Greenhouse Gas Emissions as Affected by Fertilization Type (Pig Slurry vs. Mineral) and Soil Management in Mediterranean Rice Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Sites Description and Experimental Design
2.2. Greenhouse Gas Measurements and Analyses
2.3. Soil Sampling and Analysis
2.4. Calculations and Statistical Analysis
3. Results
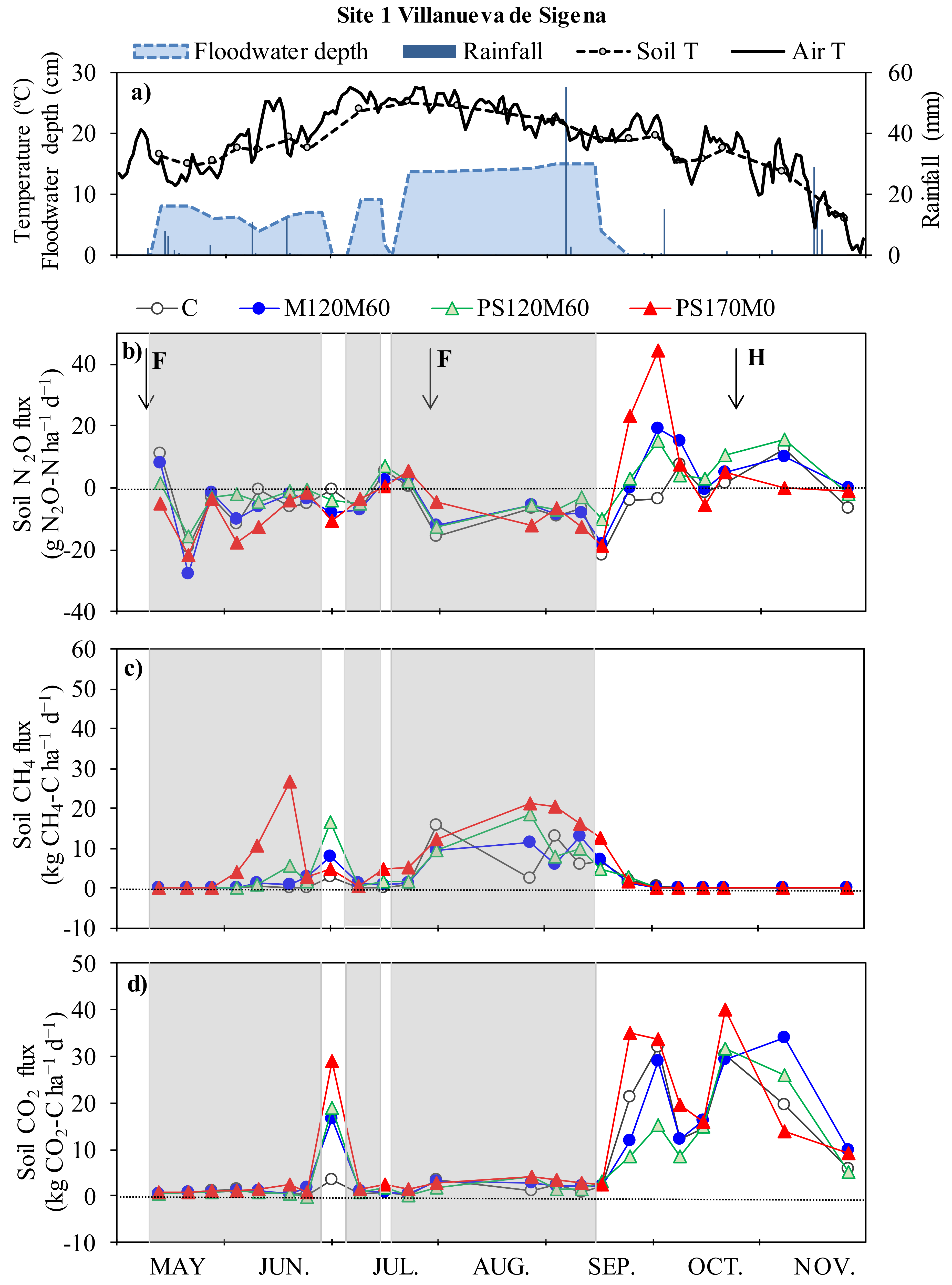
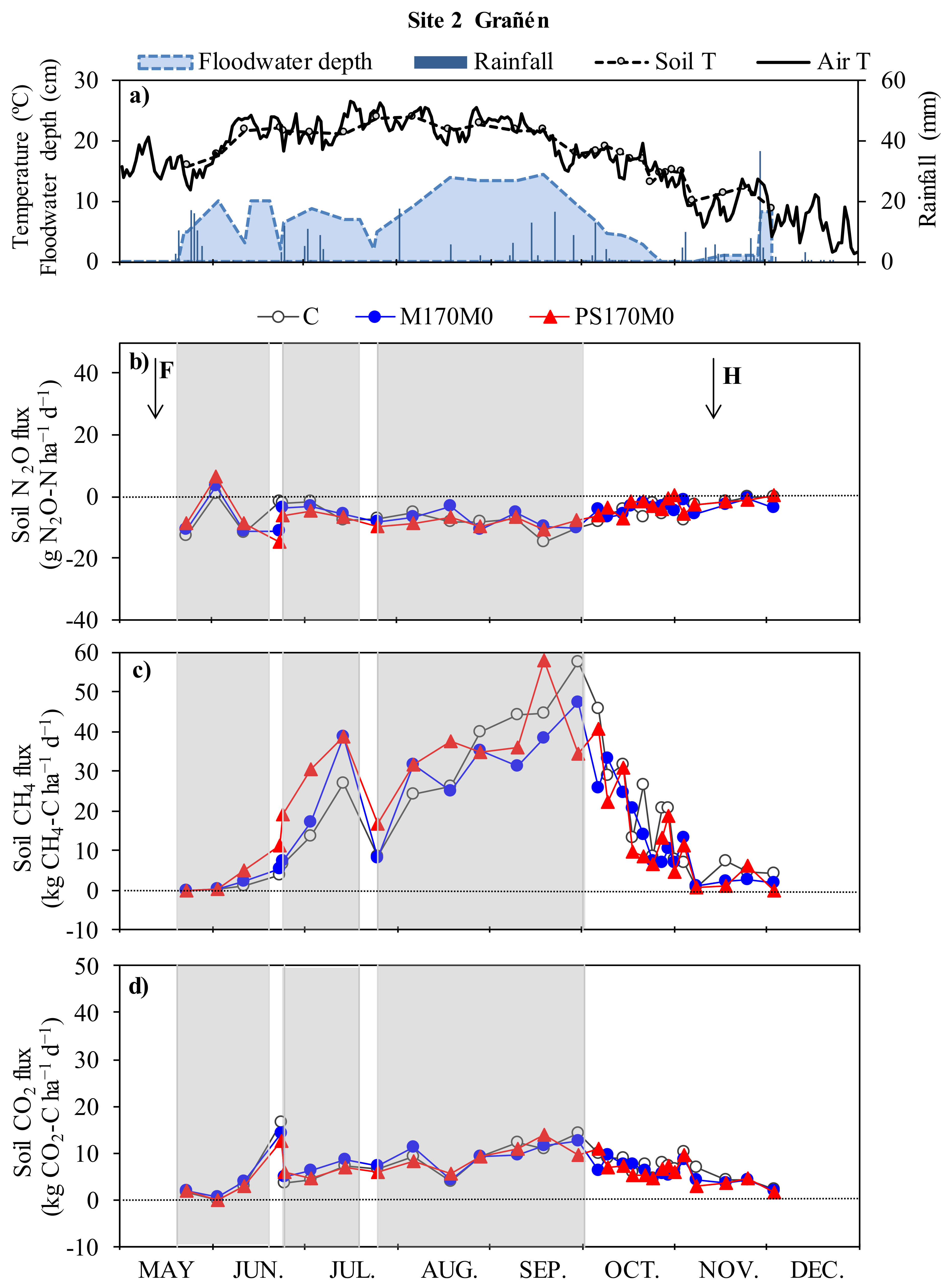
3.1. Meteorological Conditions and Drainage of the Plots
3.2. Nitrous Oxide Fluxes and Cumulative Emissions
3.3. Methane Fluxes and Cumulative Emissions
3.4. Carbon Dioxide Fluxes and Cumulative Emissions
3.5. Soil Ammonium and Nitrate Concentration
4. Discussion
4.1. Nitrous Oxide Fluxes and Cumulative Emissions
4.2. Methane Fluxes and Cumulative Emissions
4.3. Carbon Dioxide Fluxes and Cumulative Emissions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.A.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; et al. Agriculture, Forestry and Other Land Use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adier, A., Baum, I., Brunner, S., Eickemeier, P., Eds.; Cambrige University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Ciais, P.; Sabine, C.; Baia, G.; Boop, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. Carbon and Other Biogeochemical Cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assesment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Win, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Chambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Linquist, B.A.; van Groenigen, K.J.; Adviento-Borbe, M.A.; Pittelkow, C.; Kessel, C. An Agronomic Assessment of Greenhouse Gas Emissions from Major Cereal Crops. Glob. Chang. Biol. 2012, 18, 194–209. [Google Scholar] [CrossRef]
- Cai, Z.C.; Xing, G.X.; Yan, X.Y.; Xu, H.; Tsuruta, H.; Yagi, K.; Minami, K. Methane and Nitrous Oxide Emissions from Rice Paddy Fields as Affected by Nitrogen Fertilisers and Water Management. Plant Soil 1997, 196, 7–14. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Agnelli, A.E.; Linquist, B.; Adviento-Borbe, M.A.; Agnelli, A.; Gavina, G.; Ravaglia, S.; Ferrara, R.M. Alternate Wetting and Drying of Rice Reduced CH4 Emissions but Triggered N2O Peaks in a Clayey Soil of Central Italy. Pedosphere 2016, 26, 533–548. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Jiang, J.; Zheng, X.; Sass, R.L. A 3-Year Field Measurement of Methane and Nitrous Oxide Emissions from Rice Paddies in China: Effects of Water Regime, Crop Residue, and Fertilizer Application. Glob. Biogeochem. Cycles 2005, 19, 1–9. