Abstract
Fruit ripening involves changes in physical, physiological and metabolic activities through the actions of enzymes and regulatory genes. This study was initiated to identify the genes related to the ripening of kiwifruit. Gold ‘Haegeum’ kiwifruit is a yellow-fleshed kiwifruit cultivar usually used for fresh marketing. The fruit is harvested at a physiologically mature but unripe stage for proper storage, marketing distribution and longer shelf life. To identify the differentially expressed genes (DEGs) during ripening, fruit treated with ethylene were compared with control fruit that ripened naturally without ethylene treatment. Firmness, respiration rate, ethylene production rate, total soluble solids (TSS), titratable acidity (TA), brix acid ratio (BAR) and overall acceptability were taken during the study as fruit ripening indicators. Total mRNAs were sequenced by Illumina high-throughput sequencing platform and the transcriptome gene set was constructed by de novo assembly. We identified 99,601 unigenes with an average length of 511.77 bp in transcriptome contigs. A total of 28,582 differentially expressed unigenes were identified in the ethylene treatment vs. control. Of these 28,582 unigenes, 13,361 and 15,221 genes were up- and downregulated, respectively, in the treated fruit. The results also showed that 1682 and 855 genes were up- and downregulated, respectively, more than 2-fold at p < 0.05 in fruit treated with ethylene as compared with the control fruit. Moreover, we identified 75 genes showing significantly different expression; 42 were upregulated, and 33 were downregulated. A possible category of the identified ripening-related genes was also made. The findings of this study will add to the available information on the effect of ethylene treatment on ripening and the related changes of kiwifruit at the genomic level, and it could assist the further study of genes related to ripening for kiwifruit breeding and improvement.
1. Introduction
Kiwifruit (Actinidia spp.) is a perennial deciduous warm-temperate fruit that belongs to the family Actinidiaceae, and it is native to Northern China, Korea, Siberia, and Japan [1]. According to FAOSTAT [2], worldwide annual production of kiwifruit was 4.32 million Mt from the total planted area of 247,793 ha, out of which the Republic of Korea took a share of 7991 Mt from the planted area of 492 ha. In Korea, kiwifruit is mostly cultivated in the southeast region due to warm winters [3,4,5].
More than 70 species were recorded within the genus Actinidia; A. deliciosa and A. chinensis are the major commercially cultivated species [6,7]. Several cultivars were then bred in New Zealand including the most popular ‘Hayward’ and ‘Hort16A’ cultivars which belong to A. deliciosa and A. chinensis, respectively [7]. The introduction of kiwifruit into Korea was started with the green-fleshed ‘Hayward’ in the 1970s, while the yellow-fleshed ‘Hort16A’ has been cultivated since 2004. Afterwards, several cultivars were imported from China and New Zealand, and the yellow-fleshed kiwifruit cultivars such as ‘Haegeum’, ‘Jecygold’, and ‘Halla-gold’ were bred in Korea [7].
Commercially, kiwifruit is harvested at physiological maturity but at an unripe stage and stored at low temperature (0 °C) for proper storage, marketing distribution and longer shelf life [8]. However, consumers prefer to purchase “ready to eat” kiwifruit. To fulfill the consumers’ preference, it is inevitable to use ripening techniques like exogenous ethylene treatment at harvest or along the storage period.
Various ripening related studies on kiwifruit have been reported, including effects of exogenous ethylene treatment at 100 μL kg−1 [9], polyamines [10], treatments with salicylic acid [11] and high-temperature stress of propylene treated fruit [12]. Matalo et al. [13] also reported the expression of some ripening-related genes that are involved in ethylene-independent low-temperature-modulated ripening in ‘Rainbow Red’ kiwifruit during storage and on-vine. In addition, Richardson et al. [14] studied kiwifruit development from anthesis through to fruit senescence for Actinidia chinensis ’Hort16A’ and reported eight genes with differential expression over fruit development. The effects of ethylene on ripening and the observable ripening related changes of kiwifruit have been known from these studies, but the understanding of regulatory mechanism of kiwifruit ripening remains quite limited.
