Preliminary Study of Somatic Variants of Palomino Fino (Vitis vinifera L.) Grown in a Warm Climate Region (Andalusia, Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Grapevine Samples
2.2. Genetic Analysis
2.3. Ampelographic Description
2.4. Grape Musts Physicochemical Characterization
2.5. Statistical Analysis
3. Results
3.1. Genetic Analysis
3.2. Ampelographical Description
3.3. Grape Must Physicochemical Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dzhambazova, T.; Tsvetkov, I.; Atanassov, I.; Rusanov, K.; Martínez-Zapater, J.M.; Atanassov, A.; Hvarleva, T. Genetic diversity in native Bulgarian grapevine germplasm (Vitis vinifera L.) based on nuclear and chloroplast microsatellite polymorphisms. Vitis 2009, 48, 115–121. [Google Scholar]
- Organisation Internationalde de la Vigne et du Vin. OIV World Vitivinicultural Statistics 2013–2014; Organisation Internationalde de la Vigne et du Vin: Paris, France, 2014. [Google Scholar]
- McGorven, P.E. Ancient Wine: The Search for the Origins of Viticulture, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2003; p. 57. [Google Scholar]
- Laucou, V.; Lacombe, T.; Dechesne, F.; Siret, R.; Bruno, J.P.; Dessup, M.; Dessup, J.; Ortigosa, P.; Parra, P.; Roux, C.; et al. High throughput analysis of grape diversity as a tool for germplasm collection management. Appl. Genet. 2011, 122, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Billiard, R. La Vigne Dans L’Antiquité; Jean Laffite Editions: Marseille, France, 1913. [Google Scholar]
- Moncada, X.; Pelsy, F.; Merdinoglu, D.; Hinrichsen, P. Genetic diversity and geographical dispersal in grapevine clones revealed by microsatellite markers. Genome 2006, 49, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Bejerano, P.; Royo, C.; Mauri, N.; Ibáñez, J.; Martínez-Zapater, J.M. Somatic variation and cultivar innovaion in grapevine. In Advances in Grape and Wine Biotechnology, 1st ed.; Morata, A., Loira, I., Eds.; Intechopen: London, UK, 2016; p. 8. [Google Scholar]
- Anon. Estadísticas 2012. SeVi 2012, 3379, 1031. [Google Scholar]
- Buhner-Zaharieva, T.; Moussaoui, S.; Lorente, M.; Andreu, J.; Núñez, R.; Ortiz, J.M.; Gorgocena, Y. Preservation and molecualr characterization of ancient varieties in Spanish grapevine germplasm collections. Am. J. Enol. Vitic. 2010, 61, 557–562. [Google Scholar] [CrossRef]
- Zinelabidine, L.H.; Cunha, J.; Eiras-Dias, J.E.; Cabello, F.; Martínez-Zapater, J.M.; Ibáñez, J. Pedigree analysis of the Spanish grapevine cultivar ‘Hebén’. Vitis 2015, 54, 81–86. [Google Scholar]
- Martinez-Zapater, J.M.; Carmona, M.J.; Díaz-Riquelme, J.; Fernández, L.; Lijavetzky, D. Grapevine genetics after the genome sequence: Challenges and limitations. Aust. J. Grape Wine Res. 2010, 16, 33–46. [Google Scholar] [CrossRef]
- Compés, R.; Sotés, V. El Sector Vitivinícola Frente al Desafío del Cambio Climático, 1st ed.; Monografías Cajamar: Murcia, Spain, 2018; pp. 45–64. [Google Scholar]
