Use of Aloe Vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetal Material
2.2. Coating Formulations
2.3. Experimental Design
2.4. Physical Analysis
2.5. Chemical Analysis
2.6. Proximate Composition
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussions
3.1. Weight Loss
3.2. Color Analysis
3.3. Firmness
3.4. Soluble Solids Content
3.5. Titratable Acidity and pH
3.6. Proximate Compounds
3.7. Vitamin Content
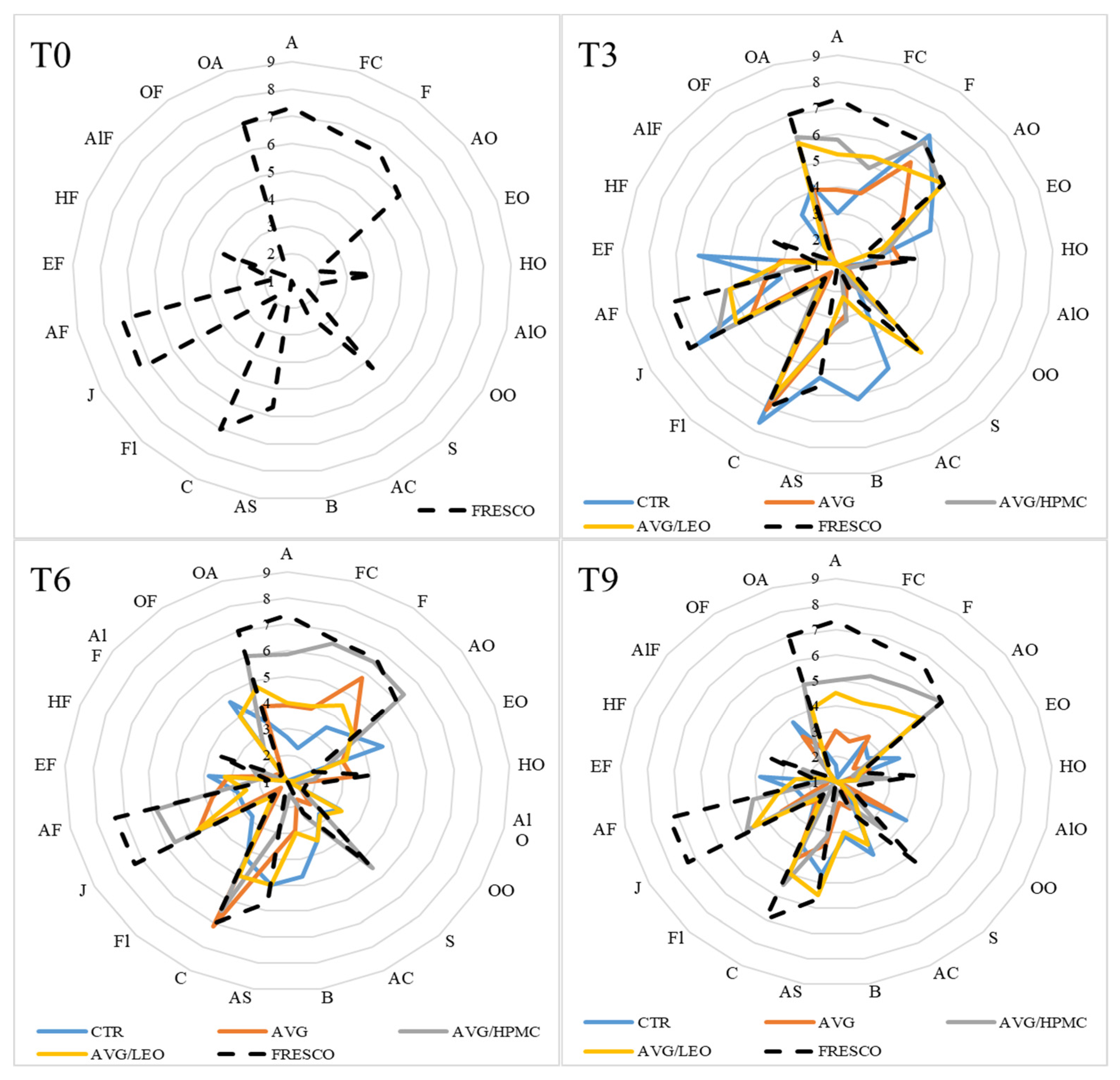
3.8. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiloyannis, C.; Montanaro, G.; Mininni, A.N.; Dichio, B. Sustainable production systems in fruit tree orchards. II Int. Symp. Hortic. Eur. 2012, 1099, 319–324. [Google Scholar] [CrossRef]
- Parajuli, R.; Thoma, G.; Matlock, M.D. Environmental sustainability of fruit and vegetable production supply chains in the face of climate change: A review. Sci. Total Environ. 2019, 650, 2863–2879. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Jobling, J.J.; McGlasson, W.B. A comparison of ethylene production, maturity and controlled atmosphere storage life of Gala, Fuji and Lady Williams apples (Malus domestica, Borkh.). Postharvest Biol. Technol. 1995, 6, 209–218. [Google Scholar] [CrossRef]
- Francaviglia, D.; Farina, V.; Avellone, G.; Bianco, R.L. Fruit yield and quality responses of apple cvars Gala and Fuji to partial rootzone drying under Mediterranean conditions. J. Agric. Sci. 2013, 151, 556–569. [Google Scholar] [CrossRef]
- Schupp, J.R.; Fallahi, E.; Chun, I.J. Effect of particle film on fruit sunburn, maturity and quality of Fuji’ and Honey crisp’ apples. Horttechnology 2002, 12, 87–90. [Google Scholar] [CrossRef]
- Wijewardane, R.M.N.A.; Guleria, S.P.S. Effect of post-harvest coating treatments on apple storage quality. J. Food Sci. Technol. Mysore 2009, 46, 549–553. [Google Scholar] [CrossRef]
- Luo, Y.; Barbosa-Cánovas, G.V. Enzymatic browning and its inhibition in new apple cultivars slices using 4-hexylresorcinol in combination with ascorbic acid/Pardeamiento enzimático y su inhibición en rodajas de manzanas de nuevas variedades utilizando 4-hexilresorcinol en combinación con ácido ascórbico. Food Sci. Technol. Int. 1997, 3, 195–201. [Google Scholar] [CrossRef]
- Krochta, J.M. Protein as raw materials for films and coatings: Definitions, current status, and opportunities. In Protein-Based Films and Coatings; Gennadios, A., Ed.; CRC Press: Boca Raton, FL, USA, 2002; Volume 425, pp. 1–41. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; Del Rio, M.A.; Pérez-Gago, M.B. Performance of hydroxypropyl methylcellulose (HPMC)-lipid edible coatings with antifungal food additives during cold storage of ‘Clemenules’ mandarins. LWT Food Sci. Technol. 2011, 44, 2342–2348. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zachwieja, Z.; Folta, M.; Barton, H.; Piotrowicz, J.; Zemser, M.; Màrtín-Belloso, O. Comparative contents of dietary fiber, total phenolics, and minerals in persimmons and apples. J. Agric. Food Chem. 2001, 49, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of re-crystallization on tensile, optical and water vapour barrier properties of corn starch films containing fatty acids. Food Hydrocoll. 2012, 26, 302–310. [Google Scholar] [CrossRef]
- Park, H.J.; Weller, C.L.; Vergano, P.J.; Testin, R.F. Permeability and mechanical properties of cellulose-based edible films. J. Food Sci. 1993, 58, 1361–1364. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S. Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT Food Sci. Technol. 2005, 38, 617–624. [Google Scholar] [CrossRef]
