Profitability of Artificial Pollination in ‘Manzanillo’ Olive Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Orchard Location
2.2. Experimental Design and Treatments
2.3. Initial and Final Fruit Set and Size
3. Results
Fruit Set, Size and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cuevas, J. Las Variedades de Olivo Cultivadas en España; Rallo, L., Barranco, D., Caballero, J.M., del Río, C., Martín, A., Tous, J., Trujillo, I., Eds.; Junta de Andalucía, Mundi-Prensa y COI: Sevilla, Spain, 2004; pp. 303–308. [Google Scholar]
- Guerin, J.; Sedgley, M. Cross-Pollination in Olive Cultivars; Rural Industries Research and Development Corporation Barton: Canberra, Australia, 2007. [Google Scholar]
- Lavee, S.; Datt, A.C. The necessity of cross-pollination for fruit set of Manzanillo olives. J. Hortic. Sci. 1978, 53, 261–266. [Google Scholar] [CrossRef]
- Cuevas, J.; Polito, V.S. Compatibility relationships in ‘Manzanillo’ olive. HortScience 1997, 32, 1056–1058. [Google Scholar] [CrossRef]
- Rejano, L.; Montaño, A.; Casado, F.J.; Sánchez, A.H.; de Castro, A. Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 5–15. [Google Scholar]
- Bradley, M.; Griggs, W.H.; Hartmann, H.T. Studies on self-and cross-pollination of olives under varying temperature conditions. Calif. Agric. 1961, 15, 4–5. [Google Scholar]
- Lavee, S. Handbook of Fruit Set and Development; Monselise, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 261–276. [Google Scholar]
- Morettini, A. Olivocoltura; REDA: Rome, Italy, 1972. [Google Scholar]
- Sánchez-Estrada, A.; Cuevas, J. Pollination strategies to improve fruit set in orchards of ‘Manzanillo’ olive in a nontraditional producing country, Mexico. HortTechnology 2019, 1, 1–7. [Google Scholar]
- Vuletin-Selak, G.; Cuevas, J.; Ban, S.G.; Perica, S. Pollen tube performance in assessment of compatibility in olive (Olea europaea L.) cultivars. Sci. Hortic. 2014, 165, 36–43. [Google Scholar] [CrossRef]
- Cuevas, J.; Díaz-Hermoso, A.J.; Galián, D.; Hueso, J.J.; Pinillos, V.; Prieto, M.; Sola, D.; Polito, V.S. Response to cross pollination and choice of pollinators in olive cultivars (Olea europaea L.) “Manzanillo”, “Hojiblanca” and “Picual”. Olivae 2001, 85, 26–35. [Google Scholar]
- Mookerjee, S.; Guerin, J.; Collins, G.; Ford, C.; Sedgley, M. Paternity analysis using microsatellite markers to identify pollen donors in an olive grove. Appl. Genet. 2005, 111, 1174–1182. [Google Scholar] [CrossRef]
- Gianni, T.; Michelotti, V. Pollination in Plants; Mokwala, P.W., Ed.; IntechOpen Ltd.: London, UK, 2018; pp. 59–83. [Google Scholar]
- Sibbett, G.S.; Freeman, M.; Ferguson, L.; Polito, V.S. Effect of topically applied ‘Sevillano’ pollen on normal-seeded and parthenocarpic “shotberry” fruit set of ‘Manzanillo’ olive. HortTechnology 1992, 2, 228–230. [Google Scholar] [CrossRef]
- Pinillos, V.; Cuevas, J. Artificial pollination in tree crop production. Hortic. Rev. 2008, 34, 239–276. [Google Scholar]
- Ayerza, R.; Coates, W. Supplemental pollination: Increasing olive (Olea europaea L.) yields in hot, arid environments. Exp. Agric. 2004, 40, 481–491. [Google Scholar] [CrossRef]
- Grijalva-Contreras, R.L.; Macías-Duarte, R.; López-Carvajal, A.; Martínez-Díaz, G.; Nuñez-Ramírez, F.; Robles-Contreras, F. Supplemental pollination with different sources of pollen in olive (Olea europaea) ‘Manzanilla’ under hot and arid environment. Ann. Res. Rev. Biol. 2015, 363–369. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M.; Reeve, E. Domestication of plants in the old world. Genet. Res. 1995, 66, 181–182. [Google Scholar]