- González-Andrés, F.; Martín, J.P.; Yuste, J.; Rubio, J.A.; Arranz, C.; Ortiz, J.M. Identification and molecular biodiversity of autochthonous grapevine cultivars in the “Comarca del Bierzo”, León, Spain. Vitis 2007, 46, 71–76. [Google Scholar]
- Casanova, J.; Mozas, P.; Ortiz, J.M. Ampelography and microsatellite DNA analysis of autochthonous and endagered grapevine cultivars in the province of Huesca (Spain). Span. J. Agric. Res. 2011, 9, 790–800. [Google Scholar] [CrossRef]
- Balda, P.; Ibáñez, J.; Sancha, C.; de Toda, F.M. Characterization and identification of minority red grape varieties recovered in Rioja, Spain. Am. J. Enol. Vitic. 2014, 65, 148–152. [Google Scholar] [CrossRef]
- Jiménez-Cantizano, A.; Lara, M.; Serrano, M.J.; Puig, A.; García de Luján, A. Caracterización de diferentes tipos de la variedad Palomino. In Proceedings of the XXIV Jornadas de Viticultura y Enología Tierra de Barros, Almendralejo, Spain, 6–10 May 2002. [Google Scholar]
- Jiménez-Cantizano, A.; Puertas, B.; Serrano, M.J. Adaptation and selection of cultivars of grapevine quality wines in warm climate. In Proceedings of the II International Symposium on Tropical Wines, Petrolina, Brasil, 25–28 May 2010. [Google Scholar]
- De Herrera, G.A. Agricultura General, 1st ed.; Editorial Real Sociedad Económica Matritense: Madrid, Spain, 1819. [Google Scholar]
- Cabello, F.; Ortiz, J.; Muñoz-Organero, G.; Rodríguez-Torres, I.; Benito, A.; Rubio, C.; García-Muñoz, S.; Sáiz, R. Variedades de Vid en España, 2nd ed.; AMV Ediciones: Madrid, Spain, 2019. [Google Scholar]
- Serrano, J.; Valcárcel, M.C. Variabilidad del Palomino Fino (Vitis Vinifera), 1st ed.; Consejería de Agricultura y Pesca, Junta de Andalucía: Seville, Spain, 1997; p. 11. [Google Scholar]
- De Bobadilla, G.F. Viníferas Jerezanas y de Andalucía Occidental; Instituto Nacional de Investigaciones Agronómicas: Madrid, Spain, 1956. [Google Scholar]
- González-Moreno, J.M.; Bustillo-Barroso, J.M.; Lara Benítez, M.; García de Luján, A. Catálogo de Clones de Variedades de vid en Andalucía, 1st ed.; Consejería de Agricultura y Pesca, Junta de Andalucía: Seville, Spain, 2004; pp. 145–188. [Google Scholar]
- Red de Alerta e Información Fitosanitaria de Andalucía (RAIF). Available online: https://www.juntadeandalucia.es/agriculturapescaydesarrollorural/raif (accessed on 11 February 2020).
- Santesteban, L.G.; Miranda, C.; Royo, J.B. Vegetative growth, reproductive development and vineyard balance. In Methodologies and Results in Grapevine Research, 1st ed.; Delrot, S., Medrano, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer: New York, NY, USA, 2010; pp. 45–56. [Google Scholar]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Identification and characterization of white grape varieties autochthonous of a warm climate region (Andalusia, Spain). Agronomy 2020, 10, 205. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Genetical, morphological and physicochemical characterization of the autochthonous cultivar ‘Uva Rey’ (Vitis vinifera L.). Agronomy 2019, 9, 563. [Google Scholar]
- Vitis. International Variety Catalogue. Available online: www.vivc.de (accessed on 21 January 2020).