Transcript profile during fruit ripening and softening have been studied for other fruits such as tomato [15], apple [16], banana [17] and persimmon [18]. Similarly, transcriptome profiling of kiwifruit has also been done on different developmental stages [19]. However, molecular data indicating postharvest changes during ripening of kiwifruit is insufficient compared with those of other fruits. Hence, in this study, we treated kiwifruit with exogenous ethylene application and compared it with control (untreated fruit) to study the changes related to ripening.
The main objective of the present study is to identify candidate genes related to ripening and ripening-related changes. The DEGs in ethylene-treated kiwifruit were analyzed and identified to show the candidate genes that could be involved in softening and ripening-related changes. The transcriptome profile from our study could provide the information needed for further functional genomics study of kiwifruit.
2. Materials and Methods
2.1. Plant Material and Ethylene Treatment
Gold ‘Haegeum’ kiwifruit cultivar, one of the commonly grown cultivars in Jangheung, Korea was selected for this study. Pollination was performed during anthesis in May 2018 when the flower buds open completely and harvesting was done randomly from the ready vines at 170 days after full bloom (DAFB) on 20 October, 2018. After harvesting, fruit were transported to the postharvest quality management laboratory at the Department of Horticultural Sciences, Kangwon National University within 12 h of harvest. Carefully selected, uniform-size fruit free from physical defects were then treated with ethylene at 100 μL kg−1 [9,16] in a sealed 62 L container for 3 days at 25 °C; air in the sealed containers was ventilated and distributed by a fan (Coolertec CT8025L12RA-3P, Zhengzhou, China). Control fruit were treated at similar conditions without ethylene. Sixty fruit were placed in each category to collect data from fifteen biological replicates for firmness, respiration rate, ethylene production rate, total soluble solids (TSS), titratable acidity (TA) and overall acceptability of the fresh fruit at the beginning and on the third day of the experiment. Fruit flesh samples were collected from both the treated and control groups on the third day and frozen by liquid nitrogen. The frozen samples were stored in a deep freezer (−80 °C) until transcriptome analysis.
2.2. RNA Extraction and Sequencing Using Illumina Truseq Stranded mRNA Library Prep Kit
Total RNA was extracted and pooled according to Park et al. [20]. RNA purity and integrity were checked as described by [21], and mRNA sequencing was done by the method followed by [22].
2.3. Mapping Reads on a Reference Genome and Calculating Expression between Samples
Reads for each sample were mapped to the reference genome (Actinidia chinensis Red5) by Tophat (v2.0.13) (http://ccb.jhu.edu/software/tophat/). The aligned results were then added to Cuffdiff (v2.2.0) (http://cole-trapnell-lab.github.io/cufflinks/cuffdiff/) to report differentially expressed genes. For library normalization and dispersion estimation, geometric and pooled methods (http://cole-trapnell-lab.github.io/cufflinks/cuffdiff/) were applied.
2.4. Identification of DEGs and Functional Enrichment Analysis
The method used by [22] was implemented to detect DEGs between control and ethylene-treated fruit after applying two filtering processes. For ontology analysis, genes after 2-fold change were picked and applied to DAVID (http://david.abcc.ncifcrf.gov/) as an input to get a comprehensive set of functional annotation.
2.5. Measurement of Firmness, Respiration Rate and Ethylene Production Rate
Kiwifruit firmness was measured from fifteen fruits, two measurements per fruit, by a puncture at the equator of the fruit with a maximum force of 10 kg and a 3 mm diameter round stainless steel probe having a flat end [23]. Respiration rate and ethylene production rate of kiwifruit was measured and expressed as described by [24,25].
2.6. Measurement of Total Soluble Solids (TSS), Titratable Acidity (TA), Brix Acid Ratio (BAR) and Overall Acceptability
TSS and TA were measured according to Tilahun et al. [25,26] from fifteen sample fruits. To measure TSS from the sample fruits at 20 °C, digital refractometer (Atago Co. Ltd., Tokyo, Japan) was used, and expressed in percent. Kiwifruit juice was diluted (1 mL juice to 19 mL distilled water) and DL22 food and beverage analyzer (Mettler Toledo Ltd., Zurich, Switzerland) was used for titration of the diluted juice by 0.1 N NaOH up to pH 8.1 to obtain TA and expressed as mg of citric acid per kg of fresh kiwifruit weight. BAR was determined by dividing the TSS with titratable acidity [8]. The overall acceptability of kiwifruit during the ripening period was evaluated as the mean value of the subjective scale made by 10 trained panels of graduate students for flavor, sweetness, chewiness and appearance according to a subjective scale by [27] from bad (1) to excellent (5).