- Sharif, Z.I.M.; Subuki, I.; Zaki, N.A.M.; Mustapha, F.A.; Yusof, N.M.; Jai, J. Turmeric (Curcuma longa L.) oil as antioxidant agent in starch-based edible coating film for fresh-cut fruits. Chron. Complement. Altern. Integr. Med. 2019, 1. [Google Scholar] [CrossRef]
- Fahs, A.; Brogly, M.; Bistac, S.; Schmitt, M. Hydroxypropyl methylcellulose (HPMC) formulated films: Relevance to adhesion and friction surface properties. Carbohydr. Polym. 2010, 80, 105–114. [Google Scholar] [CrossRef]
- European Commission. European Parliament and council directive No. 95/2/EC of 20 February 1995 on additives other than colours and sweeteners for use in foodstuffs. Off. J. 1995, L61, 1–40. [Google Scholar]
- Burdock, G.A. Safety assessment of hydroxypropyl methylcellulose as a food ingredient. Food Chem. Toxicol. 2007, 45, 2341–2351. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53. [Google Scholar] [CrossRef]
- Azevedo, A.N.; Buarque, P.R.; Cruz, E.M.O.; Blank, A.F.; Alves, P.B.; Nunes, M.L.; de Aquino Santana, L.C.L. Response surface methodology for optimisation of edible chitosan coating formulations incorporating essential oil against several foodborne pathogenic bacteria. Food Control 2014, 43, 1–9. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. 2019, 59, 2467–2480. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Aghaei, K.; Ghajarbeygi, P. The Effect of Edible Chitosan Coatings with Cinnamon Essential Oil on the Shelf Life of Strawberry. Ph.D. Thesis, Qazvin University of Medical Sciences, Qazvin, Iran, 2019. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003, 95, 853–860. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Rojas-Graü, M.A.; Mosqueda-Melgar, J.; Martín-Belloso, O. Comparative study on essential oils incorporated into an alginate-based edible coating to assure the safety and quality of fresh-cut Fuji apples. J. Food Prot. 2008, 71, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- US EPA (1994) Reregistration Eligibility Decision (RED) Limonene. US Environmental Protection Agency, Prevention, Pesticides and Toxic Substances (Report No. EPA-738-R-94-034).
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. J. Int. 2008, 163, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Song, H.Y.; Jo, W.S.; Song, N.B.; Min, S.C.; Song, K.B. Quality change of apple slices coated with Aloe vera gel during storage. J. Food Sci. 2013, 78, C817–C822. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, O.P.; Raju, P.S.; Singh, A.; Bawa, A.S. Shellac and aloe-gel-based surface coatings for maintaining keeping quality of apple slices. Food Chem. 2011, 126, 961–966. [Google Scholar] [CrossRef]
- Supapvanich, S.; Mitrsang, P.; Srinorkham, P.; Boonyaritthongchai, P.; Wongs-Aree, C. Effects of fresh Aloe vera gel coating on browning alleviation of fresh cut wax apple (Syzygium samarangenese) fruit cv. Taaptimjaan. J. Food Sci. Technol. Mysore 2016, 53, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.E.; McAnalley, B.H. A Drink Containing Mucilaginous Polysaccharides and Its Preparation. U.S. Patent No. 5,443,830, 22 August 1995. [Google Scholar]
- Farina, V.; Passafiume, R.; Tinebra, I.; Scuderi, D.; Saletta, F.; Gugliuzza, G.; Gallotta, A.; Sortino, G. Postharvest application of aloe vera gel-based edible coating to improve the quality and storage stability of fresh-cut papaya. J. Food Qual. 2020. [Google Scholar] [CrossRef]
- AOACOfficial methods of analysis. Arlington, VA, USA: Association of Official Analytical Chemists1985aMethods 22.026, 22:362.