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of Nitrifier Denitrification in the Production of Nitrous Oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Coyne, M.S. Biological Denitrification. In Nitrogen in Agricultural Systems; Schepers, J.S., Raun, W.R., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2008; pp. 201–253. [Google Scholar]
- Helmut, S.; Seiler, W.; Conrad, R. Processes Involved in Formation and Emission of Methane in Rice Paddies. Biogeochemistry 1989, 7, 33–53. [Google Scholar]
- Wassmann, R.; Aulakh, M.S. The Role of Rice Plants in Regulating Mechanisms of Methane Emissions. Biol. Fertil. Soils 2000, 31, 20–29. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The Chemistry of Submerged Soils. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1972. [Google Scholar]
- Goyal, S.; Sakamoto, K.; Inubushi, K.; Kamewada, K. Long-Term Effects of Inorganic Fertilization and Organic Amendments on Soil Organic Matter and Soil Microbial Properties in Andisols. Arch. Agron. Soil Sci. 2006, 52, 617–625. [Google Scholar] [CrossRef]
- Buresh, R.J.; Reddy, K.R.; van Kessel, C. Nitrogen Transformations in Submerged Soils. In Nitrogen in Agricultural Systems; Schepers, J.S., Raun, W.R., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2008; pp. 401–436. [Google Scholar]
- Conrad, R. Soil Microorganisms as Controllers of Atmospheric Trace Gases (H2, CO, CH4, OCS, N2O, and No). Microbiol. Rev. 1996, 60, 609–640. [Google Scholar] [CrossRef]
- Linquist, B.A.; Adviento-Borbe, M.A.; Pittelkow, C.M.; van Kessel, C.; van Groenigen, K.J. Fertilizer Management Practices and Greenhouse Gas Emissions from Rice Systems: A Quantitative Review and Analysis. Field Crops Res. 2012, 135, 10–21. [Google Scholar] [CrossRef]
- Neue, H.U.; Wassmann, R.; Lantin, R.S.; Alberto, M.C.R.; Aduna, J.B.; Javellana, A.M. Factors Affecting Methane Emission from Rice Fields. Atmos. Environ. 1996, 30, 1751–1754. [Google Scholar] [CrossRef]
- Bossio, D.A.; Horwath, W.R.; Mutters, R.G.; van Kessel, C. Methane Pool and Flux Dynamics in a Rice Field Following Straw Incorporation. Soil Biol. Biochem. 1999, 31, 1313–1322. [Google Scholar] [CrossRef]