- Jiménez-Cantizano, A. Caracterización Molecular del Banco de Germoplasma de vid del Rancho de la Merced. Ph.D. Thesis, Universidad de Cádiz, Cádiz, Spain, 2014. [Google Scholar]
- Organisation Internationale de la Vigne et du Vin (OIV). OIV Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; OIV: Paris, France, 2009. [Google Scholar]
- Benito, A.; Muñoz-Organero, G.; de Andrés, M.T.; Ocete, R.; García-Muñoz, S.; López, M.A.; Arroyo-García, R.; Cabello, F. Ex situ ampelographical characterisation of wild Vitis vinifera from fifty-one Spanish populations. Aust. J. Grape Wine Res. 2017, 23, 143–152. [Google Scholar] [CrossRef]
- OIV Office International de la Vigne et du Vin. Recuéil des Méthodes Internationales D’Analyse des vins et des Moûts; OIV Office International de la Vigne et du Vin: Paris, France, 2014. [Google Scholar]
- Hidalgo-Togores, J. Vendimia. Recepción de uva en la bodega. Índices de maduración químicos. In Tratado de Enología, 5th ed.; Hernández-Úbeda, I., Ed.; Editorial Mundi-Prensa: Madrid, Spain, 2019; Volume I, pp. 238–240. [Google Scholar]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Use of multiflora bee pollen as a flor velum yeast growth activator in biological aging wines. Molecules 2019, 24, 1763. [Google Scholar] [CrossRef] [PubMed]
- Aerny, J. Composés azotés des moûts et des vins. Rev. Suisse Vitic. Arboric. Hortic. 1997, 28, 161–168. [Google Scholar]
- This, P.; Jung, A.; Boccacci, P.; Borrego, J.; Botta, R.; Constantini, K.; Crespan, M.; Dangl, G.S.; Eisenheid, C.; Ferreira-Monteiro, F.; et al. Development of a standard set of microsatellite reference alleles for identification of grape cultivars. Theor. Appl. Genet. 2004, 109, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–5199. [Google Scholar] [CrossRef]
- Tattersall, I.; Desalle, R. A Natural History of Wine, 2nd ed.; Yale University Press: New Haven, CT, USA, 2015; pp. 62–90. [Google Scholar]
- Jiménez-Cantizano, A.; Lara, M.; Ocete, M.E.; Ocete, R. Short communication. Characterization of the relic Almuñécar grapevine cultivar. Span. J. Agric. Res. 2012, 10, 454–460. [Google Scholar]
- Thompson, M.M.; Olmo, H.P. Cytohistological studies of cytochimeric and tetraploid grapes. Am. J. Bot. 1963, 50, 901–907. [Google Scholar] [CrossRef]
- Torregrosa, L.; Fernandez, L.; Bouquet, A.; Bourisquot, J.M.; Pelsy, F.; Martinez-Zapater, J.M. Origins and consequences of somatic variation in grapevine. In Genetics, Genomics and Breeding of Grapes, 2nd ed.; Adam-Blondon, A.F., Martinez-Zapater, J.M., Kole, C., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 68–92. [Google Scholar]
- García-Muñoz, S.; Muñoz-Organero, G.; De Andrés, M.T.; Cabello, F. Ampelography: An old technique with future uses, the case of minor varieties of Vitis vinifera L. from the Balearic Islands. J. Int. Sci. Vigne Vin 2011, 45, 125–137. [Google Scholar] [CrossRef]
- Iriarte-Chiapusso, M.J.; Ocete-Perez, C.A.; Hernández-Beloqui, B.; Ocete-Rubio, R. Vitis vinífera in the Iberian Peninsula: A review. Plant Biosyst. 2017, 151, 245–257. [Google Scholar] [CrossRef]
- Gago, P.; Conéjéro, G.; Martínez, M.C.; Boso, S.; This, P.; Verdeil, J.-L. Microanatomy of leaf trichomes: Oportuities for improved ampelographic discrimination of grapevine (Vitis vinifera L.) cultivars. Aust. J. Grape Wine Res. 2016, 22, 494–503. [Google Scholar] [CrossRef]
- De Orduña, R.M. Climate change associate effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Coombe, B. Influence of temperature on composition and quality of grapes. In Proceedings of the International Symposium on Grapevine Canopy and Vigor Management, Davis, CA, USA, 11 August 1986. [Google Scholar]
- Winkler, A.J.; Cook, J.A.; Kliewer, W.M.; Lider, L.A. General Viticulture; University California Press: Berkeley, CA, USA, 1974. [Google Scholar]
- García-Romero, J.P. Impacto y adaptación al cambio climático en España. In El Sector Vitivinícola Frente al Desafío del Cambio Climático, 1st ed.; Compés, R., Sotés, V., Eds.; Monografías Cajamar: Murcia, Spain, 2018; pp. 265–268. [Google Scholar]
- Tesnière, C.; Brice, C.; Blondin, B. Responses of Saccharomyces cerevisiae to nitrogen starvation in wine alcoholic fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 7025–7034. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.C.; de Toda, F.M. Variability of tempranillo grape composition in the rioja DOCa (Spain) related to soil and climatic characteristics. J. Sci. Food Agric. 2019, 99, 1153–1165. [Google Scholar] [CrossRef]
- Andrades-Rodríguez, M.S. Influencias Climáticas Sobre el Proceso de Maduración del Fruto de Vitis Vinífera, 1st ed.; Consejería de Agricultura y Alimentación Ed, Gobierno de La Rioja: Logroño, Spain, 1991.
