitrous Oxide Emission from Acid Sulfate Sugarcane Soils on the Coastal Lowlands; Australian Society of Sugar Cane Technologists: Queensland, Australia, 2007; pp. 95–105. [Google Scholar]
- Denmead, O.T.; Macdonald, B.; Naylor, T.; Wang, W.; Salter, B.; White, I.; Wilson, S.; Griffith, D.; Moody, P. Whole-of-Season Greenhouse Gas Emissions from Australian Sugarcane Soils; Australian Society of Sugar Cane Technologists: Queensland, Australia, 2008; pp. 105–114. [Google Scholar]
- IPCC. Mitigation of Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Spokas, K.A.; Reicosky, D.C. Impacts of sixteen different biochars on soil greenhouse gas production. Ann. Environ. Sci. 2009, 3, 179–193. [Google Scholar]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef]
- Shepherd, J.G.; Joseph, S.; Sohi, S.P.; Heal, K.V. Biochar and enhanced phosphate capture: Mapping mechanisms to functional properties. Chemosphere 2017, 179, 57–74. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Bornø, M.L.; Müller-Stöver, D.S.; Liu, F. Contrasting effects of biochar on phosphorus dynamics and bioavailability in different soil types. Sci. Total Environ. 2018, 627, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Liu, S.; Meng, J.; Jiang, L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Chen, B. Biochar impacts on soil silicon dissolution kinetics and their interaction mechanisms. Sci. Rep. 2018, 8, 8040. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, B.; Chen, M. Novel alleviation mechanisms of aluminum phytotoxicity via released biosilicon from rice straw-derived biochars. Sci. Rep. 2016, 6, 29346. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Faucherre, S.; Joss, H.; Obst, M.; Goeckede, M.; Planer-Friedrich, B.; Peiffer, S.; Gilfedder, B.; Elberling, B. Silicon increases the phosphorus availability of arctic soils. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Masulili, A.; Utomo, W.H.; Syechfani, M. Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia. J. Agric. Sci. 2010, 2, 39–47. [Google Scholar] [CrossRef]
- Yao, L.; Yu, X.; Huang, L.; Zhang, X.; Wang, D.; Zhao, X.; Li, Y.; He, Z.; Kang, L.; Li, X.; et al. Responses of phaseolus calcaltus to lime and biochar application in an acid soil. PeerJ 2019, 7, e6346. [Google Scholar] [CrossRef]
- Petter, F.A.; Borges de Lima, L.; Marimon Júnior, B.H.; Alves de Morais, L.; Marimon, B.S. Research article: Impact of biochar on nitrous oxide emissions from upland rice. J. Environ. Manag. 2016, 169, 27–33. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef]
- Watanabe, A.; Ikeya, K.; Kanazaki, N.; Makabe, S.; Sugiura, Y.; Shibata, A. Five crop seasons’ records of greenhouse gas fluxes from upland fields with repetitive applications of biochar and cattle manure. J. Environ. Manag. 2014, 144, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chen, H.; Li, B.; Xiong, Z. Biochar reduces yield-scaled emissions of reactive nitrogen gases from vegetable soils across China. Biogeosciences 2017, 14, 2851–2863. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.; Lee, R.D.; Morris, L.A.; Fisher, D.S. Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef]
- Hammes, K.; Smernik, R.J.; Skjemstad, J.O.; Schmidt, M.W. Characterisation and evaluation of reference materials for black carbon analysis using elemental composition, colour, BET surface area and 13C NMR spectroscopy. Appl. Geochem. 2008, 23, 2113–2122. [Google Scholar] [CrossRef]