- Bronson, K.F.; Neue, H.U.; Singh, U.; Abao, E.B. Automated Chamber Measurements of Methane and Nitrous Oxide Flux in a Flooded Rice Soil.1. Residue, Nitrogen, and Water Management. Soil Sci. Soc. Am. J. 1997, 61, 981–987. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Khera, T.S.; Doran, J.W.; Bronson, K.F. Denitrification, N2O and CO2 Fluxes in Rice-Wheat Cropping System as Affected by Crop Residues, Fertilizer N and Legume Green Manure. Biol. Fertil. Soils 2001, 34, 375–389. [Google Scholar] [CrossRef]
- Denier van der Gon, H.A.C.; Neue, H.U. Influence of Organic Matter Incorporation on the Methane Emission from a Wetland Rice Field. Glob. Biogeochem. Cycles 1995, 9, 11–22. [Google Scholar] [CrossRef]
- Liang, X.Q.; Li, H.; Wang, S.X.; Ye, Y.S.; Ji, Y.J.; Tian, G.M.; van Kessel, C.; Linquist, B.A. Nitrogen Management to Reduce Yield-Scaled Global Warming Potential in Rice. Field Crops Res. 2013, 146, 66–74. [Google Scholar] [CrossRef]
- Maris, S.C.; Teira-Esmatges, M.R.; Bosch-Serra, A.D.; Moreno-García, B.; Català, M.M. Effect of Fertilising with Pig Slurry and Chicken Manure on GHG Emissions from Mediterranean Paddies. Sci. Total Environ. 2016, 569, 306–320. [Google Scholar] [CrossRef]
- Win, A.T.; Toyota, K.; Win, K.T.; Motobayashi, T.; Ookawa, T.; Hirasawa, T.; Chen, D.; Lu, J. Effect of Biogas Slurry Application on CH4 and N2O Emissions, Cu and Zn Uptakes by Whole Crop Rice in a Paddy Field in Japan. Soil Sci. Plant Nutr. 2014, 60, 411–422. [Google Scholar] [CrossRef]
- Schütz, H.; Holzapfel-Pschorn, A.; Conrad, R.; Rennenberg, H.; Seiler, W. A 3-Year Continuous Record on the Influence of Daytime, Season, and Fertilizer Treatment on Methane Emission Rates from an Italian Rice Paddy. J. Geophys. Res. Atmos. 1989, 94, 16405–16416. [Google Scholar] [CrossRef]
- Wang, J.Y.; Jia, J.X.; Xiong, Z.Q.; Khalil, M.A.K.; Xing, G.X. Water Regime-Nitrogen Fertilizer-Straw Incorporation Interaction: Field Study on Nitrous Oxide Emissions from a Rice Agroecosystem in Nanjing, China. Agric. Ecosyst. Environ. 2011, 141, 437–446. [Google Scholar] [CrossRef]
- Tinarelli, A. El Arroz; Mundi-Prensa: Madrid, Spain, 1989. [Google Scholar]
- Yagüe, M.R. El Purín Como Fertilizante: Agronomía E Implicaciones Ambientales. Ph.D. Thesis, Universidad de Lleida, Lleida, Spain, 2006. [Google Scholar]
- Sasada, Y.; Win, K.T.; Nonaka, R.; Win, A.T.; Toyota, K.; Motobayashi, T.; Hosomi, M.; Dingjiang, C.; Lu, J. Methane and N2O Emissions, Nitrate Concentrations of Drainage Water, and Zinc and Copper Uptake by Rice Fertilized with Anaerobically Digested Cattle or Pig Slurry. Biol. Fertil. Soils 2011, 47, 949–956. [Google Scholar] [CrossRef]