- de Luján, A.G.; Peña, B.; Morales, M. Comportamiento de la Variedad de vid Palomino Fino con Distintos Tipos de Poda, 1st ed.; Instituto Nacional de Tecnología Agraria y Alimentaria: Madrid, Spain, 1989. [Google Scholar]
- Catalina, L. Estudio de la maduración del fruto de la vid. In Proceedings of the II Jornadas Universitarias Sobre el Jerez (Universidad de Cádiz), Cádiz, Spain, 24–28 May 1982. [Google Scholar]
- Gerber, C. Researches sur la maduration des fruits charnus. Ann. Sci. Nat. Bot. 1897, 8, 1–16. [Google Scholar]
- Mullins, M.G.; Bouquet, A.; Williams, L.E. The Biology of the Grapevine, 1st ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 80–146. [Google Scholar]
- Pérez-Rodríguez, L. Formación y evolución de alcoholes superiores y otros componentes en vinos de Jerez. Ph.D. Thesis, Universidad de Sevilla, Seville, Spain, 1979. [Google Scholar]
- Sepúlveda, G.; Kliewer, W.M. Effects of high temperature on grapevines (Vitis vinifera L.). II: Distribution of soluble sugars. Am. J. Enol. Vitic. 1986, 37, 20–25. [Google Scholar]
- Puertas, B. Estudio Sobre el Potencial Vitícola y Enológico de Quince Variedades Blancas de vid en la Zona del Jerez. Ph.D. Thesis, Universidad de Cadiz, Cadiz, Spain, 1989. [Google Scholar]
- de Álvaro, J.S. Evolución de los principales ácidos orgánicos durante el periodo de maduración de la uva en la Denominación de Origen Jerez-Xérèz-Sherry. Bachelor’s Thesis, Universidad de Cádiz, Cadiz, Spain, 1986. [Google Scholar]