2.7. Statistical Analysis of Quality Parameters
Results of the considered quality parameters were expressed as means ± standard errors. SAS statistical software (SAS/STAT 9.1; SAS Institute Inc., USA) was implemented for statistical analyses, and tests for significance were done using t-test.
3. Results and Discussion
3.1. Assembly and Annotation
The summaries of the mapping and sequencing results are presented in Table 1 and Table 2. The RNA sequence analysis was made by using Illumina Truseq Stranded mRNA library prep kit and Novaseq 6000 for sequencing. A total of 51,331,356 and 50,615,434 reads were generated from the control and ethylene libraries, with mapped read numbers of 41,747,134 and 41,801,951, respectively (Table 1). We identified 99,274 unigenes with an average length of 513.5 bp in transcriptome contigs. The average unigene length for kiwifruit in this study was comparable to those observed in other species such as persimmon (643.2 bp) [18], Oncidium (493 bp) [28], and Pinus contorta (500 bp) [29].

Table 1.
Summary of mapping result.

Table 2.
Summary of the sequencing result.
The identified unigenes were classified into three functional categories; 45,275 genes were assigned to biological processes, 8223 to molecular function and 13,224 to the cellular component. Further subcategories were also observed within each main category of the GO classification scheme and the dominant 10 subcategories from each main category are presented in Figure 1. Kiwifruit annotation in the present study gained more descriptive information (67.2%) than those observed in other species such as Diospyros kaki (61.1%) [18], Bupleurum chinense (52.6%) [30] and Dendrocalamus latiflorus (54.9%) [31].

Figure 1.
The number of genes in different gene ontology (GO) subcategories.
3.2. DEGs in the Comparison of Ethylene-Treated vs. Control Kiwifruit
A total of 28,582 unigenes were differentially expressed during the comparison of ethylene-treated vs. control ‘Haegeum’ gold kiwifruit. From the differentially expressed unigenes, 13,361 were upregulated and 15,221 were downregulated in the ethylene-treated fruit. The number of genes that induced expression of more than 2-fold change at p < 0.05 was 1682, and the number of genes that revealed inhibited expression of more than 2-fold change at p < 0.05 was 855 (Table 3). The mainly expressed genes during ethylene treatment of ‘Haegeum’ gold kiwifruit were the genes involved in cellular and metabolic process, the genes involved in response to stimulus in the biological process, the genes involved in binding and catalytic activities in the molecular function and the genes involved in cell and cell parts in the cellular component (Figure 2, Figure 3 and Figure 4).

Table 3.
Summary of differentially expressed genes (DEGs) during the comparison of control vs. ethylene-treated ‘Haegeum’ gold kiwifruit.

Figure 2.
Summary of upregulated (outside) and downregulated (inner side) genes that involved in biological process in ethylene-treated ‘Haegeum’ gold kiwifruit.

Figure 3.
Summary of upregulated (outside) and downregulated (inner side) genes that involved in molecular function in ethylene-treated ‘Haegeum’ gold kiwifruit.

Figure 4.
Summary of upregulated (outer side) and downregulated (inner side) genes that involved in cellular function in ethylene-treated ‘Haegeum’ gold kiwifruit.