- Huang, H.Y.; Cao, J.L.; Wu, H.S.; Ye, X.M.; Ma, Y.; Yu, J.G.; Shen, Q.R.; Chang, Z.Z. Elevated Methane Emissions from a Paddy Field in Southeast China Occur after Applying Anaerobic Digestion Slurry. Glob. Chang. Biol. Bioenergy 2014, 6, 465–472. [Google Scholar] [CrossRef]
- Moreno-García, B.; Guillén, M.; Quílez, D. Response of Paddy Rice to Fertilisation with Pig Slurry in Northeast Spain: Strategies to Optimise Nitrogen Use Efficiency. Field Crops Res. 2017, 208, 44–54. [Google Scholar] [CrossRef]
- Piccinini, S.; Bortone, G. The Fertilizer Value of Agricultural Manure: Simple Rapid Methods of Assessment. J. Agric. Eng. Res. 1991, 49, 197–208. [Google Scholar] [CrossRef]
- Yagüe, M.R.; Quílez, D. On-Farm Measurement of Electrical Conductivity for the Estimation of Ammonium Nitrogen Concentration in Pig Slurry. J. Environ. Qual. 2012, 41, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Arias-Navarro, C.; Díaz-Pinés, E.; Kiese, R.; Rosenstock, T.S.; Rufino, M.C.; Stern, D.; Neufeldt, H.; Verchot, L.V.; Butterbach-Bahl, K. Gas Pooling: A Sampling Technique to Overcome Spatial Heterogeneity of Soil Carbon Dioxide and Nitrous Oxide Fluxes. Soil Biol. Biochem. 2013, 67, 20–23. [Google Scholar] [CrossRef]
- Spanish Association for Standardization (AENOR). ISO 13395:1997: Water Quality. Determination of Nitrite Nitrogen and Nitrate Nitrogen and the Sum of Both by Flow Analysis (CFA and FIA) and Spectrometric Detection. ISO Standard; Spanish Association for Standardization (AENOR): Madrid, Spain, 1997. [Google Scholar]
- Spanish Association for Standardization (AENOR). ISO 11732:2005: Water Quality—Determination of Ammonium Nitrogen—Method by Flow Analysis (CFA and FIA) and Spectrometric Detection. ISO Standard; Spanish Association for Standardization (AENOR): Madrid, Spain, 2005. [Google Scholar]
- Janssen, M.; Lennartz, B. Horizontal and Vertical Water and Solute Fluxes in Paddy Rice Fields. Soil Tillage Res. 2007, 94, 133–141. [Google Scholar] [CrossRef]
- Lennartz, B.; Horn, R.; Duttmann, R.; Gerke, H.H.; Tippkötter, R.; Eickhorst, T.; Janssen, I.; Janssen, M.; Rüth, B.; Sander, T.; et al. Ecological Safe Management of Terraced Rice Paddy Landscapes. Soil Tillage Res. 2009, 102, 179–192. [Google Scholar] [CrossRef]
- Berger, S.; Jang, I.; Seo, J.; Kang, H.; Gebauer, G. A Record of N2O and CH4 Emissions and Underlying Soil Processes of Korean Rice Paddies as Affected by Different Water Management Practices. Biogeochemistry 2013, 115, 317–332. [Google Scholar] [CrossRef]