- Johansen, C. Artificial pollination of apples with bee-collected pollen. J. Econ. Entomol. 1956, 49, 825–828. [Google Scholar] [CrossRef]
- McGregor, S.E. Insect Pollination of Cultivated Crop Plants; Agricultural Handbook; USDA: Washington, DC, USA, 1976. [Google Scholar]
- Sedgley, M.; Griffin, A.R. Sexual Reproduction of Tree Crops; Academic Press: London, UK, 1989. [Google Scholar]
- Westwood, N.F. Temperate-Zone Pomology; Timber Press: Portland, OR, USA, 1993. [Google Scholar]
- Williams, R.R.; Legge, A.P. Pollen application by mechanical dusting in English apple orchards. J. Hortic. Sci. 1979, 54, 67–74. [Google Scholar] [CrossRef]
- Pritchard, K.D.; Edwards, W. Supplementary pollination in the production of custard apple (Annona sp.)—The effect of pollen source. J. Hortic. Sci. Biotechnol. 2006, 81, 78–83. [Google Scholar] [CrossRef]
- Awad, M.A. Pollination of date palm (Phoenix dactylifera L.) cv. Khenazy by pollen grain-water suspension spray. J. Food Agric. Environ. 2010, 8, 313–317. [Google Scholar]
- Abu-Zahra, T.R.; Al-Abbadi, A.A. Effects of artificial pollination on pistachio (Pistacia vera L.) fruit cropping. J. Plant Sci. 2007, 2, 228–232. [Google Scholar]
- Bennett, J.; Koflanovich, T.; Stahmann, W. Pecan growers’ experiences with artificial pollination. In Proceedings of the 20th Western Pecan Conference, Las Cruces, NM, USA, 6–10 March 1986. [Google Scholar]
- Ellena, M.; Sandoval, P.; Gonzalez, A.; Galdames, R.; Jequier, J.; Contreras, M.; Azocar, G. Preliminary results of supplementary pollination on hazelnut in south Chile. Acta Hortic. 2012, 1052, 121–127. [Google Scholar] [CrossRef]
- Rejón, J.D.; Suárez, C.G.; Alche, J.D.; Castro, A.J.; Rodriguez-García, M.I. Evaluación de diferentes métodos para estimar la calidad del polen en distintos cultivares de olivo (Olea europaea L.). Polen 2010, 20, 61–72. [Google Scholar]
- Griggs, W.; Vansell, G.H.; Lwakiri, B.T. Pollen storage: High viability of pollen obtained after storage in home freezer. Calif. Agric. 1953, 7, 12. [Google Scholar]
- Pinney, K.; Polito, V.S. Olive pollen storage and in vitro germination. Acta Hortic. 1990, 286, 207–210. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Pinillos, V.; Cuevas, J. Standardization of the fluorochromatic reaction test to assess pollen viability. Biotech. Histochem. 2008, 83, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.; Hueso, J.J.; Rallo, L. Polinización artificial en olivo. Actas Hortic. 1999, 26, 13–18. [Google Scholar]
- Ruíz-Corral, J.A.; Medina, G.G.; Grajeda Silva, S.M.M.; Díaz, P.G. Estadísticas Climatológicas Básicas del Estado de Sonora (Periodo 1961–2003); Libro Técnico No. 1; INIFAP-CIRNO-SAGARPA: Sonora, Mexico, 2005. [Google Scholar]
- Barranco, D.; Milona, G.; Rallo, L. Épocas de floración de cultivares de olivo en Córdova. Investig. Agraria Prod. Prot. Veg. 1994, 9, 213–220. [Google Scholar]
- Cuevas, J.; Pinillos, V. Polinización artificial en olivo: Recolección de polen. Agric. Rev. Agropecu. 2006, 885, 418–425. [Google Scholar]
- Olive Fantastic. Available online: http://olivefantastic.com/2015/10/selling-your-fruit-for-table-olives-or-oil-and-where-can-you-make-more-money-also-2015-table-olive-olive-oil-pricing/ (accessed on 25 June 2019).
