Abscission of Orange Fruit (Citrus sinensis (L.) Osb.) in the Mediterranean Basin Depends More on Environmental Conditions Than on Fruit Ripeness
Abstract
1. Introduction
2. Results
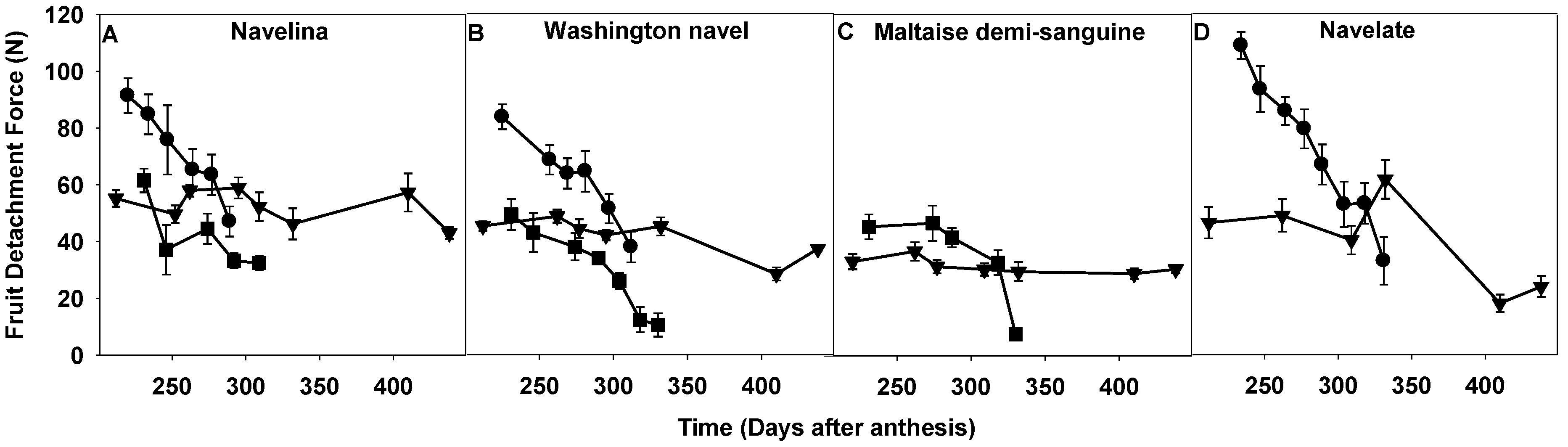
2.1. Comparison of Fruit Detachment Force in Corsica, Spain and Tunisia
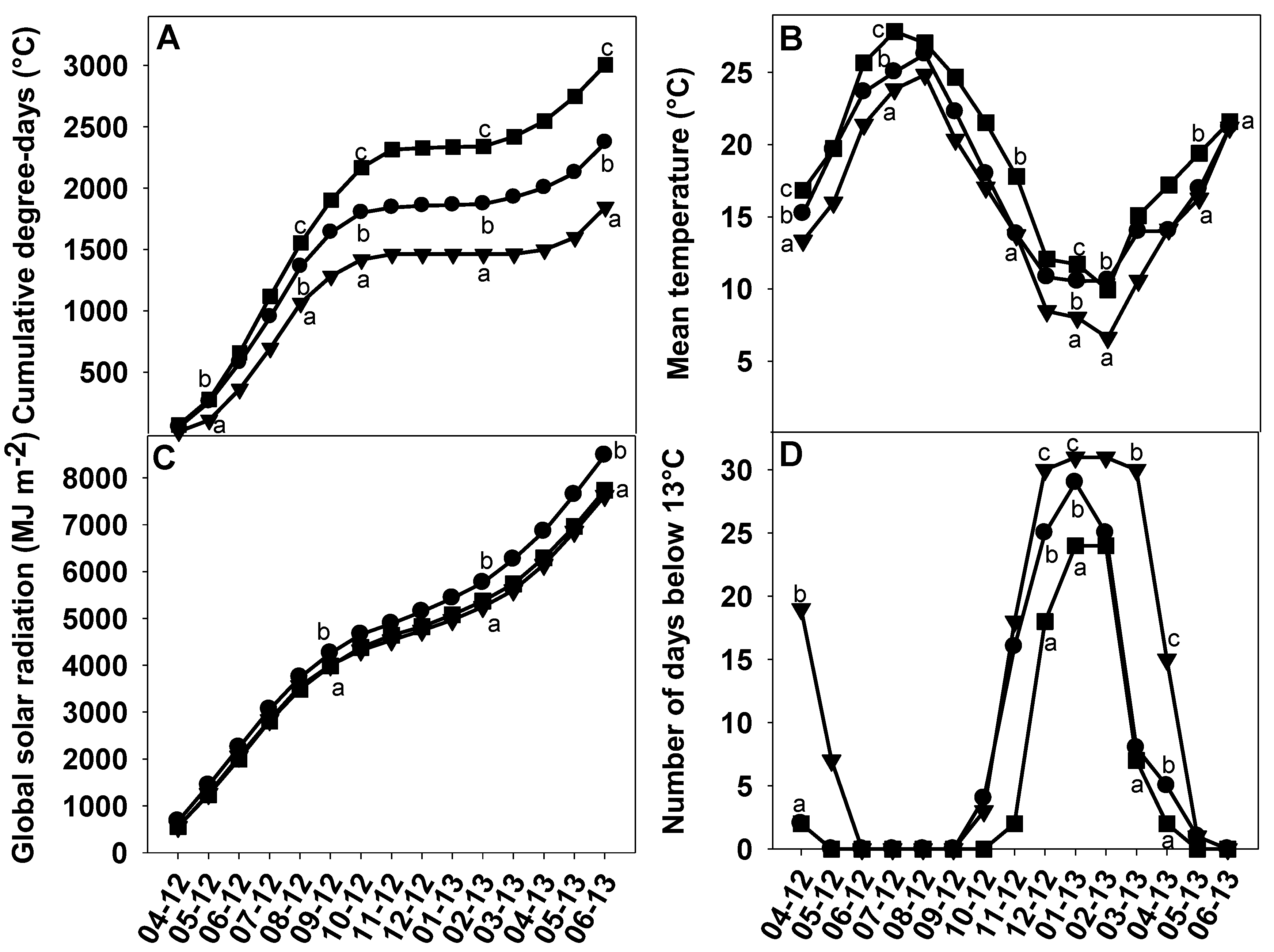
2.2. Soil and Climatic Conditions in Corsica, Spain and Tunisia
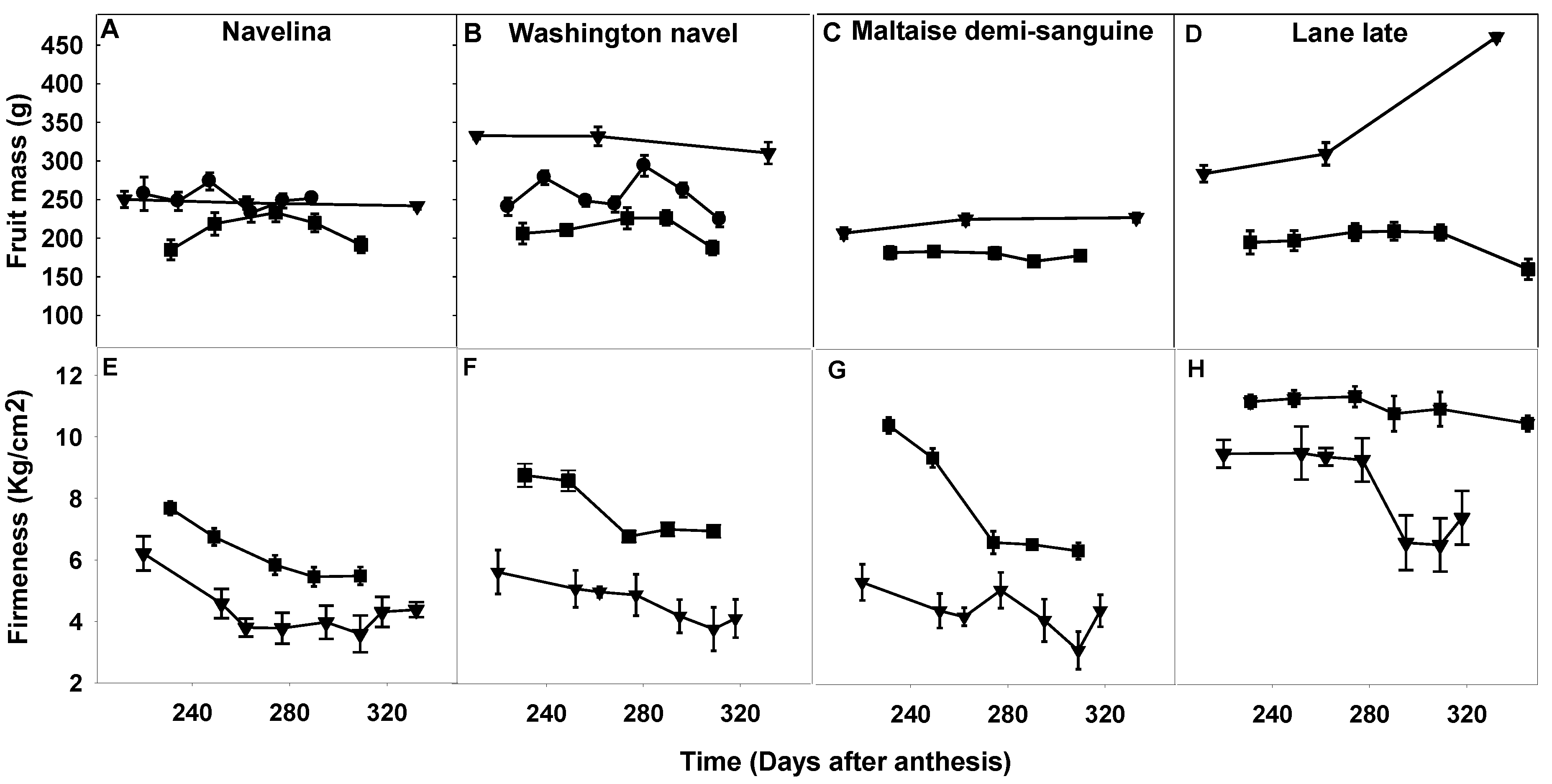
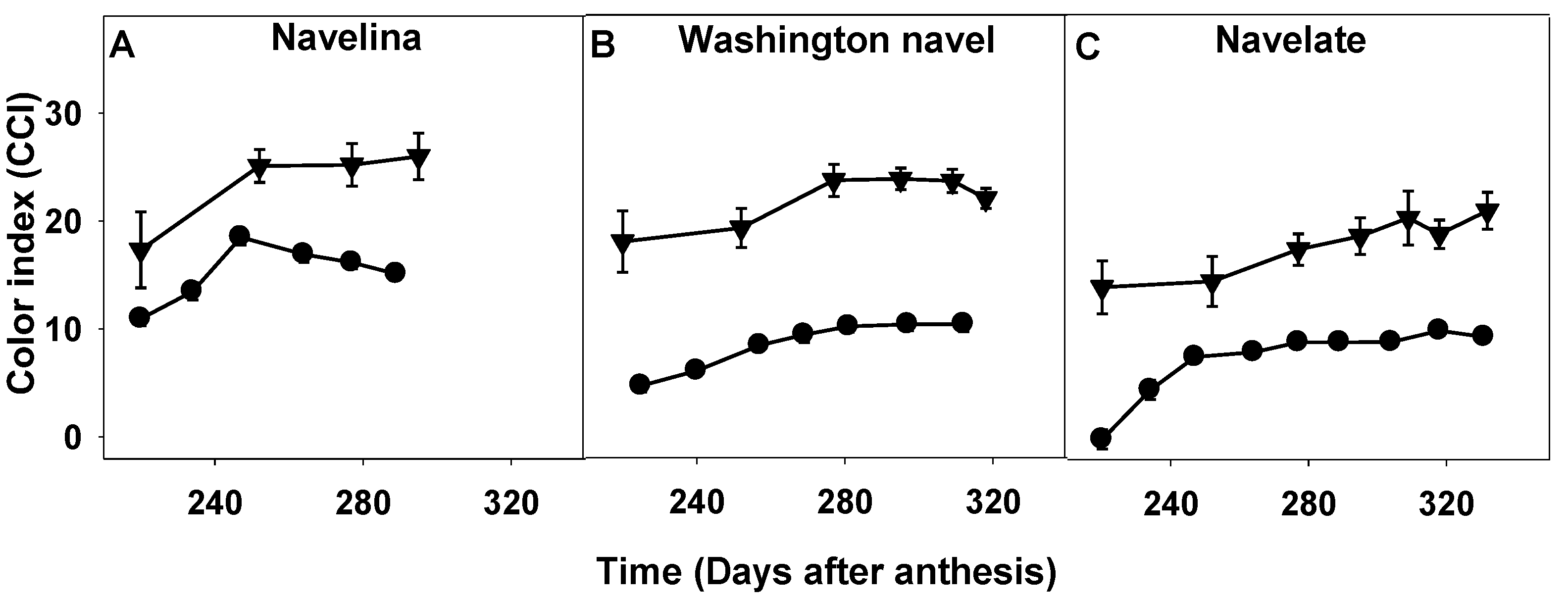
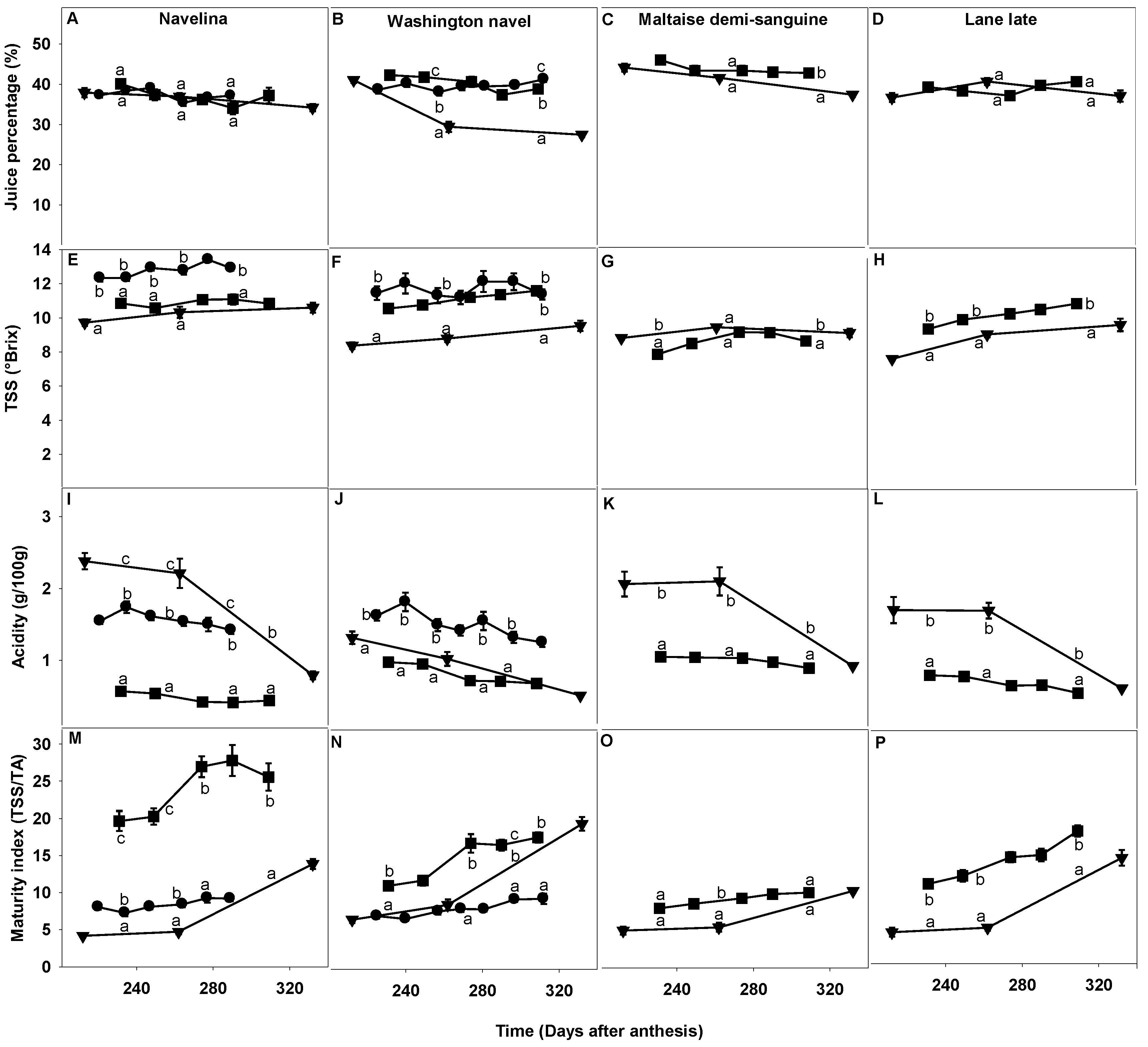
2.3. Comparison of Fruit Maturity Attributes and Their Evolution during the Fruit Maturation and Fruit Drop Period
2.3.1. Fruit Mass, Firmness and Color Index
2.3.2. Juiciness, TSS, Acidity and Fruit Maturity Index
2.3.3. Correlations between Maturation, Environment and Fruit Abscission
3. Discussion
3.1. FDF Measurement and Fruit Shedding
3.2. Impact of the Environment on Abscission Evaluated via Changes in Fruit Traits and in Fruit Maturation
3.3. Impact of the Environment on Abscission
4. Materials and Methods
4.1. Plant Material
4.2. Climatic Data
4.3. Soil Conditions
4.4. Evaluation of External and Internal Fruit Maturity
4.5. Measurement of the Fruit Detachment Force (FDF)
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Production. 2016. Available online: http://faostat3.fao.org/home/E (accessed on 10 March 2020).
- Syvertsen, J.; Levy, Y. Salinity interactions with other abiotic and biotic stresses in citrus. Hort. Technol. 2005, 15, 100–103. [Google Scholar] [CrossRef]
- Terol, J.; Conesa, A.; Colmenero, J.M.; Cercos, M.; Tadeo, F.; Agustí, J.; Alós, E.; Andres, F.; Soler, G.; Brumos, J. Analysis of 13000 unique Citrus clusters associated with fruit quality, production and salinity tolerance. BMC Genom. 2007, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Jacquemond, C.; Curk, F.; Heuzet, M. Les Clémentiniers et Autres Petits Agrumes. In Editions Quae; Collection Savoir-Faire: Versaille, France, 2013; pp. 1–363. [Google Scholar]
- Patt, J.; Carmeli, D.; Zafrir, I. Influence of soil physical conditions on root development and on productivity of citrus trees. Soil Sci. 1966, 102, 82–84. [Google Scholar] [CrossRef]
- Spiegel-Roy, P.; Goldschmidt, E.E. Reproductive physiology: Flowering and fruiting. In The Biology of Citrus; Cambridge University Press: Cambridge, UK, 1996; pp. 70–118. [Google Scholar]