- Hrazdina, G. Physiological and biochemical events during development and maturation on the grape berries. Am. J. Enol. Vitic. 1984, 35, 220–227. [Google Scholar]
- Iland, P.G.; Coombe, B.G. Malate, tartrate, potassium and sodium in flesh and skin of shiraz grapes during ripening concentration and compartmentation. Am. J. Enol. Vitic. 1988, 39, 71–76. [Google Scholar]
- Lakso, A.N.; Kliewer, W.M. The influence of temperature on malic acid metabolism in grape berries. II. Temperature responses of net dark CO2 fixation and malic acids pools. Am. J. Enol. Vitic. 1977, 29, 145–148. [Google Scholar]

| Grapevine Variety Code | PF, PJ, PG, PP | CS | CH | MPGB | PN | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Microsatellite Locus | ||||||||||
| VVIB01 | 291 | 307 | 291 | 291 | 289 | 295 | 291 | 295 | 289 | 295 |
| VMC1b11 | 184 | 188 | 184 | 184 | 166 | 184 | 184 | 188 | 166 | 172 |
| VMC4F31 | 176 | 206 | 174 | 178 | 174 | 180 | 168 | 206 | 174 | 180 |
| VVMD5 | 226 | 238 | 228 | 236 | 232 | 236 | 226 | 234 | 226 | 236 |
| VVMD7 | 236 | 246 | 236 | 236 | 236 | 240 | 232 | 246 | 236 | 240 |
| VVMD21 | 243 | 249 | 249 | 257 | 249 | 249 | 249 | 265 | 249 | 249 |
| VVMD24 | 209 | 209 | 209 | 217 | 209 | 219 | 213 | 217 | 215 | 217 |
| VVMD25 | 240 | 240 | 238 | 246 | 238 | 252 | 240 | 246 | 238 | 246 |
| VVMD27 | 186 | 194 | 176 | 190 | 182 | 190 | 180 | 194 | 186 | 190 |
| VVMD28 | 238 | 250 | 236 | 238 | 220 | 230 | 248 | 270 | 220 | 238 |
| VVMD32 | 254 | 256 | 238 | 238 | 238 | 270 | 262 | 270 | 238 | 270 |