- Codex Alimentarius. Codex Standard for Table Olives. FAO. STAN 66 1981. Available online: https://bit.ly/2XCs5nA (accessed on 25 June 2019).
- Sáez, A.; Negri, P.; Viel, M.; Aiknza, M.A. Pollination efficiency of artificial and bee pollination practices in kiwifruit. Sci. Hortic. 2019, 246, 1017–1021. [Google Scholar] [CrossRef]
- Griggs, W.; Hartmann, H.; Bradley, M.V.; Iwakiri, B.T.; Whisler, J. Olive pollination in California. Bull. Calif. Agric. Exp. Stn. 1975, 869, 49. [Google Scholar]
- Grijalva-Contreras, R.L.; Grijalva-Durón, S.A.; Macías-Duarte, R.; López-Carvajal, A.; Robles-Contreras, F. Response of the artificial pollination and biostimulant application on olive tree productivity under desertic conditions of Sonora. Biotecnia 2012, 3, 39–44. [Google Scholar]
- Hueso, J.J. Polinización Artificial en el Cultivar de Olivo (Olea europaea L.) ‘Picual’. Trabajo Profesional Fin de Carrera (Master’s Thesis), Escuela Técnica Superior Ingeniería Agronómica y de Montes, Universidad de Córdoba, Córdoba, Spain, 1999. [Google Scholar]
- Vaknin, Y.; Gan-Mor, S.; Bechar, A.; Ronen, B.; Eisikowitch, D. Electrostatic pollination of pistachio (Pistacia vera L.)–A novel technique of pollen supplementation in agriculture. Cah. Options Méditer. 2001, 56, 53–57. [Google Scholar]
- Iqbal, M. Effect of different pollination techniques on fruit set, pomological characters and yield of Dhakki date palm (Phoenix dactylifera L.) in Dera Ismail Khan, KP. J. Agric. 2010, 26, 515–551. [Google Scholar]
- Pinillos, V.; Cuevas, J. Open-pollination provides sufficient levels of cross-pollen in Spanish monovarietal olive orchards. HortScience 2009, 44, 499–502. [Google Scholar] [CrossRef]
- Vuletin-Selak, G.; Perica, S.; Goreta-Ban, S.; Radumic, M. Reproductive success after self-pollination and cross-pollination of olive cultivars in Croatia. HortScience 2011, 46, 186–191. [Google Scholar] [CrossRef]
- Sánchez-Estrada, A.; Cuevas, J. ‘Arbequina’ olive is self-incompatible. Sci. Hortic. 2018, 230, 50–55. [Google Scholar] [CrossRef]
- Cuevas, J.; Polito, V.S. The role of staminate flowers in the breeding system of Olea europaea (Oleaceae): An andromonoecious, wind-pollinated taxon. Ann. Bot. 2004, 93, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-de Santa Pau, M.; Rallo, L. Las Variedades de Olivo Cultivadas en España; Rallo, L., Barranco, D., Caballero, J.M., del Río, C., Martín, A., Tous, J., Trujillo, I., Eds.; Junta de Andalucía, Mundi-Prensa y COI: Sevilla, Spain, 2005; pp. 311–314. [Google Scholar]
- Suárez, M.P.; Fernández-Escobar, R.; Rallo, L. Competition among fruits in olive II. Influence of inflorescence or fruit thinning and cross-pollination on fruit set components and crop efficiency. Acta Hortic. 1984, 149, 131–144. [Google Scholar] [CrossRef]
- Cuevas, J.; Rallo, L.; Rapoport, H.F. Crop load effects on floral quality in olive. Sci. Hortic. 1994, 59, 123–130. [Google Scholar] [CrossRef]
- Denney, J.O. Xenia includes metaxenia. HortScience 1992, 7, 722–728. [Google Scholar] [CrossRef]
- Cuevas, J.; Oller, R. Olive seed set and its impact on seed and fruit weight. Acta Hortic. 2002, 586, 485–488. [Google Scholar] [CrossRef]
- Farinelli, D.; Pierrantozzi, P.; Palese, A.M. Pollenizer and cultivar influence seed number and fruit characteristics in Olea europaea L. HortScience 2012, 47, 1430–1437. [Google Scholar] [CrossRef]
- Sánchez-Estrada, A.; Cuevas, J. Pollination designs in ‘Manzanillo’ olive orchards. Sci. Hortic. 2020, 261, 108918. [Google Scholar] [CrossRef]
 large size (160/180 fruit kg−1),
large size (160/180 fruit kg−1),  medium size (200/240 fruit kg−1),
medium size (200/240 fruit kg−1),  small size (280/320 fruit kg−1) and
small size (280/320 fruit kg−1) and  rejected (petite size). Adaptation from [39]. (A) one pollination; (B) two pollinations; (C) four pollinations. Trial 2017.