- Bain, J.M. Morphological, anatomical, and physiological changes in the developing fruit of the Valencia orange, Citrus sinensis (L.) Osbeck. Aust. J. Bot. 1958, 6, 1–23. [Google Scholar]
- Soule, J.; Grierson, W. Maturity and grade standards. In Fresh Citrus Fruits; Wardowski, W.F., Nagy, S., Grierson, W., Eds.; AVI Pub. Co.: Westport, CT, USA, 1986; pp. 23–48. [Google Scholar]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R. Physiology of citrus fruiting. Braz. J. Plant Pysiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- Roongruangsri, W.; Rattanapanone, N.; Leksawasdi, N.; Boonyakiat, D. Physico-chemical changes during growth and maturation of tangerine fruit cv. ‘Sai Nam Phueng’and ‘See Thong’. Chiang Mai Univ. J. Nat. Sci. 2013, 12, 59–72. [Google Scholar]
- Baldwin, E. Citrus fruit. In Biochemistry of Fruit Ripening; Seymour, G.B., Taylor, J.E., Tucker, G.A., Eds.; Springer Netherlands: London, UK, 1993; pp. 107–149. [Google Scholar]
- Kimball, D.A.; Box, C. Factors affecting the rate of maturation of citrus fruits. Proc. Fla. State Hort. Soc. 1984, 97, 40–44. [Google Scholar]
- Barry, G.H.; van Wyk, A.A. Low-temperature cold shock may induce rind colour development of ‘Nules Clementine’ mandarin (Citrus reticulata Blanco) fruit. Postharvest Biol. Technol. 2006, 40, 82–88. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Fanciullino, A.-L.; Dubois, C.; Ollitrault, P. Effect of genotype and environment on citrus juice carotenoid content. J. Agric. Food Chem. 2009, 57, 9160–9168. [Google Scholar] [CrossRef]
- El-Otmani, M.; Ait-Oubahou, A.; Zacarias, L. Citrus spp.: Orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon and lime. In Postharvest Biology and Technology of Tropical and Subtropical Fruit; Yahia, E., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 437–515. [Google Scholar]
- Chelong, I.; Sdoodee, S. Effect of climate variability and degree-day on development, yield and quality of shogun (Citrus reticulata Blanco) in southern Thailand. Kasetsart J. 2013, 47, 333–341. [Google Scholar]
- Reuther, W.; Nauer, E.; Summers, L. Effects of seasonal temperature regimes on development and maturation of citrus fruits. Proc. Intl. Soc. Citricult. 1973, 3, 63–71. [Google Scholar]
- Goldschmidt, E. Reassessment of climatic effects on fruit maturation in Citrus towards the development of a fruit maturation and quality model. Proc. Int. Soc. Citricult. 2000, 2, 300–302. [Google Scholar]