- Ferré, C.; Zechmeister-Boltenstern, S.; Comolli, R.; Andersson, M.; Seufert, G. Soil Microbial Community Structure in a Rice Paddy Field and Its Relationships to CH4 and N2O Fluxes. Nutr. Cycl. Agroecosystems 2012, 93, 35–50. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W.; Cao, Z.; Fiedler, S.; Frenzel, P.; Jahn, R.; Kalbitz, K.; Kölbl, A.; Schloter, M. Biogeochemistry of Paddy Soils. Geoderma 2010, 157, 1–14. [Google Scholar] [CrossRef]
- Poth, M.; Focht, D.D. 15N Kinetic Analysis of N2O Production by Nitrosomonas Europaea: An Examination of Nitrifier Denitrification. Appl. Environ. Microbiol. 1985, 49, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen Losses from the Soil/Plant System: A Review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Adviento-Borbe, M.A.; Hill, J.E.; Six, J.; van Kessel, C.; Linquist, B.A. Yield-Scaled Global Warming Potential of Annual Nitrous Oxide and Methane Emissions from Continuously Flooded Rice in Response to Nitrogen Input. Agric. Ecosyst. Environ. 2013, 177, 10–20. [Google Scholar] [CrossRef]
- Schneiders, M.; Scherer, H.W. Fixation and Release of Ammonium in Flooded Rice Soils as Affected by Redox Potential. Eur. J. Agron. 1998, 8, 181–189. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Scherer, H.W. Ammonium Fixation by Clay Minerals in Different Layers of Two Paddy Soils after Flooding. Biol. Fertil. Soils 1999, 29, 152–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Scherer, H.W. Mechanisms of Fixation and Release of Ammonium in Paddy Soils after Flooding II. Effect of Transformation of Nitrogen Forms on Ammonium Fixation. Biol. Fertil. Soils 2000, 31, 517–521. [Google Scholar] [CrossRef]
- Stucki, J.W.; Golden, D.C.; Roth, C.B. Effects of Reduction and Reoxidation of Structural Iron on the Surface Charge and Dissolution of Dioctahedral Smectites. Clays Clay Miner. 1984, 32, 350–356. [Google Scholar] [CrossRef]
- Mengel, K.; Horn, D.; Tributh, H. Availability of Interlayer Ammonium as Related to Root Vicinity and Mineral Type. Soil Sci. 1990, 149, 131–137. [Google Scholar] [CrossRef]
- Iida, T.; Deb, S.K.; Kharbuja, R.G. Nitrous Oxide Emission Measurement with Acetylene Inhibition Method in Paddy Fields under Flood Conditions. Paddy Water Environ. 2007, 5, 83–91. [Google Scholar] [CrossRef]