rejected (petite size). Adaptation from [39]. (A) one pollination; (B) two pollinations; (C) four pollinations. Trial 2017.
 large size (160/180 fruit kg−1),
large size (160/180 fruit kg−1),  medium size (200/240 fruit kg−1),
medium size (200/240 fruit kg−1),  small size (280/320 fruit kg−1) and
small size (280/320 fruit kg−1) and  rejected (petite size). Adaptation from [39]. (A) one pollination; (B) two pollinations; (C) four pollinations. Trial 2017.
rejected (petite size). Adaptation from [39]. (A) one pollination; (B) two pollinations; (C) four pollinations. Trial 2017.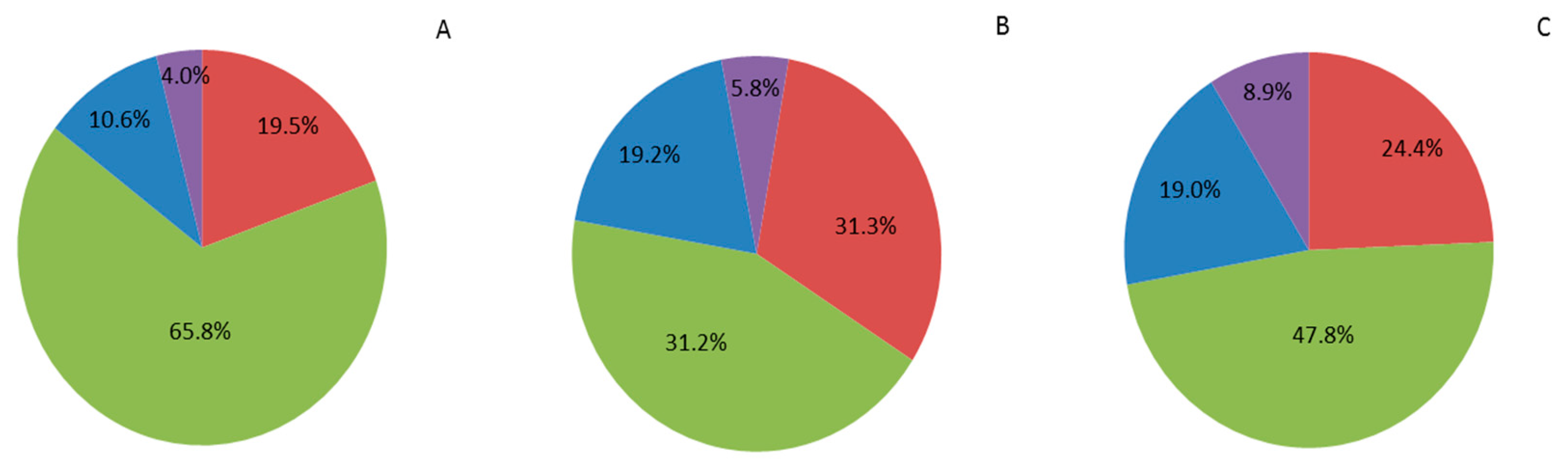
 extra-large size (130/150)
extra-large size (130/150)  large size (160/180 fruit kg−1),
large size (160/180 fruit kg−1),  medium size (200/240 fruit kg−1),
medium size (200/240 fruit kg−1),  small size (280/320 fruit kg−1) and
small size (280/320 fruit kg−1) and  rejected (petite size). Adaptation from [39]. (A) one pollination; (B) two pollinations; (C) four pollinations. Trial 2018.