- Stenzel, N.M.C.; Neves, C.S.V.J.; Marur, C.J.; Scholz, M.B.d.S.; Gomes, J.C. Maturation curves and degree-days accumulation for fruits of ‘Folha Murcha’ orange trees. Sci. Agric. 2006, 63, 219–225. [Google Scholar] [CrossRef][Green Version]
- Harcourt, D.A. Thermal summation model for predicting seasonal occurrence of the alfalfa weevil, Hypera postica (Coleoptera: Curculionidae), in southern Ontario. Can. Entomol. 1981, 113, 601–605. [Google Scholar] [CrossRef]
- Niederhuth, C.E.; Cho, S.K.; Seitz, K.; Walker, J.C. Letting Go is Never Easy: Abscission and Receptor-Like Protein Kinases. J. Integr. Plant Biol. 2013, 55, 1251–1263. [Google Scholar] [CrossRef]
- Addicott, F.T. Environmental factors in the physiology of abscission. Plant. Physiol. 1968, 43, 1471. [Google Scholar]
- El-Otmani, M.; M’Barek, A.A.; Coggins, C.W., Jr. GA3 and 2,4-D prolong on-tree storage of citrus in Morocco. Sci. Hortic. 1990, 44, 241–249. [Google Scholar] [CrossRef]
- Tadeo, F.; Simó, A.; Agustí, J.; Merelo, P.; Fuente, D.J.I. La abscisión de frutos maduros en naranjos dulces del grupo Navel correlaciona con la acumulación de azúcares en el zumo. Levante Agric. Rev. Int. Cítr. 2007, 389, 393–402. [Google Scholar]
- Hartmond, U.; Yuan, R.; Burns, J.K.; Grant, A.; Kender, W.J. Citrus fruit abscission induced by methyl-jasmonate. J. Am. Soc. Hortic. Sci. 2000, 125, 547–552. [Google Scholar] [CrossRef]
- Aragón-Rodríguez, F.; Castro-García, S.; Sola-Guirado, R.R.; Gil-Ribes, J.A. Fruit abscission pattern of ‘Valencia’ orange with canopy shaker. Sci. Hortic. 2019, 246, 916–920. [Google Scholar] [CrossRef]
- Pozo, L.; Malladi, A.; John-Karuppiah, K.J.; Lluch, Y.; Alferez, F.; Burns, J.K. Daily fluctuation in fruit detachment force of ‘Valencia’ orange is related to time of day, temperature, relative humidity, fruit weight and juice percentage. Proc. Fla. State Hortic. Soc. 2007, 120, 41–44. [Google Scholar]
- Kender, W.J.; Hartmond, U. Variability in detachment force and other properties of fruit within orange tree canopies. Fruit Var. J. 1999, 53, 105–109. [Google Scholar]
- Kumar, N.; Ebel, R.C. Oxidative metabolism in ‘Valencia’ sweet orange [Citrus sinensis (L.) Osbeck] flavedo tissue treated with the abscission agent 5-chloro-3-methyl-4-nitro-1H-pyrazole (CMNP). J. Hortic. Sci. Biotechnol. 2015, 90, 413–418. [Google Scholar] [CrossRef]