3.3. Firmness and Genes related to Softening
Ethylene treatment efficiently hastens the ripening process as observed on the firmness (Figure 5). The firmness in the treated ‘Haegeum’ gold kiwifruit decreased sharply from 26.99 N at harvest to 4.82 N on day 3, in contrast to 23.01 N in control fruit on the same day (Figure 5). Table 4 shows the expression levels of the significantly upregulated unigenes involved in the ripening of kiwifruit. Physiological processes are associated with the complex fruit softening processes and result in the remodeling of cell wall structure [32]. Cell wall hydrolytic enzymes such as polygalacturonase (PG), pectate lyase (PL), pectin acetylesterase and β-galactosidase (β-gal) are involved in the ripening process by solubilizing pectin polysaccharides of fruits [33,34,35,36]. Interestingly, in this study, all PG, PL, pectin acetylesterase and β-gal genes were upregulated due to ethylene treatment, in turn hastening the action of pectin-digesting enzymes that lead to softening of kiwifruit. Besides, xyloglucan endotransglucosylase/hydrolase and oligopeptide transporter-like were upregulated in our study. According to Schroder and Atkinson [37], xyloglucans are thought to be part of a cellulose–hemicellulose framework that stabilizes the cell wall. So, the upregulation of the xyloglucan endotransglucosylase/hydrolase gene could lead to the ripening and softening of kiwifruit. The upregulation of an oligopeptide transporter-like which encodes integral membrane proteins to translocate substrates into the cytosol [38] could be related to the solubilization of pectin polysaccharides during ripening. Furthermore, β-1, 4-mannosyl-glycoprotein-like which is involved in cell wall modification and degradation [39] and cinnamoyl CoA-reductase (CCR), an enzyme that catalyzes key steps in the biosynthesis of lignin, were also upregulated due to ethylene treatment. Consistent with this study, Li et al. [40] reported the increase in lignin content during cold storage implying the fact that stimulation of lignin biosynthesis could be due to stresses like low temperature and exogenous application of ethylene.
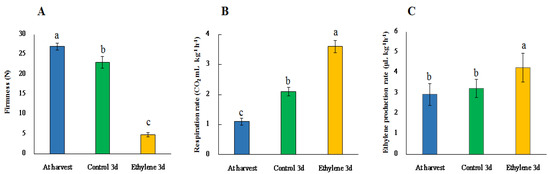
Figure 5.
Firmness (A), respiration rate (B) and ethylene production rate (C) of ‘Haegeum’ gold kiwifruit as affected by ethylene treatment during 3 days of ripening at 25 °C. Vertical bars represent standard errors of the means (n = 15). Different letters indicate significant difference between different groups by Tukey’s test at p ≤ 0.05.

Table 4.
List of DEGs in ‘Haegeum’ gold kiwifruit (upregulated) in the comparison of ethylene treated vs. control.
An increased amount of trehalose, nonreducing disaccharide, in fruits has been reported to improve shelf life [41]. In this study, the gene that encodes trehalase (α-glucoside-1-glucohydrolase), a hydrolytic cell wall-bound enzyme that cleaves trehalose into two glucose moieties [41] was upregulated due to ethylene treatment. This phenomenon degrades apoplastic trehalose which in turn causes softening of the fruit (Figure 5).
Contrarily, the genes that encode cellulose synthase A catalytic subunit 8 (UDP-forming)-like, which takes part in synthesizing cellulose [42], expansin-A1-like, cell wall proteins that affect cell wall composition [43] were downregulated implying that catabolic process could dominate the anabolic process during ripening of fruits. Mannan endo-1,4-beta-mannosidase, which is involved in the cell wall hydrolysis [44], and caffeoylshikimate esterase (CSE), which plays an essential role in lignin biosynthesis [45], were also downregulated due to ethylene treatment probably due to full ripening of kiwifruit before 3 days after treatment with exogenous ethylene. In addition, the genes that encode aquaporin PIP1-4-like and aquaporin PIP2-7-like were downregulated probably due to the reduction of the transport of water and solutes across the cell membrane [46] as the fruit ripened.