rejected (petite size). Adaptation from [39]. (A) one pollination; (B) two pollinations; (C) four pollinations. Trial 2018.
 extra-large size (130/150)
extra-large size (130/150)  large size (160/180 fruit kg−1),
large size (160/180 fruit kg−1),  medium size (200/240 fruit kg−1),
medium size (200/240 fruit kg−1),  small size (280/320 fruit kg−1) and
small size (280/320 fruit kg−1) and  rejected (petite size). Adaptation from [39]. (A) one pollination; (B) two pollinations; (C) four pollinations. Trial 2018.
rejected (petite size). Adaptation from [39]. (A) one pollination; (B) two pollinations; (C) four pollinations. Trial 2018.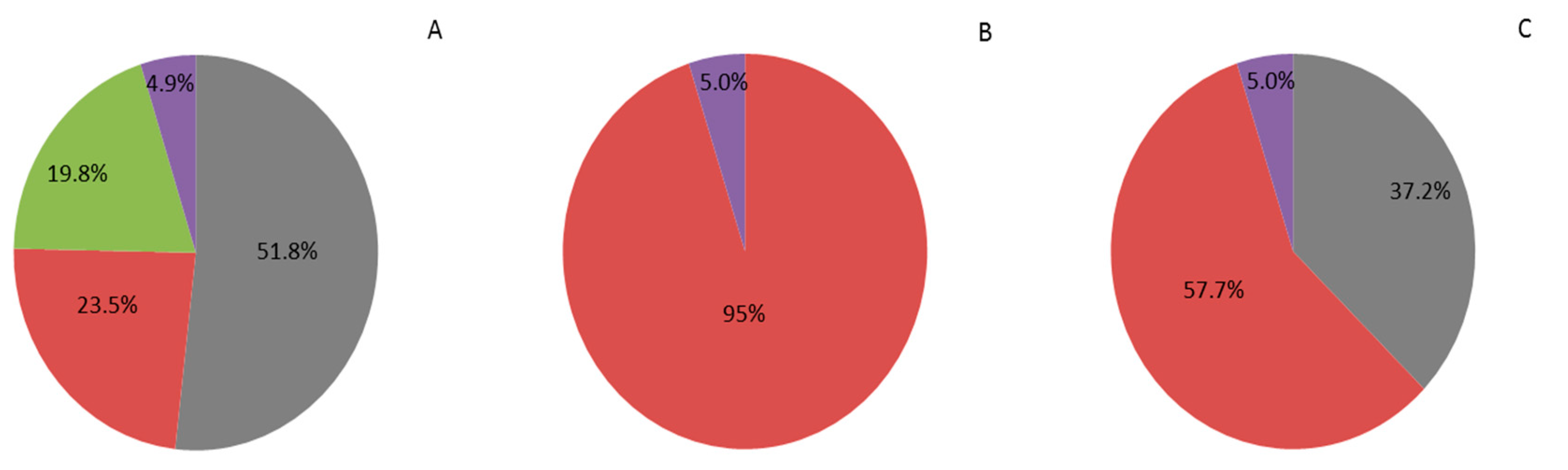
| Treatments (No. Pollinations) | 2017 (“On”) | 2018 (“Off”) | ||||
|---|---|---|---|---|---|---|
| Initial Fruit Set (Fruit Panicle−1) | Final Fruit Set (Fruit Panicle−1) | Fruit Mass (g) | Initial Fruit Set (Fruit Panicle−1) | Final Fruit Set (Fruit Panicle−1) | Fruit Mass (g) | |
| 0 | 0.87 c a | 0.07 c | 3.69 a | 0.68 a | 0.06 b | 3.93 b |
| 1 | 1.23 b | 0.22 b | 3.47 a | 0.27 a | 0.12 a | 4.93 a |
| 2 | 1.72 a | 0.24 b | 3.60 a | 0.30 a | 0.12 a | 4.63 a |
| 4 | 1.62 a | 0.38 a | 3.42 a | 0.31 a | 0.10 a | 4.66 a |
| Treatments (No. Pollinations) | Fruit Size | Price (USD kg−1) a | Yield per Size (kg Tree−1) b | Yield (kg Tree−1) c | Pollen Cost (USD Tree−1) | Income (USD Tree−1) d | Income (USD ha−1) e |
|---|---|---|---|---|---|---|---|
| 0 | ----- | ------ | ND g | 3.88 | 0.00 | ND | ND |
| 1 | Big | 1.40 | 6.26 | 31.25 | 0.90 | 41.33 | 5166 |
| Medium | 1.40 | 20.70 | |||||
| Small | 1.05 | 3.44 | |||||
| Petite f | 0.70 | 1.25 | |||||
| 2 | Big | 1.40 | 11.06 | 35.40 | 1.80 | 43.90 | 5488 |
| Medium | 1.40 | 15.46 | |||||
| Small | 1.05 | 6.78 | |||||
| Petite | 0.70 | 2.07 | |||||