- Wilson, W. Problems encountered using cycloheximide to produce abscission of oranges. Hortscience 1973, 8, 323–324. [Google Scholar]
- Estornell, L.H.; Agustí, J.; Merelo, P.; Talón, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199–200, 48–60. [Google Scholar] [CrossRef]
- Merelo, P.; Agustí, J.; Arbona, V.; Costa, M.L.; Estornell, L.H.; Gómez-Cadenas, A.; Coimbra, S.; Gómez, M.D.; Pérez-Amador, M.A.; Domingo, C.; et al. Cell wall remodeling in abscission zone cells during ethylene-promoted fruit abscission in citrus. Front. Plant Sci. 2017, 8, 126. [Google Scholar]
- Patharkar, O.R.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef]
- Marsh, K.; Richardson, A.; Macrae, E. Early-and mid-season temperature effects on the growth and composition of satsuma mandarins. J. Hortic. Sci. Biotechnol. 1999, 74, 443–451. [Google Scholar] [CrossRef]
- Utsunomiya, N.; Yamada, H.; Kataoka, I.; Tomana, T. The effect of fruit temperatures on the maturation of satsuma mandarin (Citrus unshiu Marc.) fruits. J. Jpn. Soc. Hortic. Sci. 1982, 51, 135–141. [Google Scholar] [CrossRef][Green Version]
- Richardson, A.C.; Marsh, K.; Macrae, E. Temperature effects on satsuma mandarin fruit development. J. Hortic. Sci. 1997, 72, 919–929. [Google Scholar] [CrossRef]
- Hutton, R.J.; Landsberg, J.J. Temperature sums experienced before harvest partially determine the post-maturation juicing quality of oranges grown in the Murrumbidgee Irrigation Areas (MIA) of New South Wales. J. Sci. Food Agric. 2000, 80, 275–283. [Google Scholar] [CrossRef]
- Hodgson, R.W. Horticultural varieties of citrus. In The Citrus Industry; Reuther, W., Webber, H.J., Batchelor, L.D., Eds.; Univ. of California Press: Riverside, CA, USA, 1967; pp. 431–591. [Google Scholar]
- Castle, W.; Tucker, D.; Krezdorn, A.; Youtsey, C. Rootstocks for Florida Citrus: Rootstock Selection, The First Step Success, 2nd ed.; University of Florida, Inst. Food Agric. Sci.: Gainesville, FL, USA, 1993. [Google Scholar]
- Wirch, J.; Kappel, F.; Scheewe, P. The effect of cultivars, rootstocks, fruit maturity and gibberellic acid on pedicel retention of sweet cherries (Prunus avium L.). J. Am. Pomol. Soc. 2009, 63, 108–114. [Google Scholar]