- Simmonds, M.B.; Anders, M.; Adviento-Borbe, M.A.; van Kessel, C.; McClung, A.; Linquist, B.A. Seasonal Methane and Nitrous Oxide Emissions of Several Rice Cultivars in Direct-Seeded Systems. J. Environ. Qual. 2015, 44, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, S.; Guo, Y.; Liu, Q.; Zou, J. Methane and Nitrous Oxide Emissions from Organic and Conventional Rice Cropping Systems in Southeast China. Biol. Fertil. Soils 2010, 46, 825–834. [Google Scholar] [CrossRef]
- Kimura, M.; Murase, J.; Lu, Y. Carbon Cycling in Rice Field Ecosystems in the Context of Input, Decomposition and Translocation of Organic Materials and the Fates of Their End Products (CO2 and CH4). Soil Biol. Biochem. 2004, 36, 1399–1416. [Google Scholar] [CrossRef]
- Watanabe, A.; Takeda, T.; Kimura, M. Evaluation of Origins of Ch4 Carbon Emitted from Rice Paddies. J. Geophys. Res. 1999, 104, 23623–23629. [Google Scholar] [CrossRef]
- Wassmann, R.; Neue, H.U.; Alberto, M.C.R.; Lantin, R.S.; Bueno, C.; Llenaresas, D.; Arah, J.R.M.; Papen, H.; Seiler, W.; Rennenberg, H. Fluxes and Pools of Methane in Wetland Rice Soils with Varying Organic Inputs. Environ. Monit. Assess. 1996, 42, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Neue, H.U. Fluxes of Methane from Rice Fields and Potential for Mitigation. Soil Use Manag. 1997, 13, 258–267. [Google Scholar] [CrossRef]
- Cicerone, R.J.; Shetter, J.D.; Delwiche, C.C. Seasonal Variation of Methane Flux from a California Rice Paddy. J. Geophys. Res. Ocean. 1983, 88, 11022–11024. [Google Scholar] [CrossRef]
- Sanchis, E.; Ferrer, M.; Torres, A.G.; Cambra-López, M.; Calvet, S. Effect of Water and Straw Management Practices on Methane Emissions from Rice Fields: A Review through a Meta-Analysis. Environ. Eng. Sci. 2012, 29, 1053–1062. [Google Scholar] [CrossRef]
- Banker, B.C.; Kludze, H.K.; Alford, D.P.; DeLaune, R.D.; Lindau, C.W. Methane Sources and Sinks in Paddy Rice Soils: Relationship to Emissions. Agric. Ecosyst. Environ. 1995, 53, 243–251. [Google Scholar] [CrossRef]
- Tyler, S.C.; Bilek, R.S.; Sass, R.L.; Fisher, F.M. Methane Oxidation and Pathways of Production in a Texas Paddy Field Deduced from Measurements of Flux, Δl3c, and Δd of CH4. Glob. Biogeochem. Cycles 1997, 11, 323–348. [Google Scholar] [CrossRef]
- Iqbal, J.; Castellano, M.J.; Parkin, T.B. Evaluation of Photoacoustic Infrared Spectroscopy for Simultaneous Measurement of N2O and CO2 Gas Concentrations and Fluxes at the Soil Surface. Glob. Chang. Biol. 2013, 19, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Yamulki, S.; Jarvis, S.C. Automated Chamber Technique for Gaseous Flux Measurements: Evaluation of a Photoacoustic Infrared Spectrometer-Trace Gas Analyzer. J. Geophys. Res. D Atmos. 1999, 104, 5463–5469. [Google Scholar] [CrossRef]