3.4. Ethylene Production and Respiration Rates and Related Genes
As observed in Figure 5, respiration and ethylene production rates were also higher on the third day in ethylene-treated fruit as compared with the control. The final step in ethylene biosynthesis is catalyzed by 1-aminocyclopropane-1-carboxylate oxidase (ACCO) [18,47]. We observed significant upregulation of ACCO and downregulation of LRR receptor-like serine/threonine-protein kinase that mediates 1-aminocyclopropane-1-carboxylic acid (ACC) synthase [48] due to ethylene treatment for ripening of kiwifruit. The gene that encodes methyltransferase, an enzyme that functions as a barrier to prevent senescence [49] was also downregulated due to ethylene treatment. The above results imply that exogenous ethylene application could stimulate more ethylene biosynthesis which in turn causes a rise in respiration rate, biochemical changes, color changes and softening (Figure 5, Figure 6 and Figure 7). Besides, ethylene-related gene families such as ethylene-responsive transcription factor RAP2-10, ethylene-responsive transcription factor RAP2-3 and ethylene-responsive transcription factor 4 were upregulated, which consequently lead to higher respiration rate and faster ripening [18]. Moreover, downregulation of zinc finger protein-like expression could cause accelerated ripening [50].
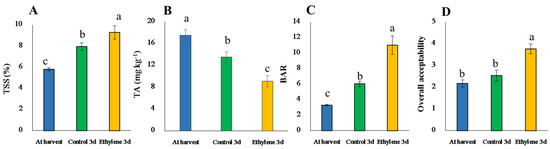
Figure 6.
TSS (A), TA (B), BAR (C) and overall acceptability (D) of gold ‘Haegeum’ kiwifruit as affected by ethylene treatment during 3 days of ripening at 25 °C. Vertical bars represent standard errors of the means (n = 15). Different letters indicate significant difference between different groups by Tukey’s test at p ≤ 0.05.

Figure 7.
Gold ‘Haegeum’ kiwifruit at harvest and after 3 days of ripening with ethylene treatment or without ethylene treatment (control) at 25 °C.
However, the gene that encodes S-adenosylmethionine decarboxylase (SAMDC) was also upregulated due to exogenous ethylene treatment. The biosynthesis of polyamines, i.e., spermidine and spermine, are catalyzed by SAMDC [51]. Polyamines are effective inhibitors of ethylene synthesis during the early stages of fruit ripening by inhibiting the conversion of methionine and ACC to ethylene [52]. The result implies that ethylene-treated kiwifruit could respond to the exogenous stimuli by upregulation of the gene that encodes SAMDC immediately after treatment to inhibit further ethylene synthesis. Additionally, ethylene treatment caused the downregulation of ethylene-responsive transcription factor TINY-like in kiwifruit (Table 5). This could be due to its role before harvest in plant growth and development in the enhancement of a ‘tiny’ phenotype rather than its action after harvest [53]. Generally, ethylene affects ripening through the regulation of ethylene receptors and triggering signal transduction reactions which ultimately control relevant gene expression in the fruit [20].

Table 5.
List of DEGs in ‘Haegeum’ gold kiwifruit (downregulated) in the comparison of ethylene treated vs. control.
Ethylene bursts during ripening and causes the climacteric rise of respiration (Figure 5), which could be a death signal that prepares fruit for seed dispersal through the breakdown of cellular components [47]. In this study, the genes that encoded glycolysis and TCA cycling enzymes, which mediate climacteric respiration, such as ribose-5-phosphate isomerase and pyruvate kinase, were upregulated in ethylene-treated fruit. The results imply that the response of the enzymes after ethylene treatment could lead to faster ripening as compared with the control. The gene that encodes 6-phosphogluconolactonase, the protein related to fruit respiratory pathway and quality [54], was also upregulated due to ethylene treatment. This could be explained by the respiration burst caused by the higher ethylene concentration.
3.5. TSS, TA, BAR and Sensory Evaluation and the Related Genes
TSS, TA, BAR and the sensory evaluation in the present study reveal that ethylene-treated fruit was preferred over the control on the third day of ripening (Figure 6). Phosphoenolpyruvate carboxylase (PEPC) is involved in organic acid synthesis [55]. The gene that encodes PEPC was downregulated after ethylene treatment due to the reduction of acidity (Figure 6) during ripening that might be associated with the conversion of organic acids into sugars and their derivatives or their utilization in respiration [25]. β-Amylase (BAM), one of the key genes related to starch degradation during the ripening of fruit [18], was also upregulated in our study. BAM breaks starch into maltose, resulting in the sweet flavor of ripe fruit [56]. Furthermore, the upregulation of the sucrose-phosphate synthase (SPS) encoding gene could be related to an increase in TSS, BAR and sensory quality (Figure 6). Similarly, Duque et al. [57] reported the accumulation of soluble sugars coincided with a gradual activation of SPS after harvest during cold storage. Besides, glucose-1-phosphate adenylyltransferase large subunit 2/amyloplastic-like protein, which plays a role in the synthesis of starch was downregulated, implying that fruit ripening involves catabolic processes rather than anabolic processes.