- Zacarias, L.; Talon, M.; Ben-Cheick, W.; Lafuente, M.T.; Primo-Millo, E. Abscisic acid increases in non-growing and paclobutrazol treated fruits of seedless mandarins. Physiol. Plant. 1995, 4, 613–619. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Tadeo, F.R.; Talon, M.; Primo-Millo, E. Leaf abscission induced by ethylene in water stressed intact seedlings of (Citrus reshni Hort. ex Tan.) requires previous abscissic acid accumulation in roots. Plant. Physiol. 1996, 112, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Addicott, F.T. Abscission; University of California Press: Berkeley, CA, USA, 1982; p. 370. [Google Scholar]
- Greenberg, J.; Goren, R.; Riov, J. The role of cellulase and polygalacturonase in abscission of young and mature Shamouti orange fruits. Physiol. Plant. 1975, 34, 1–7. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Prinsen, E.; Van Der Straeten, D.; Vandenbussche, F. Hormone-controlled UV-B responses in plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar] [CrossRef]
- Rakitina, T.Y.; Vlasov, P.V.; Rakitin, V.Y. Hormonal aspects of different resistance of Arabidopsis thaliana mutants to ultraviolet radiation. Russ. J. Plant Phys. 2001, 48, 353–358. [Google Scholar] [CrossRef]
- Predieri, S.; Norman, H.A.; Krizek, D.T.; Pillai, P.; Mirecki, R.M.; Zimmerman, R.H. Influence of UV-B radiation on membrane lipid-composition and ethylene evolution in Doyenne-d’Hiver pear shoots grown in-vitro under different photosynthetic photon fluxes. Environ. Exp. Bot. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Yelenosky, G. Freeze survival of Citrus trees in Florida. In Proceedings of the International Plant Cold Hardiness Seminar, St. Paul, MN, USA, 2–4 November 1977; 1978; Volume 1, pp. 297–311. [Google Scholar]
- Yuan, R.; Burns, J.K. Temperature factor affecting the abscission response of mature fruit and leaves to CMN-Pyrazole and Ethephon in ‘Hamlin’ oranges. J. Am. Soc. Hortic. Sci. 2004, 129, 287–293. [Google Scholar] [CrossRef]
- Bustan, A.; Goldschmidt, E. Estimating the cost of flowering in a grapefruit tree. Plant Cell Eviron. 1998, 21, 217–224. [Google Scholar] [CrossRef]
- Crane, J.C. The role of hormones in fruit set and development. HortScience 1969, 4, 1969–1970. [Google Scholar]
- Goldschmidt, E. Endogenous growth substances of Citrus tissues. HortScience 1976, 11, 95–99. [Google Scholar]
- Hofman, P.J. Abscisic acid and gibberellins in the fruitlets and leaves of ‘Valencia’ orange in relation to fruit growth and retention. Sci. Hortic. 1990, 42, 257–267. [Google Scholar] [CrossRef]