| 4 | Big | 1.40 | 12.96 | 53.26 | 3.60 | 64.08 | 8010 |
| Medium | 1.40 | 25.47 | |||||
| Small | 1.05 | 10.08 | |||||
| Petite | 0.70 | 4.71 |
| Treatments (No. Pollinations) | Fruit size | Price (USD kg−1) a | Yield Per Size (kg Tree−1) b | Yield (kg Tree−1) c | Pollen Cost (USD Tree−1) | Income (USD Tree−1) d | Income (USD ha−1) e |
|---|---|---|---|---|---|---|---|
| 0 | -------- | ------ | ND g | 4.83 | 0.00 | ND | ND |
| 1 | Extra large | 1.30 | 9.04 | 11.41 | 0.90 | 16.37 | 2046 |
| Big | 1.40 | 1.96 | |||||
| Medium | 1.40 | 1.68 | |||||
| Small | 1.05 | 0.00 | |||||
| Petite f | 0.70 | 0.57 | |||||
| 2 | Extra large | 1.30 | 0.00 | 10.12 | 1.80 | 13.81 | 1726 |
| Big | 1.40 | 9.62 | |||||
| Medium | 1.40 | 0.00 | |||||
| Small | 1.05 | 0.00 | |||||
| Petite | 0.70 | 0.50 | |||||
| 4 | Extra large | 1.30 | 3.57 | 8.48 | 3.60 | 11.22 | 1403 |
| Big | 1.40 | 4.49 | |||||
| Medium | 1.40 | 0.00 | |||||
| Small | 1.05 | 0.00 | |||||
| Petite | 0.70 | 0.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Estrada, A.; Cuevas, J. Profitability of Artificial Pollination in ‘Manzanillo’ Olive Orchards. Agronomy 2020, 10, 652. https://doi.org/10.3390/agronomy10050652
Sánchez-Estrada A, Cuevas J. Profitability of Artificial Pollination in ‘Manzanillo’ Olive Orchards. Agronomy. 2020; 10(5):652. https://doi.org/10.3390/agronomy10050652
Chicago/Turabian StyleSánchez-Estrada, Alberto, and Julián Cuevas. 2020. "Profitability of Artificial Pollination in ‘Manzanillo’ Olive Orchards" Agronomy 10, no. 5: 652. https://doi.org/10.3390/agronomy10050652
APA StyleSánchez-Estrada, A., & Cuevas, J. (2020). Profitability of Artificial Pollination in ‘Manzanillo’ Olive Orchards. Agronomy, 10(5), 652. https://doi.org/10.3390/agronomy10050652