- Rosenstock, T.S.; Diaz-Pines, E.; Zuazo, P.; Jordan, G.; Predotova, M.; Mutuo, P.; Abwanda, S.; Thiong’o, M.; Buerkert, A.; Rufino, M.C.; et al. Accuracy and Precision of Photoacoustic Spectroscopy Not Guaranteed. Glob. Chang. Biol. 2013, 19, 3565–3567. [Google Scholar] [CrossRef] [PubMed]
- Tirol-Padre, A.; Rai, M.; Gathala, M.; Sharma, S.; Kumar, V.; Sharma, P.C.; Sharma, D.K.; Wassmann, R.; Ladha, J. Assessing the Performance of the Photo-Acoustic Infrared Gas Monitor for Measuring CO2, N2O, and CH4 Fluxes in Two Major Cereal Rotations. Glob. Chang. Biol. 2013. [Google Scholar]
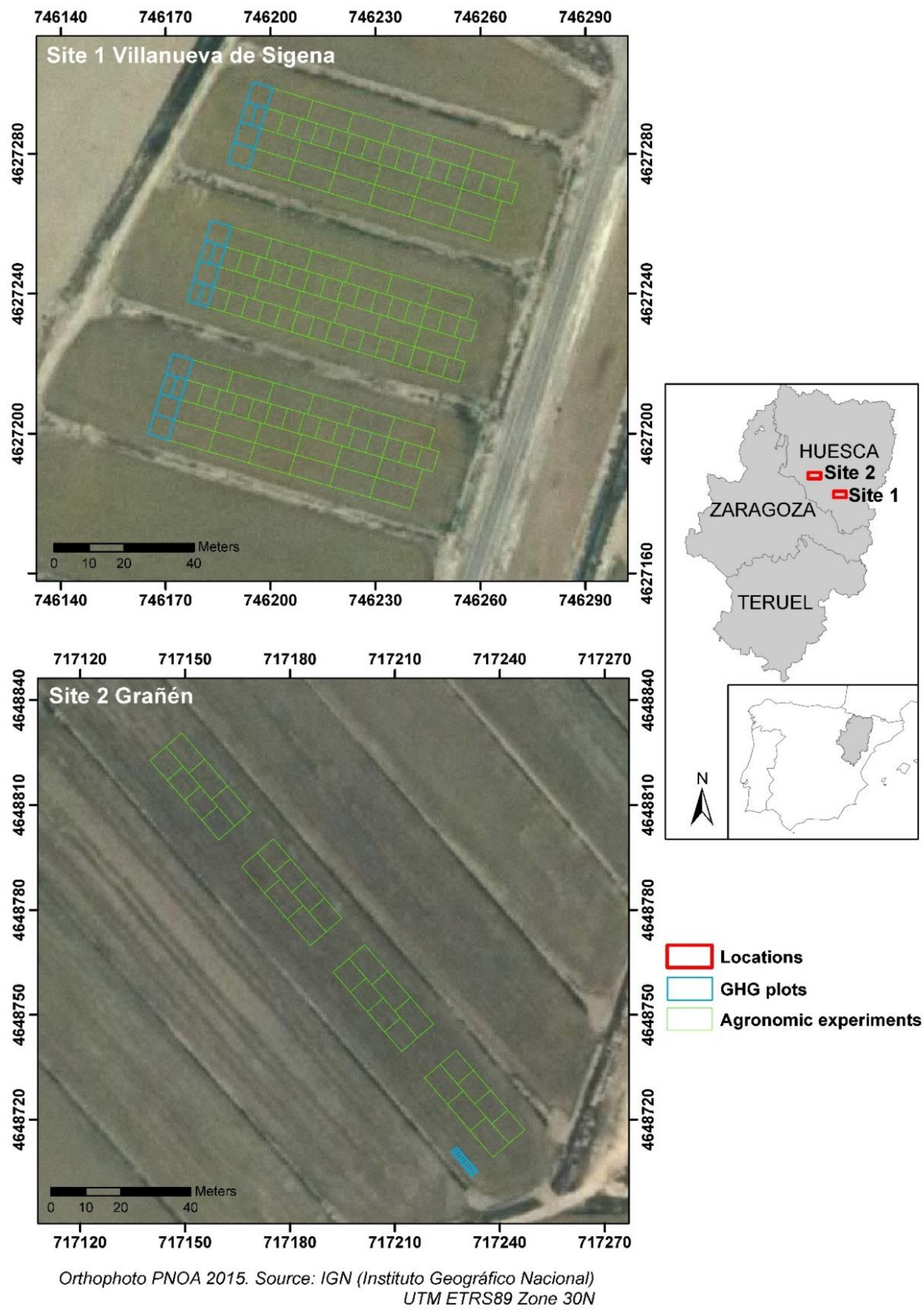

| Site and Soil Characteristics | Site 1 Villanueva de Sigena | Site 2 Grañén |
|---|---|---|
| Previous years growing rice | 3 | >15 |
| Puddling | No | Yes |
| Latitude | 41° 45’ 31.87’’ N | 41° 57’ 29.97’’ N |
| Longitude | 0° 2’ 18.16’’ W | 0° 22’ 37.56’’ W |
| Elevation (m) | 250 | 332 |
| Annual precipitation (mm) † | 347 | 334 |
| Mean annual air temperature (°C) † | 14.6 | 13.5 |
| Annual ET0 (mm) † | 1201 | 1194 |
| pH (1:2.5, water extract) | 8.5 | 8.3 |
| Electrical conductivity of saturated paste extract (ECe, dS m−1) | 0.8 | 4.9 |
| Organic matter (Walkley–Black; % dry matter) | 1.01 | 2.06 |
| Calcium carbonate eq. (% dry matter) | 29 | 24 |
| NO3− (potassium chloride extract; mg kg−1 dry soil) | 11.79 | 14.99 |
| NH4+ (potassium chloride extract; mg kg−1 dry soil) | 6.07 | 10.87 |
| Olsen P (mg kg−1 dry soil) | 6 | 38.2 |
| K (ammonium acetate extract; mg kg−1 dry soil) | 81 | 224 |
| Particle size distribution (%) | ||
| Sand (2000–50 µm) | 13.4 | 16.4 |
| Silt (50–2 µm) | 66.2 | 54.1 |
| Clay (<2 µm) | 20.4 | 29.5 |
| USDA textural class | Silty loam | Silty clay loam |
| NH4+-N | Organic N | Organic C | NH4+-N | NH4+-N | Total N | ||
|---|---|---|---|---|---|---|---|
| kg ha−1 | kg ha−1 | kg ha−1 | |||||
| Site | Treatments | Before Seeding | Topdressing | Growing Season | |||
| 1 | Control (C) | -- | -- | -- | -- | -- | -- |
| M120M60 | 120 † | -- | -- | 60 † | 180 | 180 | |
| PS120M60 | 109 ‡ | 92 | 663 | 60 † | 169 | 261 | |
| PS170M0 | 165 ‡ | 140 | 1007 | -- | 165 | 305 | |
| 2 | Control (C) | -- | -- | -- | -- | -- | -- |
| M170M0 | 170 ¥ | -- | -- | -- | 170 | 170 | |
| PS170M0 | 171 ‡ | 45 | 824 | -- | 171 | 216 | |
| Site 1 Villanueva de Sigena | Site 2 Grañén | |