Methanol O-anthraniloyltransferase is an enzyme responsible for the production of O-methyl anthranilate, an important aroma and flavor compound in the grapefruit [58]. Similarly, methanol O-anthraniloyltransferase gene was upregulated due to ethylene treatment in this study, implying that O-methyl anthranilate could be produced during ripening, which can directly influence the aroma and flavor of ‘ready to eat’ kiwifruit. Hence, the methanol O-anthraniloyltransferase gene could be responsible for the development of aroma and flavor of kiwifruit during ripening. In this study, the gene that encodes arogenate dehydrogenase, an enzyme that catalyzes the oxidative decarboxylation of arogenate into the aromatic amino acid tyrosine [59], was also upregulated due to exogenous ethylene treatment, indicating that kiwifruit ripening is also associated with changes in aroma of the fruit. Interestingly, the genes that encode linoleate 13S-lipoxygenase and linoleate 9S-lipoxygenase, lipoxygenases that may contribute to aldehyde generation to produce grassy aroma during kiwifruit ripening [60], were downregulated due to ethylene treatment. The results of overall sensory evaluation and BAR in the current study (Figure 6) could also be related to the above changes. However, 4-coumarate-CoA ligase-like, an enzyme that involved in phenylpropanoid-derived compound (PDC) pathway [61], carboxylesterase (CXE) which regulates plant bioactive metabolism through the control of esterases to release volatile carboxyl esters [62], UDP-glycosyltransferase which catalyzes the production of glycosides (a potential source of aroma and flavor in fruits) [63] and α-glucosidase, the key enzyme that catalyzes the final step in the digestive process of carbohydrates [64], were downregulated in the present study after 3 days of ethylene treatment, probably due to early action of these enzymes at the onset of ethylene treatment and reduced action afterward.
L-Ascorbate oxidase gene was upregulated due to ethylene treatment. L-ascorbic acid (AA), one of the major antioxidants in fruits and vegetables, known as vitamin C by most consumers, is oxidized to L-dehydroascorbic acid (DHAA), mainly due to the activity of L-ascorbate oxidase [65]. The biological activity of DHAA has been considered to be equivalent to AA as it can be converted readily into AA in the human body [66]. Besides, the enzyme that catalyzes the terminal step in the biosynthetic pathway of AA [67] could be enhanced due to the upregulation of L-gulonolactone oxidase (Table 4). Moreover, the other ascorbate-related genes in kiwifruit, including galacturonosyltransferase and UDP-glucose dehydrogenase, were upregulated due to ethylene treatment.
3.6. Stress-Related Genes Due to Ethylene Treatment
Ethylene treatment also caused the upregulation of transcription factor MYB1R1 (Table 4). Shin et al. [68] reported that the expression of transcription factor MYB1R1 in potato plants improved plant tolerance to drought stress via regulation of water loss. Peptide methionine sulfoxide reductase and universal stress protein A-like protein genes that could be involved in the protective roles were also upregulated. In line with our results, Lopez et al. [69] and Tkaczuk et al. [70] reported the upregulation of peptide methionine sulfoxide reductase and universal stress protein A-like protein genes to overcome stress conditions. Moreover, there was an upregulation of the gene that encodes peroxidase, the enzyme that catalyzes the reduction of peroxides and the polymerization of toxic compounds [71]. Hence, the upregulation of transcription factor MYB1R1, peptide methionine sulfoxide reductase, universal stress protein A-like protein genes and peroxidase in the present study could be the response of the fruit to the stress caused by the exogenous ethylene treatment.