- AOACOfficial methods of analysis. Arlington, VA, USA: Association of Official Analytical Chemists1985bMethods 3.140, 3: 50.
- AOACOfficial methods of analysis. Arlington, VA, USA: Association of Official Analytical Chemists1985cMethods 14.018, 3: 69.
- AOACOfficial methods of analysis. Arlington, VA, USA: Association of Official Analytical Chemists1985dMethods 22.080, 22:369.
- AOACOfficial methods of analysis. Arlington, VA, USA: Association of Official Analytical Chemists1985eMethods 22.080, 22:360.
- Saritha, V.; Anilakumar, K.R.; Khanum, F. Antioxidant and antibacterial activity of Aloe vera gel extracts. Int. J. Pharm. Biol. Arch. 2010, 1, 376–384. [Google Scholar] [CrossRef]
- Loewus, F.A. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Palazzolo, E.; Letizia Gargano, M.; Venturella, G. The nutritional composition of selected wild edible mushrooms from Sicily (southern Italy). Int. J. Food Sci. Nutr. 2012, 63, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Morand, P.; Gullo, J.L. Minéralisation des tissus végétaux en vue du dosage de P, K, Ca, Mg, Na. Ann. Agron. 1970, 21, 229–236. [Google Scholar]
- Fogg, D.N.; Wilkinson, A.N. The colorimetric determination of phosphorus. Analyst 1958, 83, 406–414. [Google Scholar] [CrossRef]
- Rapisarda, P.; Intelisano, S. Sample preparation for vitamin C analysis of pigmented oral juices. Ital. J. Food Sci. 1996, 8, 251–256. [Google Scholar]
- Gianguzzi, G.; Mazzaglia, A.; Sortino, G.; Farina, V. Instrumental and sensory evaluation of seven apple (Malus domestica Borkh.) cultivars under organic cultivation in Sicily. J. Agric. Res. 2017, 15, 1878–1889. [Google Scholar] [CrossRef]
- Bai, J.; Hagenmaier, R.D.; Baldwin, E.A. Coating selection for ‘Delicious’ and other apples. Postharvest Biol. Technol. 2003, 28, 381–390. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Klangmuang, P.; Sothornvit, R. Barrier properties, mechanical properties and antimicrobial activity of hydroxypropyl methylcellulose-based nanocomposite films incorporated with Thai essential oils. Food Hydrocoll. 2016, 61, 609–616. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Martins, M.M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria x ananassa cv. Camarosa) under commercial storage conditions. Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.L.; Del Rio, M.A.; Krochta, J.M.; Perez-Gago, M.B. Fatty acid effect on hydroxypropyl methylcellulose-beeswax edible film properties and postharvest quality of coated ‘ortanique’ mandarins. J. Agric. Food Chem. 2008, 56, 10689–10696. [Google Scholar] [CrossRef]
- Perez-Gago, M.B.; Rojas, C.; DelRio, M.A. Effect of lipid type and amount of edible hydroxypropyl methylcellulose-lipid composite coatings used to protect postharvest quality of mandarins cv. Fortune. J. Food Sci. 2002, 67, 2903–2910. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Bras, J.; Williams, T.; Sénechal, T.; Orts, W. HPMC reinforced with different cellulose nano-particles. Carbohydr. Polym. 2011, 86, 1549–1557. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve Food Quality, 2nd ed.; Baldwin, E.A., Hagenmaier, R., Bai, J., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G.; Lopez, M.L. Fruit color development, anthocyanin content, standard quality, volatile compound emissions and consumer acceptability of several ‘Fuji’ apple strains. Sci. Hortic. 2012, 137, 138–147. [Google Scholar] [CrossRef]