- Talon, M.; Zacarias, L.; Primo-Millo, E. Hormonal changes associated with fruit set and development in mandarins differing in their parthenocarpic ability. Physiol. Plant. 1990, 79, 400–406. [Google Scholar] [CrossRef]
- Kojima, K. Changes of abscisic acid, indole-3-acetic acid and gibberellin-like substances in the flowers and developing fruitlets of citrus cultivar ‘Hyuganatsu’. Sci. Hortic. 1996, 65, 263–272. [Google Scholar] [CrossRef]
- Tadeo, F.R.; Cercós, M.; Colmenero-Flores, J.M.; Iglesias, D.J.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R. Molecular physiology of development and quality of citrus. Adv. Bot. Res. 2008, 47, 147–223. [Google Scholar]
- Yuan, R.; Kender, W.J.; Burns, J.K. Young Fruit and Auxin Transport Inhibitors Affect the Response of MatureValencia’Oranges to Abscission Materials via Changing Endogenous Plant Hormones. J. Am. Soc. Hortic. Sci. 2003, 128, 302–308. [Google Scholar] [CrossRef]
- Plummer, J.A.; Mullins, M.G.; Vine, J.H. Seasonal changes in endogenous ABA and IAA and the influence of applied ABA and auxin in relation to shoot growth and abscission in Valencia Orange (Citrus sinensis (L.) Osbeck). Plant Growth Regul. 1991, 10, 139–151. [Google Scholar] [CrossRef]
- Yuan, R.; Hartmond, U.; Kender, W.J. Physiological factors affecting response of mature ‘Valencia’ orange fruit to CMN–pyrazole. II. Endogenous concentrations of indole-3-Acetic acid, abscisic acid, and ethylene. J. Am. Soc. Hortic. Sci. 2001, 126, 420–426. [Google Scholar] [CrossRef]
- Monteith, J. Evaporation and environment. Symp. Soc. Exp. Biol. 1965, 19, 205–234. [Google Scholar]
- Bevington, K.B.; Castle, W.S. Annual root growth pattern of young citrus trees in relation to shoot growth, soil temperature, and soil water content. J. Am. Soc. Hortic. Sci. 1985, 110, 840–845. [Google Scholar]
- Mendel, K. The influence of temperature and light on the vegetative development of citrus trees. In Proceedings of the International Citrus Symposium, Riverside, CA, USA, 16–26 March 1968; pp. 259–265. [Google Scholar]
- Jiménez-Cuesta, M.; Cuquerella, J.; Martinez-Javaga, J. Determination of a color index for citrus fruit degreening. In Proceedings of the International Society of Citriculture. International Citrus Congress, Tokyo, Japan, 9–12 November 1981; 1982; pp. 750–753. [Google Scholar]
- Burns, J.K.; Pozo, L.V.; Arias, C.R.; Hockema, B.; Rangaswamy, V.; Bender, C.L. Coronatine and abscission in citrus. J. Am. Soc. Hortic. Sci. 2003, 128, 309–315. [Google Scholar] [CrossRef]