Phenylalanine ammonia-lyase (PAL), one of the key enzymes in the phenylpropanoid pathway [72], and 3-ketoacyl-CoA synthase (KCS) were also upregulated due to ethylene treatment (Table 4). The phenylpropanoid pathway produces phenolic compounds that contribute to fruit pigmentation and the disease resistance response during the ripening process of fruits [73]. Han et al. [74] reported increased transcript levels of the KCS gene, a gene that involves very long-chain fatty acid biosynthesis of lipid pathway following the application of abscisic acid (ABA) for wound suberization. Furthermore, there was an upregulation of the gene that encodes β-carotene hydroxylase, an enzyme in the zeaxanthin biosynthetic pathway which could help for protection against stress [75]. Therefore, ethylene treatment could intensify the phenylpropanoid metabolism, lipid metabolism and zeaxanthin biosynthesis through activating PAL, KCS and beta-carotene hydroxylase enzymes during kiwifruit ripening.
In contrast to Park et al. [18] who reported the downregulation of polyphenol oxidases (PPOs) after treatment of persimmon fruit, treatment of kiwifruit with ethylene in the present study upregulated PPOs. PPOs are involved in production of brown pigments in wounded tissues by catalyzing the oxidation of phenolic compounds to quinones [76]. In this study, the treatment of kiwifruit with exogenous ethylene hastens the softening as compared with control (Figure 5 and Figure 7), and the fruit could be liable to physical damage which may result in wounded tissues. Besides, superoxide dismutase (SOD), the first line of defense against oxidative stress [77], subtilisin-like protease which is associated with pathogen resistance and plant immunity [78], ABC transporter G family member 3-like which is mainly involved in detoxification process and response to abiotic stress [79] and receptor-like protein kinase (RLK), the transmembrane proteins that perceive the signals of environmental conditions [80], were downregulated. Hence, care should be taken not to ripen the kiwifruit over three days after treatment with ethylene as it may lead to oversoftening that favors postharvest loss and browning.
The gene that encodes glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an essential component of the glycolytic pathway which converts glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate was upregulated in this study. In addition to its key role in glycolysis, GAPDH is involved in abiotic stress resistance in plants [81]. The gene that encodes adenylate isopentenyltransferase, a cytokinin (CK) biosynthesis enzyme, was also upregulated with ripening due to ethylene treatment. In line with our results, [82] indicated the increasing levels of CK in green and gold kiwifruit during ripening. Ha et al. [83] reported that CK regulates responses to environmental stresses in plants. Besides, NAC transcription factor was upregulated in this study. Stress-inducible NAC genes are involved in abiotic stress response [84]. In this study, ethylene-induced ripening in kiwifruit also evokes the upregulation of the prolyl-4-hydroxylase gene, consistent with [85], who reported the expression of the same gene during ripening of banana, with it being involved in stress, defense and detoxification. Moreover, Chen et al. [86] suggested that WRKY transcription factors play important roles associated with stress responses in plants. Our results also showed upregulation of the WRKY transcription factor gene indicating that kiwifruit responds toward the stress caused by the exogenous application of ethylene. Zhu et al. [87] reported that exogenous niacin treatment during the growth of kiwifruit increases NADPH oxidase to increase tolerance to short-term stress conditions. In the present study, the upregulation of the gene that encodes NADPH oxidase after exogenous application of ethylene could be the response to the stress caused by ethylene treatment.
3.7. Color and Other Changes and the Related Genes
The genes that encode the light-harvesting complexes including chlorophyll a-b binding protein-like, chlorophyll a-b binding protein 3C precursor and chlorophyll a-b binding protein 37 precursor were downregulated due to ethylene treatment. Chlorophyll (ide) b reductase, which plays a central role in chlorophyll a-b binding protein degradation [88], and magnesium-protoporphyrin IX monomethyl ester (oxidative) cyclase, which has a great role in the process of chlorophyll biosynthesis [89], were also downregulated. Similarly, triose phosphate/phosphate translocator TPT-like that could catalyze the exchange of triose phosphate, 3-phosphoglycerate and inorganic phosphate across the chloroplast and plays crucial roles in photosynthesis [90] was downregulated. This could be attributed to the conversion of chloroplasts into chromoplasts, with the associated accumulation of colored carotenoids as fruits ripen [91] which causes color changes as observed in Figure 7. Additionally, the gene that encodes ribulose bisphosphate carboxylase/oxygenase (rubisco) activase, which plays a key role in photosynthesis [92], was downregulated.