- Marquina, P.; Venturini, M.E.; Oria, R.; Negueruela, A.I. Monitoring color evolution during maturity in Fuji apples. Food Sci. Technol. Int. 2004, 10, 315–321. [Google Scholar] [CrossRef]
- Hayat, I.; Masud, T.; Rathore, H.A. Effect of coating and wrapping materials on the shelf life of apple (Malus domestica cv. Borkh). J. Food Saf. 2005, 5, 24–34. [Google Scholar]
- Castillo, S.; Navarro, D.; Zapata, P.J.; Guillén, F.; Valero, D.; Serrano, M.; Martínez-Romero, D. Antifungal efficacy of Aloe vera in vitro and its use as a preharvest treatment to maintain postharvest table grape quality. Postharvest Biol. Technol. 2010, 57, 183–188. [Google Scholar] [CrossRef]
- Guillén, F.; Díaz-Mula, H.M.; Zapata, P.J.; Valero, D.; Serrano, M.; Castillo, S.; Martínez-Romero, D. Aloe arborescens and Aloe vera gels as coatings in delaying postharvest ripening in peach and plum fruit. Postharvest Biol. Technol. 2013, 83, 54–57. [Google Scholar] [CrossRef]
- Marpudi, S.K.; Ramchandaran, P.; Srividya, N. Aloe vera gel coating for post-harvest quality maintenance of fresh fig fruits. Res. J. Pharm. Biol. Chem. Sci. 2011, 4, 41–43. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of essential oils on properties of film forming emulsions and films based on hydroxypropyl methylcellulose and chitosan. J. Food Eng. 2011, 105, 246–253. [Google Scholar] [CrossRef]
- Trezza, T.A.; Krochta, J.M. The gloss of edible coatings as affected by surfactants, lipids, relative humidity, and time. J. Food Sci. 2000, 65, 658–662. [Google Scholar] [CrossRef]
- Kou, W.; Cai, C.; Xu, S.; Wang, H.; Liu, J.; Yang, D.; Zhang, T. In vitro and in vivo evaluation of novel immediate release carbamazepine tablets: Complexation with hydroxypropyl-β-cyclodextrin in the presence of HPMC. Int. J. Pharm. 2011, 409, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; Bambridge, P.A.; Scott, K.J. Use of flesh firmness and other objective tests to determine consumer acceptability of delicious apples. Aust. J. Exp. Agric. Anim. Husb. 1980, 20, 252–256. [Google Scholar] [CrossRef]
- Hussain, P.R.; Meena, R.S.; Dar, M.A.; Wani, A.M. Effect of post-harvest calcium chloride dip treatment and gamma irradiation on storage quality and shelf-life extension of Red delicious apple. J. Food Sci. Technol. 2012, 49, 415–426. [Google Scholar] [CrossRef]
- Rocha, A.M.C.N.; Morais, A.M.M.B. Shelf life of minimally processed apple (cv. Jonagored) determined by color changes. Food Control 2003, 14, 13–20. [Google Scholar] [CrossRef]
- Islam, M.; Khan, M.Z.H.; Sarkar, M.A.R.; Absar, N.; Sarkar, S.K. Changes in acidity, TSS, and sugar content at different storage periods of the postharvest mango (Mangifera indica L.) influenced by Bavistin DF. Int. J. Food Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Fonseca, G.G. Effect of L-ascorbic acid and sodium metabisulfite in the inhibition of the enzymatic browning of minimally processed apple. Int. J. Agric. Res. 2008, 3, 196–201. [Google Scholar] [CrossRef]