| VVIH54 | 166 | 166 | 166 | 182 | 164 | 168 | 166 | 166 | 164 | 168 |
| VVIN16 | 151 | 151 | 153 | 153 | 151 | 151 | 149 | 149 | 151 | 159 |
| VVIN73 | 256 | 264 | 264 | 268 | 264 | 266 | 264 | 264 | 264 | 266 |
| VVIP31 | 188 | 190 | 190 | 190 | 180 | 184 | 184 | 188 | 180 | 180 |
| VVIP60 | 318 | 322 | 306 | 314 | 318 | 322 | 318 | 318 | 318 | 320 |
| VVIQ52 | 85 | 85 | 83 | 89 | 83 | 89 | 83 | 83 | 89 | 89 |
| VVS2 | 131 | 144 | 137 | 151 | 135 | 142 | 131 | 131 | 135 | 151 |
| VVIV37 | 163 | 167 | 163 | 163 | 153 | 163 | 163 | 165 | 153 | 163 |
| VVIV67 | 364 | 366 | 364 | 372 | 364 | 372 | 364 | 375 | 364 | 372 |
| VrZAG62 | 187 | 193 | 187 | 193 | 187 | 195 | 185 | 195 | 187 | 193 |
| VrZAG79 | 250 | 260 | 246 | 246 | 242 | 244 | 250 | 254 | 238 | 244 |
| Palomino Fino | Palomino de Jerez | Palomino Gacho | Palomino Pelusón | |
|---|---|---|---|---|
| Palomino Fino | X | |||
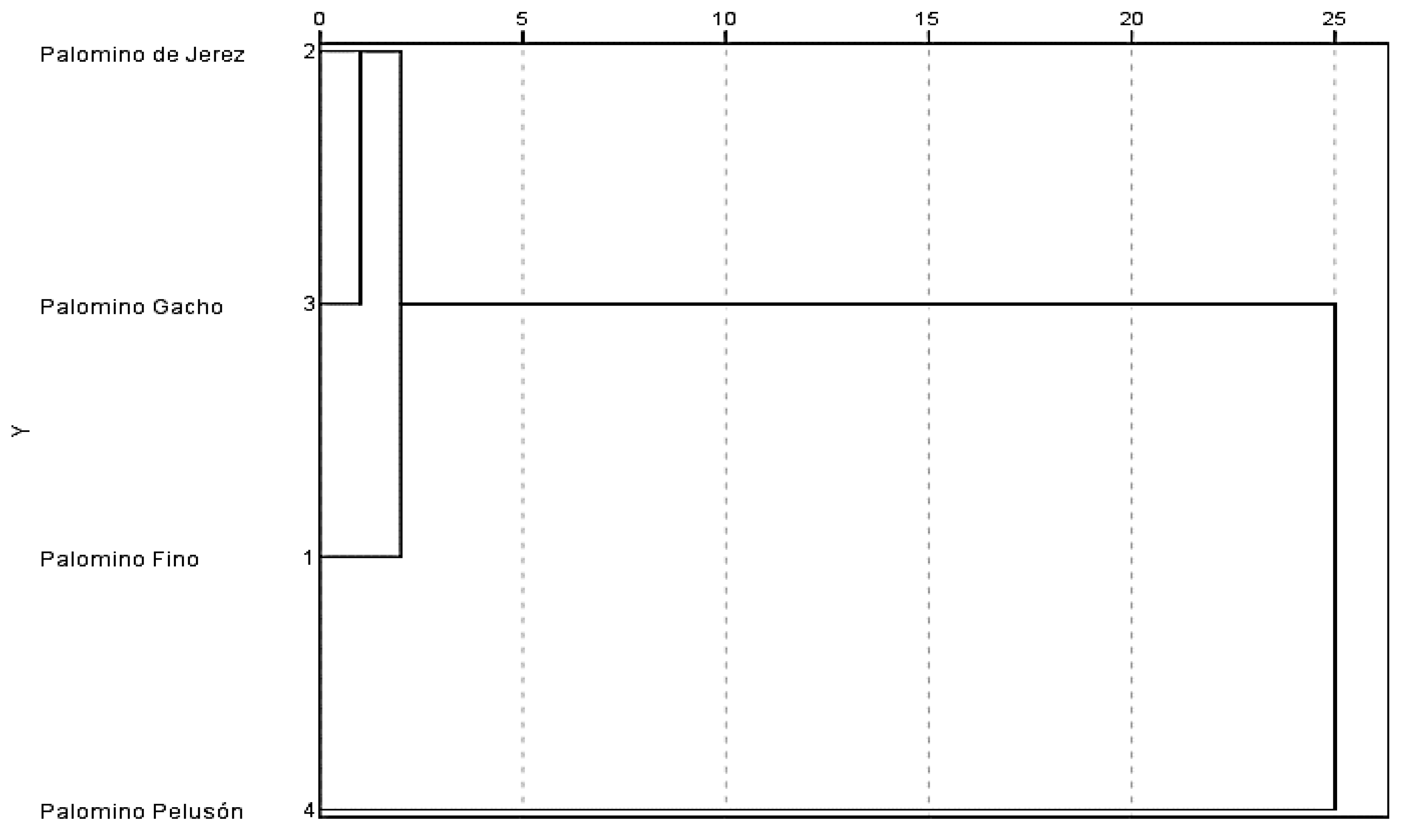
| Palomino de Jerez | 10 | X | ||
| Palomino Gacho | 8 | 10 | X | |
| Palomino Pelusón | 19 | 17 | 17 | X |
| Palomino Fino | Palomino Gacho | Palomino Pelusón | Palomino de Jerez | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
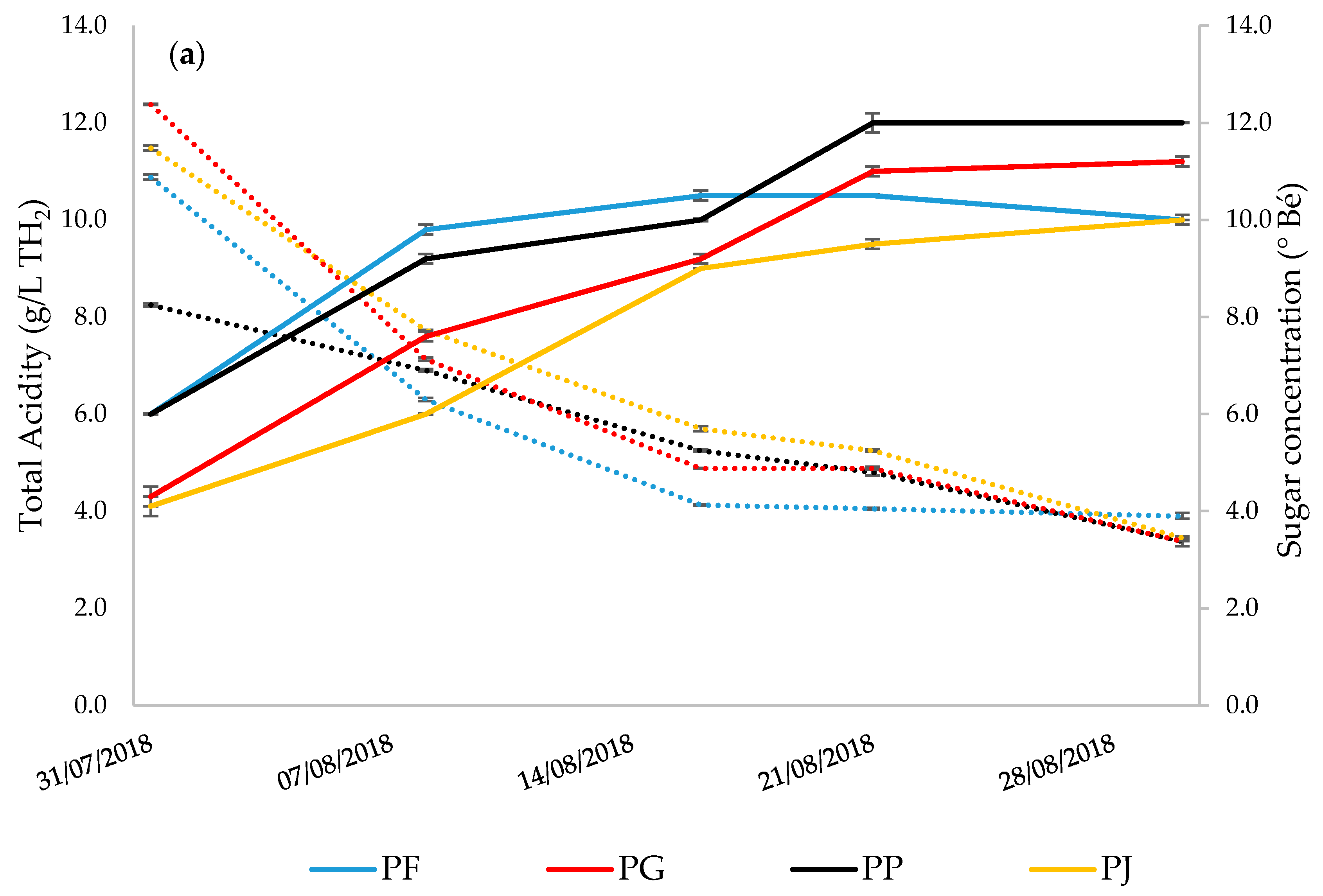
| pH | 3.87 | ± | 0.01 a | 3.53 | ± | 0.01 b | 3.61 | ± | 0.03 b | 3.53 | ± | 0.03 b |
| Baumé | 12.85 | ± | 0.00 a | 11.98 | ± | 0.09 b | 11.10 | ± | 0.01 c | 10.35 | ± | 0.10 d |
| Total Acidity (g/L) | 3.15 | ± | 0.05 a | 4.58 | ± | 0.10 b | 3.32 | ± | 0.06 a | 4.17 | ± | 0.06 c |