|---|---|---|
| Specific weight (g L−1) | 1045 | 1021 |
| Dry matter (kg mg−1) | 94 | 23 |
| Organic C (kg mg−1) | 18.58 † | 9.13 |
| Ammonium N (kg mg−1) | 3.05 | 1.89 |
| Total N (Kjeldahl, kg mg−1) | 5.63 | 2.39 |
| P2O5 (acid extraction, kg mg−1) | 4.09 | 0.3 |
| K2O (acid extraction, kg mg−1) | 3.57 | 1.96 |
| Gas Fluxes | Cumulative Emissions | |||||
|---|---|---|---|---|---|---|
| N2O-N | CH4-C | CO2-C | N2O-N | CH4-C | CO2-C | |
| g ha−1 d−1 | kg ha−1 d−1 | kg N ha−1 | kg C ha−1 | |||
| Treatments (T) | n.s | *** | n.s. | n.s. | n.s. | n.s. |
| Control (C) | −3.92 | 2.47 b | 6.76 | −0.71 | 549.1 | 1571.0 |
| M120M60 | −2.33 | 2.99 b | 7.87 | −0.45 | 665.1 | 1959.1 |
| PS120M60 | −0.45 | 3.52 b | 6.52 | −0.04 | 869.9 | 1619.6 |
| PS170M0 | −3.24 | 6.31 a | 9.15 | −0.51 | 1336.4 | 2036.1 |
| Date (D) | *** | *** | *** | |||
| T * D | n.s. | n.s. | n.s. | |||
| Gas Fluxes | Cumulative Emissions | |||||
|---|---|---|---|---|---|---|
| N2O-N | CH4-C | CO2-C | N2O-N | CH4-C | CO2-C | |
| g ha−1 d−1 | kg ha−1 d−1 | kg N ha−1 | kg C ha−1 | |||
| Treatments (T) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Control (C) | −5.45 | 18.81 | 7.04 | −1.20 | 3986.8 | 1329.1 |
| M170M0 | −5.20 | 16.43 | 6.68 | −1.09 | 3701.9 | 1330.7 |
| PS170M0 | −5.05 | 18.92 | 6.45 | −1.10 | 4326.3 | 1254.1 |
| Date (D) | *** | *** | *** | |||
| T * D | n.s. | n.s. | n.s. | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-García, B.; Guillén, M.; Quílez, D. Greenhouse Gas Emissions as Affected by Fertilization Type (Pig Slurry vs. Mineral) and Soil Management in Mediterranean Rice Systems. Agronomy 2020, 10, 493. https://doi.org/10.3390/agronomy10040493
Moreno-García B, Guillén M, Quílez D. Greenhouse Gas Emissions as Affected by Fertilization Type (Pig Slurry vs. Mineral) and Soil Management in Mediterranean Rice Systems. Agronomy. 2020; 10(4):493. https://doi.org/10.3390/agronomy10040493
Chicago/Turabian StyleMoreno-García, Beatriz, Mónica Guillén, and Dolores Quílez. 2020. "Greenhouse Gas Emissions as Affected by Fertilization Type (Pig Slurry vs. Mineral) and Soil Management in Mediterranean Rice Systems" Agronomy 10, no. 4: 493. https://doi.org/10.3390/agronomy10040493
APA StyleMoreno-García, B., Guillén, M., & Quílez, D. (2020). Greenhouse Gas Emissions as Affected by Fertilization Type (Pig Slurry vs. Mineral) and Soil Management in Mediterranean Rice Systems. Agronomy, 10(4), 493. https://doi.org/10.3390/agronomy10040493