- Olivas, G.I.; Mattinson, D.S.; Barbosa-Cánovas, G.V. Alginate coatings for preservation of minimally processed ‘Gala’apples. Postharvest Biol. Technol. 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Gutiérrez-Cortez, E.; Cornejo-Villegas, M.A.; Quintanar-Guerrero, D. The effect of nano-coatings with α-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “Red Delicious” apples. Innov. Food Sci. Emerg. Technol. 2014, 22, 188–196. [Google Scholar] [CrossRef]
- Henríquez, C.; Almonacid, S.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Cabezas, L.; Speisky, H. Determination of antioxidant capacity, total phenolic content and mineral composition of different fruit tissue of five apple cultivars grown in Chile. Chil. J. Agric. Res. 2010, 70, 523–536. [Google Scholar] [CrossRef]
- Segura, R.; Javierre, C.; Lizarraga, M.A.; Ros, E. Other relevant components of nuts: Phytosterols, folate and minerals. Br. J. Nutr. 2006, 96, S36–S44. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Levine, M. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Leahu, A.; Damian, C.; Oroian, M.; Ropciuc, S. Physico-chemical parameters of fruit juices-evolution during storage. J Lucr. Stiintifice Ser. Zooteh. 2013, 59, 213–217. [Google Scholar]
- Thornalley, P.J. The potential role of thiamine (vitamin B1) in diabetic complications. Curr. Diabetes Rev. 2005, 1, 287–298. [Google Scholar] [CrossRef]
- Clayton, M.; Biasi, W.V.; Agar, I.T.; Southwick, S.M.; Mitcham, E.J. Postharvest quality of ‘Bing’ cherries following preharvest treatment with hydrogen cyanamide, calcium ammonium nitrate, or gibberellic acid. HortScience 2003, 38, 407–411. [Google Scholar] [CrossRef]
- Schick, J.L.; Toivonen, P.M. Reflective tarps at harvest reduce stem browning and improve fruit quality of cherries during subsequent storage. Postharvest Biol. Technol. 2002, 25, 117–121. [Google Scholar] [CrossRef]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.E.; Gardini, F. Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]

| Treatment | Days of Storage | L* | a* | b* |
|---|---|---|---|---|
| CTR | 0 | 78.3 ± 1.4 Aa | −4.0 ± 0.9 Aa | 34.4 ± 0.5 Aa |
| 3 | 75.4 ± 1.2 Bab | −1.4 ± 0.5 Bb | 34.3 ± 1.2 Aa | |
| 6 | 73.3 ± 0.8 Bb | −0.9 ± 1.0 Bbc | 33.8 ± 1.3 Aa | |
| 9 | 73.2 ± 0.8 Bb | 0.0 ± 0.7 Cc | 33.5 ± 0.5 Aa | |
| AVG | 0 | 78.4 ± 0.8 Aa | −3.7 ± 0.6 Aa | 30.8 ± 2.5 Ba |
| 3 | 76.4 ± 1.5 ABb | −3.4 ± 0.3 Aa | 30.5 ± 0.9 Ba | |
| 6 | 75.7 ± 0.3 ABb | −2.2 ± 1.0 ABb | 30.3 ± 2.3 Ba | |
| 9 | 75.3 ± 1.7 ABb | −0.7 ± 0.7 Bc | 30.0 ± 1.1 Ba | |
| AVG/HPMC | 0 | 78.6 ± 0.3 Aa | −3.6 ± 0.8 ABa | 31.1 ± 0.8 Ba |
| 3 | 78.3 ± 0.5 Aa | −3.5 ± 0.5 Aa | 30.3 ± 0.4 Ba | |
| 6 | 76.9 ± 0.2 Ab | −2.4 ± 0.3 Ab | 29.6 ± 1.5 Ba | |
| 9 | 76.8 ± 0.4 Ab | −2.1 ± 0.1 Ab | 27.2 ± 1.7 Cb | |
| AVG/LEO | 0 | 77.8 ± 0.3 Aa | −3.3 ± 0.5 Ba | 29.2 ± 0.7 Ba |
| 3 | 77.6 ± 0.2 ABa | −2.9 ± 0.4 ABab | 28.7 ± 0.7 Ba | |
| 6 | 76.4 ± 0.8 ABb | −2.0 ± 0.4 ABb | 28.6 ± 2.1 Ba | |
| 9 | 76.3 ± 0.6 ABb | −0.8 ± 0.3 Bc | 28.4 ± 2.4 BCa |
| Treatment | Days of Storage | Firmness (N) | SSC (°Brix) | TA (g malic acid/L) | pH |
|---|---|---|---|---|---|
| CTR | 0 | 44.7 ± 2.5 Aa | 14.3 ± 0.6 Aa | 0.2 ± 0.0 ns | 4.0 ± 0.1 ns |
| 3 | 42.3 ± 2.5 Aab | 12.3 ± 2.1 Aa | 0.1 ± 0.0 ns | 3.9 ± 0.1 ns | |