- Burns, J.K.; Buker, R.S.; Roka, F.M. Mechanical harvesting capacity in sweet orange is increased with an abscission agent. HortTechnology 2005, 15, 758–765. [Google Scholar] [CrossRef]
| FDF (N) | Fruit Mass (g) | Juice Percentage | Firmness (Kg/cm2) | TSS (°Brix) | Acidity (g/100 g) | Maturity Index | |
|---|---|---|---|---|---|---|---|
| (A) Corsica/Spain/Tunisia | 5.9 × 10−8 *** | 2.8 × 10−9 *** | 1.1 × 10−3 ** | 1.5 × 10−4 *** | 2.3 × 10−12 *** | <2 × 10−16 *** | <2 × 10−16 *** |
| (B) Corsica/Tunisia | 5.1 × 10−13 *** | 2.2 × 10−13 *** | 1.7 × 10−6 *** | 1.0 × 10−4 *** | 2.5 × 10−2 * | <2 × 10−16 *** | <2 × 10−16 *** |
| (C) Corsica/Tunisia | 5.9 × 10−8 *** | 3.6 × 10−9 *** | 1.5 × 10−11 *** | 1.8 × 10−4 *** | 1.2 × 10−12 *** | 2.2 × 10−5 *** | 4.0 × 10−5 *** |
| Site | Cultivar | Date | Fruit Mass (g) | Juice % | Color Index | FDF (N) | TSS (°Brix) | Acidity (g/100 g) | Maturity Index | Firmness (Kg/cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Corsica | Maltaise ½ sanguine | Date1 | 175 a | 43 b | 81 a | 44 a | 9.3 a | 2.0 b | 5 a | 5 a |
| Date2 | 222 b | 38 a | 49 b | 41 a | 9.6 a | 0.9 a | 10 b | 4 a | ||
| Navelate | Date1 | 208 a | 39 b | 1 a | 57 a | 8.0 a | 1.9 b | 4 a | 6 a | |
| Date2 | 257 b | 35 a | 42 b | 57 a | 9.7 b | 0.7 a | 14 b | 5 a | ||
| Newhall navel | Date1 | 213 a | 39 b | 23 a | 55 a | 10.0 a | 1.3 b | 8 a | 8 a | |
| Date2 | 251 b | 32 a | 63 b | 41 a | 10.4 a | 0.6 a | 17 b | 4 b | ||
| Lane late | Date1 | 325 a | 37 a | −22 a | 66 a | 7.5 a | 1.7 b | 4 a | 12 b | |
| Date2 | 383 b | 36 a | 38 b | 56 a | 9.4 b | 0.6 a | 14 b | 8 a | ||
| Navelina | Date1 | 223 a | 38 b | 35 a | 59 a | 9.7 a | 2.3 b | 4 a | 6 a | |
| Date2 | 280 b | 34 a | 54 b | 46 a | 10.6 b | 0.8 a | 14 b | 4 b | ||
| Washington navel | Date1 | 291 a | 41 b | 15 a | 61 a | 8.2 a | 1.2 b | 6 a | 5 b | |
| Date2 | 362 b | 27 a | 44 b | 60 a | 9.4 b | 0.4 a | 19 b | 3 a | ||
| Spain | Navelate | Date1 | 204 a | 39 a | 0 a | 107 b | 11.1 a | 1.7 b | 6 a | - |
| Date2 | 221 a | 39 a | 9 b | 53 a | 13.8 b | 0.9 a | 14 a | - | ||
| Navelina | Date1 | 251 a | 37 a | 11 a | 91 b | 12.3 a | 1.4 a | 8 a | - | |
| Date2 | 257 a | 37 a | 15 b | 47 a | 12.9 b | 1.5 a | 9 b | - | ||
| Washington navel | Date1 | 224 a | 38 a | 5 a | 111 b | 11.2 a | 1.6 b | 7 a | - | |
| Date2 | 240 a | 41 b | 10 b | 51 a | 11.3 a | 1.2 a | 9 b | - | ||