| Ripening Index | 4.07 | ± | 0.02 a | 2.61 | ± | 0.08 b | 3.34 | ± | 0.07 c | 2.48 | ± | 0.01 b |
| Tartaric Acid (g/L) | 2.340 | ± | 0.050 a | 2.460 | ± | 0.062 b | 2.663 | ± | 0.041c | 4.002 | ± | 0.055 d |
| Malic Acid (g/L) | 0.622 | ± | 0.064 a | 0.104 | ± | 0.006 b | 0.264 | ± | 0.040 c | 0.200 | ± | 0.009 d |
| YAN (mg/L) | 200.16 | ± | 2.13 a | 247.54 | ± | 2.61 b | 189.27 | ± | 1.54 a | 196.47 | ± | 5.69 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Preliminary Study of Somatic Variants of Palomino Fino (Vitis vinifera L.) Grown in a Warm Climate Region (Andalusia, Spain). Agronomy 2020, 10, 654. https://doi.org/10.3390/agronomy10050654
Sancho-Galán P, Amores-Arrocha A, Palacios V, Jiménez-Cantizano A. Preliminary Study of Somatic Variants of Palomino Fino (Vitis vinifera L.) Grown in a Warm Climate Region (Andalusia, Spain). Agronomy. 2020; 10(5):654. https://doi.org/10.3390/agronomy10050654
Chicago/Turabian StyleSancho-Galán, Pau, Antonio Amores-Arrocha, Víctor Palacios, and Ana Jiménez-Cantizano. 2020. "Preliminary Study of Somatic Variants of Palomino Fino (Vitis vinifera L.) Grown in a Warm Climate Region (Andalusia, Spain)" Agronomy 10, no. 5: 654. https://doi.org/10.3390/agronomy10050654
APA StyleSancho-Galán, P., Amores-Arrocha, A., Palacios, V., & Jiménez-Cantizano, A. (2020). Preliminary Study of Somatic Variants of Palomino Fino (Vitis vinifera L.) Grown in a Warm Climate Region (Andalusia, Spain). Agronomy, 10(5), 654. https://doi.org/10.3390/agronomy10050654