| 6 | 39.3 ± 1.2 Abc | 11.6 ± 2.3 Aa | 0.1 ± 0.0 ns | 4.0 ± 0.1 ns | |
| 9 | 34.7 ± 0.6 Bc | 10.3 ± 3.3 Aa | 0.1 ± 0.0 ns | 4.0 ± 0.1 ns | |
| AVG | 0 | 43.0 ± 0.0 Aa | 13.3 ± 1.2 ABa | 0.2 ± 0.0 ns | 4.2 ± 0.3 ns |
| 3 | 41.0 ± 1.0 Ab | 12.2 ± 0.8 Aab | 0.1 ± 0.0 ns | 4.0 ± 0.1 ns | |
| 6 | 39.0 ± 1.0 Ac | 10.7 ± 0.6 Ab | 0.1 ± 0.0 ns | 3.9 ± 0.1 ns | |
| 9 | 37.3 ± 0.6 ABc | 10.2 ± 0.7 Ab | 0.1 ± 0.0 ns | 3.8 ± 0.1 ns | |
| AVG/HPMC | 0 | 45.3 ± 2.5 Aa | 12.3 ± 0.6 Ba | 0.2 ± 0.0 ns | 4.0 ± 0.1 ns |
| 3 | 43.7 ± 2.9 Aa | 11.8 ± 0.3 Aa | 0.1 ± 0.0 ns | 3.9 ± 0.1 ns | |
| 6 | 42.7 ± 3.1 Aa | 10.4 ± 0.5 Ab | 0.1 ± 0.0 ns | 3.9 ± 0.1 ns | |
| 9 | 41.0 ± 3.0 Aa | 9.3 ± 0.6 Ab | 0.1 ± 0.0 ns | 3.8 ± 0.1 ns | |
| AVG/LEO | 0 | 43.0 ± 1.7 Aa | 13.4 ± 0.5 ABa | 0.2 ± 0.0 ns | 3.9 ± 0.1 ns |
| 3 | 41.7 ± 1.5 Aab | 12.7 ± 0.3 Aab | 0.1 ± 0.0 ns | 3.8 ± 0.0 ns | |
| 6 | 41.3 ± 0.6 Aab | 12.1 ± 0.3 Ab | 0.1 ± 0.0 ns | 3.8 ± 0.0 ns | |
| 9 | 38.7 ± 1.5 ABb | 11.8 ± 0.3 Ab | 0.1 ± 0.0 ns | 3.7 ± 0.1 ns |
| Treatment | Days of Storage | Ca g/100g DW | % | K g/100g DW | % | Na g/100g DW | % | P g/100g DW | % | Mn g/100g DW | % | Zn g/100g DW | % | Cu g/100g DW | % | Fe g/100g DW | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR | 0 | 7.028 ± 0.001 Aba | 136.667 ± 29.023 Ab | 1.014 ± 0.576 Aa | 11.009 ± 1.943 Cb | 0.031 ± 0.001 Bb | 0.030 ± 0.006 Aa | 0.291 ± 0.001 Aa | 0.150 ± 0.026 Ac | ||||||||
| 3 | 7.310 ± 0.001 Ba | 3.9 | 138.012 ± 23.680 Ab | 1.0 | 1.345 ± 0.579 Aa | 24.6 | 12.667 ± 2.887 Cb | 13.1 | 0.029 ± 0.001 Bb | 6.5 | 0.033 ± 0.006 Ba | 10.0 | 0.031 ± 0.001 Aa | 89.3 | 0.177 ± 0.026 Ab | 15.1 | |
| 6 | 7.027 ± 0.001 Ba | 0.0 | 149.667 ± 18.339 Ca | 8.7 | 1.347 ± 0.579 Aa | 24.7 | 15.078 ± 1.000 Ba | 27.0 | 0.042 ± 0.024 Aa | 26.2 | 0.033 ± 0.006 Ba | 10.0 | 0.036 ± 0.002 Aa | 87.6 | 0.177 ± 0.025 Bb | 15.1 | |
| 9 | 7.030 ± 0.001 Ba | 0.0 | 149.667 ± 18.339 Ca | 8.7 | 1.330 ± 0.577 Ba | 23.8 | 15.099 ± 1.000 Ba | 27.1 | 0.047 ± 0.030 Aa | 34.0 | 0.033 ± 0.006 Ba | 10.0 | 0.037 ± 0.002 ABa | 87.4 | 0.180 ± 0.025 Ba | 16.7 | |
| AVG | 0 | 6.000 ± 0.764 Bb | 133.667 ± 4.509 ABb | 1.002 ± 0.500 Aa | 12.089 ± 1.052 Bb | 0.037 ± 0.006 Aa | 0.033 ± 0.040 Aa | 0.031 ± 0.001 Ab | 0.137 ± 0.026 Bc | ||||||||
| 3 | 6.833 ± 0.634 Bab | 12.2 | 134.011 ± 7.550 Bb | 0.3 | 1.045 ± 0.500 Aa | 4.1 | 13.667 ± 1.528 Bb | 11.5 | 0.040 ± 0.010 Aa | 8.3 | 0.037 ± 0.040 Ab | 9.1 | 0.033 ± 0.001 Ab | 6.1 | 0.140 ± 0.015 Cc | 2.1 | |
| 6 | 7.020 ± 0.001 Ba | 14.5 | 154.333 ± 10.599 Ba | 13.4 | 1.330 ± 0.560 Aa | 24.7 | 15.333 ± 0.577 Ba | 21.2 | 0.042 ± 0.010 Aa | 12.7 | 0.041 ± 0.006 Aa | 18.7 | 0.042 ± 0.002 Aa | 26.2 | 0.193 ± 0.012 Ab | 29.1 | |
| 9 | 7.019 ± 0.001 Ba | 14.5 | 157.333 ± 8.021 Ba | 15.0 | 1.348 ± 0.579 Ba | 25.7 | 15.337 ± 0.577 Ba | 21.2 | 0.047 ± 0.010 Aa | 22.0 | 0.044 ± 0.006 Aa | 24.2 | 0.043 ± 0.006 Bb | 28.5 | 0.200 ± 0.000 Aa | 31.5 | |
| AVG/HPMC | 0 | 8.000 ± 0.488 Ac | 123.333 ± 15.567 Cb | 1.032 ± 0.577 Aa | 12.023 ± 1.289 Bb | 0.030 ± 0.006 Bb | 0.293 ± 0.006 Aa | 0.030 ± 0.001 Ab | 0.150 ± 0.026 Bb | ||||||||
| 3 | 8.333 ± 0.577 Ac | 4.0 | 125.002 ± 13.550 Cb | 1.3 | 1.023 ± 0.574 Aa | 0.9 | 12.333 ± 0.577 Cb | 2.5 | 0.033 ± 0.006 Bb | 10.0 | 0.031 ± 0.006 Ba | 89.4 | 0.033 ± 0.002 Ab | 10.0 | 0.153 ± 0.035 Bb | 2.2 | |