- Tushar, M.S.H.K.; Mahinpey, N.; Khan, A.; Ibrahim, H.; Kumar, P.; Idem, R. Production, characterization and reactivity studies of chars produced by the isothermal pyrolysis of flax straw. Biomass Bioenergy 2012, 37, 97–105. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.; De Oliveira, L.; Chen, Y.; Ma, L. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Haseena, P.V.; Padmavathy, K.; Krishnan, R.; Madhu, G. Adsorption of ammonium nitrogen from aqueous systems using chitosan-bentonite film composite. Proc. Technol. 2016, 24, 733–740. [Google Scholar] [CrossRef]
- Hedley, M.; White, R.; Nye, P. Plant-Induced changes in the rhizosphere of rape (Brassica napus Var. Emerald) seedlings: III. Changes in L value, soil phosphate fractions and phosphatase activity. New Phytol. 1982, 91, 45–56. [Google Scholar] [CrossRef]
- Ameloot, N.; Maenhout, P.; De Neve, S.; Sleutel, S. Biochar-induced N2O emission reductions after field incorporation in a loam soil. Geoderma 2016, 267, 10–16. [Google Scholar] [CrossRef]
- De Carvalho, M.T.M.; Madari, B.E.; Bastiaans, L.; van Oort, P.A.J.; de Leal, W.G.O.; de Souza, D.M.; dos Santos, R.C.; Matsushige, I.; de Maia, A.H.N.; Heinemann, A.B.; et al. Nitrogen availability, water-filled pore space, and N2O-N fluxes after biochar application and nitrogen fertilization. Pesqui. Agropecu. Bras. 2016, 51, 1203–1212. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Liu, D.; Ding, W.; Yuan, J.; Lin, Y. Effects of biochar on nitrous oxide and nitric oxide emissions from paddy field during the wheat growth season. J. Clean. Prod. 2015, 104, 52–58. [Google Scholar] [CrossRef]
- Molla, M.A.Z.; Chowdhury, A.A.; Islam, A.; Hoque, S. Microbial mineralization of organic phosphate in soil. Plant Soil 1984, 78, 393–399. [Google Scholar] [CrossRef]
- Zulkifli, H.; Tarmizi, A. Phosphorus fractions in soil amended with empty fruit bunches and phosphate fertilizer-an incubation study. J. Oil Palm Res. 2010, 22, 823–834. [Google Scholar]
- Houba, V.J.G.; Vanderlee, J.J.; Novozamsky, I. Soil and plant analysis: A series of syllabi. In Part 5B Soil Analysis Procedures Other Procedures, 6th ed.; Wageningen Agricultural University, Department of Soil Science and Plant Nutrition: Wageningen, The Netherlands, 1995. [Google Scholar]
- ASTM. Standard test method for determination of iodine number of activated carbon. In ASTM Annual Book; ASTM International: West Conshohocken, PA, USA, 1999; Volume 4. [Google Scholar]
- Opala, P.A.; Okalebo, J.R.; Othieno, C.O. Effects of organic and inorganic materials on soil acidity and phosphorus availability in a soil incubation study. ISRN Agronomy 2012, 2012, 10. [Google Scholar] [CrossRef]
- Spychaj-Fabisiak, E.; Długosz, J.; Zamorski, R. The effect of the phosphorus dosage and incubation time on the process of retarding available phosphorus forms in a sandy soil. Pol. J. Soil Sci. 2005, 38, 23–30. [Google Scholar]
- Banerjee, A.; Sanyal, S.; Sen, S. Soil phosphatase activity of agricultural land: A possible index of soil fertility. Agric. Sci. Res. J. 2012, 2, 412–419. [Google Scholar]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243. [Google Scholar]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.T.K.; Khoi, C.M.; Van Sinh, N.; Chiem, N.H.; Toyota, K. Effects of rice husk biochar and calcium amendment on remediation of saline soil from rice-shrimp cropping system in Vietnamese Mekong Delta. JEAI 2019, 39, 1–12. [Google Scholar] [CrossRef]
- Jan, J.; Borovec, J.; Kopáček, J.; Hejzlar, J. Assessment of phosphorus associated with Fe and Al (hydr) oxides in sediments and soils. J. Soils Sed. 2015, 15, 1620–1629. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Taylor & Francis Group: London, UK; New York, NY, USA, 2015; Volume 2, pp. 421–454. [Google Scholar]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Hong, C.; Lu, S. Does biochar affect the availability and chemical fractionation of phosphate in soils? Environ. Sci. Pollut. Res. 2018, 25, 8725–8734. [Google Scholar] [CrossRef]