| Tunisia | Maltaise ½ sanguine | Date1 | 178 a | 46 b | - | 60 b | 8.3 a | 1.0 b | 8 a | 10 b |
| Date2 | 181 a | 43 a | - | 10 a | 9.1 b | 0.9 a | 10 b | 6 a | ||
| Newhall navel | Date1 | 197 a | 47 b | - | 58 b | 7.9 a | 0.7 a | 13 a | 9 b | |
| Date2 | 244 b | 41 a | - | 14 a | 9.8 b | 0.6 a | 14 b | 8 a | ||
| Lane late | Date1 | 195 a | 39 a | - | 112 b | 9.2 a | 0.8 b | 11 a | 11 b | |
| Date2 | 247 b | 38 a | - | 48 a | 12.5 b | 0.5 a | 18 b | 10 a | ||
| Navelina | Date1 | 184 a | 40 a | - | 61 b | 10.7 a | 0.5 b | 19 a | 7 b | |
| Date2 | 189 a | 37 a | - | 24 a | 10.8 a | 0.4 a | 25 b | 5 a | ||
| Washington navel | Date1 | 187 a | 42 b | - | 66 b | 10.4 a | 0.9 b | 11 a | 9 b | |
| Date2 | 206 a | 39 a | - | 14 a | 11.4 b | 0.6 a | 17 b | 7 a |
| Site | Fruit Mass (g) | Juice Percentage | TSS (°Brix) | Acidity (g/100 g) | Maturity Index | Firmness (Kg/cm2) | Color Index |
|---|---|---|---|---|---|---|---|
| Corsica | 0.05 | −0.13 | −0.12 | 0.03 | −0.06 | 0.37 | −0.41 |
| Spain | −0.09 | −0.03 | 0.13 | −0.29 | 0.25 | - | −0.31 |
| Tunisia | 0 | 0.09 | −0.35 | −0.04 | −0.03 | 0.38 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khefifi, H.; Selmane, R.; Ben Mimoun, M.; Tadeo, F.; Morillon, R.; Luro, F. Abscission of Orange Fruit (Citrus sinensis (L.) Osb.) in the Mediterranean Basin Depends More on Environmental Conditions Than on Fruit Ripeness. Agronomy 2020, 10, 591. https://doi.org/10.3390/agronomy10040591
Khefifi H, Selmane R, Ben Mimoun M, Tadeo F, Morillon R, Luro F. Abscission of Orange Fruit (Citrus sinensis (L.) Osb.) in the Mediterranean Basin Depends More on Environmental Conditions Than on Fruit Ripeness. Agronomy. 2020; 10(4):591. https://doi.org/10.3390/agronomy10040591
Chicago/Turabian StyleKhefifi, Hajer, Rim Selmane, Mehdi Ben Mimoun, Francisco Tadeo, Raphael Morillon, and François Luro. 2020. "Abscission of Orange Fruit (Citrus sinensis (L.) Osb.) in the Mediterranean Basin Depends More on Environmental Conditions Than on Fruit Ripeness" Agronomy 10, no. 4: 591. https://doi.org/10.3390/agronomy10040591
APA StyleKhefifi, H., Selmane, R., Ben Mimoun, M., Tadeo, F., Morillon, R., & Luro, F. (2020). Abscission of Orange Fruit (Citrus sinensis (L.) Osb.) in the Mediterranean Basin Depends More on Environmental Conditions Than on Fruit Ripeness. Agronomy, 10(4), 591. https://doi.org/10.3390/agronomy10040591