| 6 | 9.667 ± 0.577 Ab | 17.2 | 186.010 ± 11.533 Aa | 33.7 | 1.330 ± 0.454 Aa | 22.4 | 18.095 ± 2.000 Aa | 33.6 | 0.033 ± 0.006 Bb | 10.0 | 0.034 ± 0.006 Ba | 88.4 | 0.035 ± 0.002 Bb | 14.3 | 0.200 ± 0.035 Aa | 25.0 | |
| 9 | 13.001 ± 1.732 Aa | 38.5 | 192.000 ± 7.000 Aa | 35.8 | 2.000 ± 0.967 Ab | 48.4 | 19.333 ± 1.155 Aa | 37.8 | 0.041 ± 0.021 Aa | 26.8 | 0.035 ± 0.006 Ba | 88.1 | 0.040 ± 0.006 Aa | 25.0 | 0.200 ± 0.042 Aa | 25.0 | |
| AVG/LEO | 0 | 6.009 ± 0.576 Bb | 129.667 ± 4.509 Bc | 1.000 ± 0.544 Aa | 13.667 ± 1.052 Ac | 0.033 ± 0.006 Bb | 0.030 ± 0.004 Ab | 0.030 ± 0.001 Aa | 0.140 ± 0.026 ABb | ||||||||
| 3 | 6.333 ± 0.577 Cb | 5.1 | 134.010 ± 3.020 Bb | 3.2 | 1.346 ± 0.579 Aa | 25.7 | 15.015 ± 1.528 Ab | 9.0 | 0.037 ± 0.006 ABab | 9.1 | 0.037 ± 0.005 Aab | 18.9 | 0.031 ± 0.001 Aa | 3.2 | 0.143 ± 0.006 Cb | 2.3 | |
| 6 | 6.667 ± 0.577 Cab | 9.9 | 138.333 ± 1.528 Db | 6.3 | 1.333 ± 0.577 Aa | 25.0 | 15.333 ± 0.577 Bb | 10.9 | 0.041 ± 0.021 Aa | 18.7 | 0.039 ± 0.006 Aa | 23.1 | 0.033 ± 0.003 Ba | 10.0 | 0.190 ± 0.009 Aa | 26.3 | |
| 9 | 7.002 ± 0.563 Ba | 14.2 | 157.333 ± 8.021 Ba | 17.6 | 1.667 ± 0.576 Ab | 40.0 | 16.667 ± 1.155 ABa | 18.0 | 0.043 ± 0.021 Aa | 22.5 | 0.040 ± 0.006 Aa | 25.0 | 0.033 ± 0.006 Ba | 10.0 | 0.200 ± 0.010 Aa | 30.0 |
| Treatment | Days of Storage | Water g/100g DW | % | Protein g/100g DW | % | FAT g/100g DW | % | Carbohydrate g/100g DW | % | Sugars g/100g DW | % | Fibers g/100g DW | % | Total Polyphenols g/100g DW | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR | 0 | 83.650 ± 0.260 Ab | 0.220 ± 0.012 Aa | 0.200 ± 0.012 Aa | 13.110 ± 0.577 Aa | 9.970 ± 0.406 Aa | 2.120 ± 0.082 Aa | 0.210 ± 0.015 ABa | |||||||
| 3 | 83.867 ± 0.189 Aa | 0.3 | 0.207 ± 0.012 Aa | 6.1 | 0.193 ± 0.012 Ab | 3.3 | 12.563 ± 0.480 Cb | 4.2 | 9.657 ± 0.276 Aba | 3.1 | 2.073 ± 0.064 ABab | 2.2 | 0.203 ± 0.006 Ba | 3.2 | |
| 6 | 82.640 ± 0.332 Ac | 1.2 | 0.193 ± 0.012 Bb | 12.1 | 0.210 ± 0.017 Aa | 4.8 | 12.337 ± 0.673 Cb | 5.9 | 9.243 ± 0.537 Cab | 7.3 | 1.863 ± 0.101 Bb | 12.1 | 0.167 ± 0.024 Db | 20.6 | |
| 9 | 81.850 ± 0.510 Dd | 2.2 | 0.190 ± 0.010 Bb | 12.1 | 0.187 ± 0.012 Bb | 0.0 | 10.337 ± 0.180 Dc | 21.2 | 8.370 ± 0.202 Bb | ## | 1.737 ± 0.116 Cb | 18.1 | 0.174 ± 0.008 Db | 17.1 | |
| AVG | 0 | 84.000 ± 0.353 Aa | 0.200 ± 0.006 Ba | 0.200 ± 0.017 Ab | 12.370 ± 0.198 Cb | 9.550 ± 0.064 Bb | 2.000 ± 0.057 Ab | 0.200 ± 0.015 Bb | |||||||
| 3 | 83.323 ± 0.586 Cb | 0.8 | 0.203 ± 0.006 Aa | 1.6 | 0.190 ± 0.017 Ab | 5.0 | 12.703 ± 0.289 Ba | 2.6 | 9.490 ± 0.053 Cb | 0.6 | 2.033 ± 0.058 Bab | 1.6 | 0.197 ± 0.006 Cc | 1.7 | |
| 6 | 83.303 ± 0.119 Bb | 0.8 | 0.207 ± 0.006 Aa | 3.2 | 0.190 ± 0.017 Bb | 5.0 | 12.723 ± 0.237 Ba | 2.8 | 9.903 ± 0.076 Aa | 3.6 | 2.173 ± 0.047 Aa | 8.0 | 0.277 ± 0.025 Bb | 27.7 | |
| 9 | 82.527 ± 0.150 Cc | 1.8 | 0.203 ± 0.006 Aa | 1.6 | 0.217 ± 0.006 Aa | 7.7 | 12.693 ± 0.159 Ca | 2.5 | 9.950 ± 0.078 Aa | 4.0 | 1.953 ± 0.057 Bb | 2.3 | 0.340 ± 0.010 Aa | 41.2 | |
| AVG/HPMC | 0 | 83.550 ± 0.336 Bb | 0.180 ± 0.012 Cb | 0.200 ± 0.012 Aa | 12.970 ± 0.067 Ab | 9.670 ± 0.153 ABab | 2.000 ± 0.065 Ab | 0.200 ± 0.006 Bb | |||||||