- Clough, T.; Condron, L.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Adams, W.A. Gaseous n emission during simultaneous nitrification–denitrification associated with mineral N fertilization to a grassland soil under field conditions. Soil Biol. Biochem. 2000, 32, 1251–1259. [Google Scholar] [CrossRef]
- Skiba, U.; Ball, B. The effect of soil texture and soil drainage on emissions of nitric oxide and nitrous oxide. Soil Use Manag. 2002, 18, 56–60. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Grace, P.R.; Scheer, C.; Rowlings, D. Determining gas sampling timelines for estimating emissions in small chamber incubation experiments. IOSRJEN 2014, 4, 14–16. [Google Scholar] [CrossRef]
- Li, P.; Lang, M.; Li, C.; Hao, X. Nitrous oxide and carbon dioxide emissions from soils amended with compost and manure from cattle fed diets containing wheat dried distillers’ grains with solubles. Can. J. Soil Sci. 2016, 97, 522–531. [Google Scholar] [CrossRef]
- Malghani, S.; Kim, J.; Lee, S.-H.; Yoo, G.-Y.; Kang, H. Application of two contrasting rice-residue-based biochars triggered gaseous loss of nitrogen under denitrification-favoring conditions: A short-term study based on acetylene inhibition technique. Appl. Soil Ecol. 2018, 127, 112–119. [Google Scholar] [CrossRef]
- Takakai, F.; Takeda, M.; Kon, K.; Inoue, K.; Nakagawa, S.; Sasaki, K.; Chida, A.; Sekiguchi, K.; Takahashi, T.; Sato, T. Effects of preceding compost application on the nitrogen budget in an upland soybean field converted from a rice paddy field on gray lowland soil in Akita, Japan. Soil Sci. Plant Nutr. 2010, 56, 760–772. [Google Scholar] [CrossRef]
- Van Cleemput, O.; Samater, A.H. Nitrite in soils: Accumulation and role in the formation of gaseous N compounds. Fertil. Res. 1995, 45, 81–89. [Google Scholar] [CrossRef]

| Soil | Biochar A | Biochar B | Compost | |
|---|---|---|---|---|
| pH (H2O) (1:5) | 5.0 | 7.6 | 9.0 | 8.7 |
| Electrical conductivity (EC) (mS cm−1) (1:5) | 0.42 | 0.50 | 0.89 | 17.1 |
| NH4+-N (mg kg−1) | 14.3 | 3.69 | ND | 20,900 |
| NO3-N (mg kg−1) | 2.9 | 12.2 | ND | 2530 |
| Soluble Na (cmolc kg−1) | 1.69 | 0.21 | 0.37 | 1.75 |
| Soluble K (cmolc kg−1) | 0.18 | 2.51 | 4.17 | 41.1 |
| Soluble Ca (cmolc kg−1) | 0.15 | 0.24 | 0.78 | 2.98 |
| Exchangeable * Na (cmolc kg−1) | 1.31 | ND | 0.06 | 0.64 |
| Exchangeable * K (cmolc kg−1) | 1.08 | 2.30 | 5.43 | 14.5 |
| Exchangeable * Ca (cmolc kg−1) | 3.15 | 1.12 | 1.31 | 41.4 |
| Total C (mg g−1) | 10.4 | 479 | 471 | 154 |
| Total N (mg g−1) | 0.85 | 1.51 | 4.72 | 26.0 |
| Total P (mg kg−1) | 455 | 771 | 1100 | 11,500 |
| C:N | 12.2 | 317 | 100 | 5.9 |
| MWHC, g water g−1 | 0.70 | 5.8 | 3.4 | |
| Moisture content, % | 2.5 | 8.4 | 44.6 | 26.8 |
| Iodine number, mg g−1 | 173 | 110 |
| H2O-P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HCl-Pi | Residual-P | |
|---|---|---|---|---|---|---|---|
| Soil | 13.1 | 20.2 | 19.6 | 129 | 41.6 | 40.0 | 191 |
| Biochar A | 126 | 54.9 | ND | 112 | 83.3 | 142 | 253 |
| Biochar B | 314 | 83.3 | ND | 81.3 | 56.7 | 192 | 371 |
| Compost | 468 | 1123 | 165 | 3196 | 1544 | 4624 | 369 |
| Soil, g | Biochar A, g (Mg ha−1) | Biochar B, g (Mg ha−1) | Compost, g (Mg ha−1) | N-P2O5-K2O, mg (kg ha−1) | |
|---|---|---|---|---|---|
| Cont | 80.0 | 4.8-4.8-3.2 (60-60-40) | |||
| CharA20 | 78.4 | 1.6 (20) | 4.8-4.8-3.2 (60-60-40) | ||