| 3 | 83.473 ± 0.440 Bc | 0.1 | 0.190 ± 0.017 Bb | 5.3 | 0.193 ± 0.012 Aab | 3.3 | 12.910 ± 0.060 Ab | 0.5 | 9.553 ± 0.111 Bb | 1.2 | 2.023 ± 0.068 Bb | 1.2 | 0.200 ± 0.006 Bb | 0.0 | |
| 6 | 83.737 ± 0.232 Aa | 0.2 | 0.217 ± 0.006 Aa | 16.9 | 0.187 ± 0.015 Bab | 6.7 | 13.027 ± 0.074 Aab | 0.4 | 9.763 ± 0.196 Bab | 1.0 | 2.053 ± 0.061 Bb | 2.6 | 0.203 ± 0.006 Cb | 1.6 | |
| 9 | 83.470 ± 0.467 Ac | 0.1 | 0.203 ± 0.006 Aab | 11.5 | 0.180 ± 0.026 ABb | 10.0 | 13.273 ± 0.174 Ba | 2.3 | 9.937 ± 0.099 Aa | 2.7 | 2.240 ± 0.010 ABa | 10.7 | 0.313 ± 0.006 Ba | 36.2 | |
| AVG/LEO | 0 | 83.580 ± 0.388 Abab | 0.210 ± 0.006 ABa | 0.180 ± 0.010 Ba | 12.700 ± 0.165 Bb | 9.850 ± 0.084 Ab | 2.000 ± 0.198 Ac | 0.220 ± 0.017 Ab | |||||||
| 3 | 83.410 ± 0.357 Bb | 0.2 | 0.213 ± 0.006 Aa | 1.6 | 0.190 ± 0.010 Aa | 5.3 | 12.777 ± 0.075 ABb | 0.6 | 10.717 ± 1.858 Aa | 8.1 | 2.183 ± 0.236 Aab | 8.4 | 0.210 ± 0.017 Ab | 4.5 | |
| 6 | 83.650 ± 0.419 Aa | 0.1 | 0.187 ± 0.006 Bb | 11.1 | 0.177 ± 0.006 Cab | 1.9 | 13.393 ± 0.162 Aa | 5.2 | 9.950 ± 0.104 Aab | 1.0 | 2.163 ± 0.160 Ab | 7.6 | 0.290 ± 0.017 Aa | 24.1 | |
| 9 | 83.057 ± 0.476 Bc | 0.6 | 0.200 ± 0.000 Aab | 4.8 | 0.173 ± 0.006 Cb | 3.7 | 13.577 ± 0.254 Aa | 6.5 | 10.027 ± 0.064 Aab | 1.8 | 2.313 ± 0.059 Aa | 13.5 | 0.297 ± 0.006 Ca | 25.8 |
| Treatment | Days of Storage | Vitamin B2 Riboflavin mg/100g DW | % | Vitamin C Ascorbic Acid mg/100g DW | % |
|---|---|---|---|---|---|
| CTR | 0 | 0.040 ± 0.004 Aa | 5.100 ± 0.410 Aa | ||
| 3 | 0.039 ± 0.002 Aa | 3.3 | 4.723 ± 0.468 Bb | 7.4 | |
| 6 | 0.033 ± 0.003 Ab | 16.7 | 4.533 ± 0.416 Cc | 11.1 | |
| 9 | 0.035 ± 0.005 Ab | 11.7 | 4.810 ± 0.541 Ab | 5.7 | |
| AVG | 0 | 0.030 ± 0.010 Ba | 4.360 ± 0.400 Bc | ||
| 3 | 0.029 ± 0.010 Ba | 3.3 | 4.520 ± 0.420 Cb | 3.5 | |
| 6 | 0.029 ± 0.010 Ba | 3.3 | 4.907 ± 0.272 Ba | 11.1 | |
| 9 | 0.031 ± 0.010 Ba | 3.2 | 4.400 ± 0.200 Bbc | 0.9 | |
| AVG/HPMC | 0 | 0.031 ± 0.030 Bb | 5.000 ± 0.200 Aab | ||
| 3 | 0.030 ± 0.001 Bb | 2.2 | 5.157 ± 0.191 Aa | 3.0 | |
| 6 | 0.029 ± 0.001 Bb | 6.5 | 4.990 ± 0.385 Ab | 0.2 | |
| 9 | 0.034 ± 0.001 Aa | 8.8 | 4.467 ± 0.462 Bc | 10.7 | |
| AVG/LEO | 0 | 0.036 ± 0.010 ABa | 4.200 ± 0.200 Bc | ||
| 3 | 0.031 ± 0.010 Bb | 14.8 | 4.527 ± 0.297 BCb | 7.2 | |
| 6 | 0.030 ± 0.010 Bb | 15.7 | 4.927 ± 0.127 Ba | 14.7 | |
| 9 | 0.029 ± 0.010 Bc | 18.5 | 4.737 ± 0.456 Aab | 11.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of Aloe Vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple. Agronomy 2020, 10, 515. https://doi.org/10.3390/agronomy10040515
Farina V, Passafiume R, Tinebra I, Palazzolo E, Sortino G. Use of Aloe Vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple. Agronomy. 2020; 10(4):515. https://doi.org/10.3390/agronomy10040515
Chicago/Turabian StyleFarina, Vittorio, Roberta Passafiume, Ilenia Tinebra, Eristanna Palazzolo, and Giuseppe Sortino. 2020. "Use of Aloe Vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple" Agronomy 10, no. 4: 515. https://doi.org/10.3390/agronomy10040515
APA StyleFarina, V., Passafiume, R., Tinebra, I., Palazzolo, E., & Sortino, G. (2020). Use of Aloe Vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple. Agronomy, 10(4), 515. https://doi.org/10.3390/agronomy10040515