| CharA50 | 76.0 | 4.0 (50) | 4.8-4.8-3.2 (60-60-40) | ||
| CharB20 | 78.4 | 1.6 (20) | 4.8-4.8-3.2 (60-60-40) | ||
| CharB50 | 76.0 | 4.0 (50) | 4.8-4.8-3.2 (60-60-40) | ||
| Comp10 | 79.2 | 0.8 (10) | 4.8-4.8-3.2 (60-60-40) | ||
| Comp20 | 78.4 | 1.6 (20) | 4.8-4.8-3.2 (60-60-40) |
| H2O-P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | |
|---|---|---|---|---|---|
| 1.5 months | |||||
| Cont | 11.2 c | 23.7 d | 17.9 a,b,c | 151 c,d | 22.9 c,d |
| CharA20 | 10.4 c | 25.6 d | 16.6 b,c | 146 d,e | 29.0 c |
| CharA50 | 11.2 c | 27.6 c,d | 22.5 a,b | 144 e | 17.4 d |
| CharB20 | 5.9 e | 28.5 c,d | 22.7 a,b | 151 c | 18.8 d |
| CharB50 | 8.4 d | 31.0 c | 19.7 a,b,c | 146 e | 19.8 d |
| Comp10 | 13.4 b | 62.2 b | 15.7 c | 211 b | 44.5 b |
| Comp20 | 27.2 a | 82.5 a | 23.9 a | 218 a | 53.9 a |
| 6 months | |||||
| Cont | 1.9 c | 51.6 d | 9.2 a,b | 155 c,d | 18.3 b |
| CharA20 | 1.5 c | 60.2 c,d | 4.4b c | 152 c,d | 18.1 b |
| CharA50 | 1.8 c | 62.8 c | 3.4 c | 147 d | 19.8 b |
| CharB20 | 2.4 b,c | 60.1 c,d | 4.0 b,c | 155 c | 19.7 b |
| CharB50 | 4.7 b | 65.8 c | 3.7 c | 154 c,d | 19.6 b |
| Comp10 | 1.6 c | 102 b | 11.7 b | 209 b | 43.5 a |
| Comp20 | 8.5 a | 141 a | 12.9 a | 242 a | 39.9 a |
| Sampling Day: | 0 | 1 | 2 | 5 | 45 |
|---|---|---|---|---|---|
| NH4+-N | |||||
| Cont | 42.7 c | 50.5 b | 60.5 a | 61.4 a | 23.2 d |
| CharA20 | 44.4 c | 47.3 c | 56.0 b | 58.8 a | 17.4 d |
| CharA50 | 47.5 d | 46.3 c | 54.1 b | 59.5 a | 6.6 e |
| CharB20 | 43.3 c | 47.6 b | 62.9 a | 63.0 a | 20.2 b |
| CharB50 | 41.2 d | 46.9 c | 55.3 b | 61.0 a | 9.2 e |
| Comp10 | 313 a,b | 300 b | 313 a,b | 322 a | 161 c |
| Comp20 | 560 a | 557 a | 523 b | 540 a,b | 302 c |
| NO3−-N | |||||
| Cont | 34.4 b | 44.6 a | 43.9 a | 35.2 b | 31.3 c |
| CharA20 | 33.6 d | 42.4 b | 40.2 c | 33.9 d | 45.6 a |
| CharA50 | 32.7 d | 40.4 b | 39.2 c | 31.2 e | 46.6 a |
| CharB20 | 34.7 c | 42.0 a | 39.5 b | 29.6 d | 24.3 e |
| CharB50 | 35.4 b | 40.0 a | 36.2 b | 25.1 c | 18.6 d |
| Comp10 | 40.1 d | 50.1 b | 47.5 c | 39.9 d | 54.5 a |
| Comp20 | 46.5 c | 56.0 a | 54.4 a,b | 45.9 c | 52.1 b |
| Day 0 Incubation | Day 45 Incubation | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Total N | C:N | WFPS | NO3−-N | NH4+-N | NO3−-N | NH4+-N | pH (H2O) |
| CO2 | 0.56 ** | −0.37 ns | 0.25 ns | 0.65 ** | 0.67 ** | 0.47 ns | 0.64 ** | 0.63 ** |
| N2O | 0.73 *** | −0.74 *** | 0.68 ** | 0.80 *** | 0.79 *** | 0.42 ns | 0.79 *** | 0.75 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuong, N.T.K.; Khoi, C.M.; Ritz, K.; Sinh, N.V.; Tarao, M.; Toyota, K. Potential Use of Rice Husk Biochar and Compost to Improve P Availability and Reduce GHG Emissions in Acid Sulfate Soil. Agronomy 2020, 10, 685. https://doi.org/10.3390/agronomy10050685
Phuong NTK, Khoi CM, Ritz K, Sinh NV, Tarao M, Toyota K. Potential Use of Rice Husk Biochar and Compost to Improve P Availability and Reduce GHG Emissions in Acid Sulfate Soil. Agronomy. 2020; 10(5):685. https://doi.org/10.3390/agronomy10050685
Chicago/Turabian StylePhuong, Nguyen Thi Kim, Chau Minh Khoi, Karl Ritz, Nguyen Van Sinh, Mitsunori Tarao, and Koki Toyota. 2020. "Potential Use of Rice Husk Biochar and Compost to Improve P Availability and Reduce GHG Emissions in Acid Sulfate Soil" Agronomy 10, no. 5: 685. https://doi.org/10.3390/agronomy10050685
APA StylePhuong, N. T. K., Khoi, C. M., Ritz, K., Sinh, N. V., Tarao, M., & Toyota, K. (2020). Potential Use of Rice Husk Biochar and Compost to Improve P Availability and Reduce GHG Emissions in Acid Sulfate Soil. Agronomy, 10(5), 685. https://doi.org/10.3390/agronomy10050685