In the present study, the amino-acid-metabolizing glutamate dehydrogenase B and aspartate aminotransferase encoding genes were also upregulated due to ethylene treatment. The coordinated events of the ripening process involve the changes at physiological and biochemical levels including the changes of chloroplasts into chromoplast which accompanied the metabolism of nitrogen compounds such as amino acids [93]. Similarly, Boggio et al. [93] also reported the upregulation of glutamate dehydrogenase B and aspartate aminotransferase in fully ripened red tomato fruits. However, the genes that encode 3-hydroxyisobutyrate dehydrogenase-like which is involved in branched-chain amino acid catabolism at carbohydrate limitation [94], (-)-isopiperitenol/(-)-carveol dehydrogenase which is involved in secondary metabolism of phenylpropanoids and terpenoids [95] and flavonoid 3’-monooxygenase(F3’H) which is involved in anthocyanin biosynthesis [96] were downregulated due to ethylene treatment. Downregulation of the genes encoding the above enzymes could imply the full ripening of kiwifruit before 3 days after treatment with exogenous ethylene.
In our results, we also found that gibberellin 2-β-dioxygenase was upregulated due to ethylene treatment. Gibberellin 2-β-dioxygenase catalyzes the 2-β-hydroxylation of biologically active gibberellins, which leads to the homeostatic regulation of their endogenous level. Similarly, Onik et al. [97] also reported the upregulation of gibberellin 2-β-dioxygenase during postripening of apple fruit, which could be related to the action of ethylene on ripening. Auxin regulates every aspect of plant growth and development by controlling gene expression through auxin response factors [98]. In the present study, the gene that encodes auxin response factor-like was downregulated due to ethylene treatment. From this result, it can be presumed that these two hormones may act antagonistically during the ripening of kiwifruit.
4. Conclusions
This work presents the transcriptome sequencing analysis of ‘Haegeum’ gold kiwifruit after treatment with ethylene. Several genes were expressed differentially during the ripening of kiwifruit, and the cumulative effect brings the softening- and ripening-related changes. The results indicated that there is no single gene or enzyme that accounts for kiwifruit ripening, rather, there are many potentially responsible genes that exhibit ripening-related expressions. We identified and categorized genes related to softening and other changes during ripening. The data obtained from the present study will add to the information available on the molecular mechanisms of the effects of ethylene during ripening of kiwifruit. This study will also provide important resources for further study of the genes related to ripening for kiwifruit breeding and improvement.
Author Contributions
Conceptualization, S.T., H.R.C. and C.S.J.; methodology, S.T. and H.R.C.; execution of experiment, H.R.C., S.T. and H.K.; software, S.T. and D.S.P.; formal analysis, S.T. and H.R.C.; resources, D.S.P. and C.S.J.; original draft preparation, S.T. and H.R.C.; review and editing, D.S.P. and S.M.P.; supervision, C.S.J.; project administration, H.R.C.; funding acquisition, C.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bioindustry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116137-3).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kwack, Y.-B.; Kim, H.-L.; Choi, Y.-H.; Lee, J.-H.; Kim, J.G.; Lee, Y.-B. Fruit Quality and Fruit Locule Air Hole of Kiwifruit (Actinidia deliciosa cv. Hayward) Affected by Early Defoliation. Korean J. Environ. Agric. 2012, 31, 229–234. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations Cropping Database. 2017. Available online: http://faostat3.fao.org/home/index.html (accessed on 29 December 2018).
- Koh, Y.; Jung, J.; Hur, J. Current Status of Occurrence of Major Diseases on Kiwifruits and Their Control in KorEA. Acta Hortic. 2003, 610, 437–443. [Google Scholar] [CrossRef]
- Park, Y.; Jung, S.; Gorinstein, S. Ethylene treatment of ‘Hayward’ kiwifruits (Actinidia deliciosa) during ripening and its influence on ethylene biosynthesis and antioxidant activity. Sci. Hortic. 2006, 108, 22–28. [Google Scholar] [CrossRef]
- Kwack, Y.-B.; Kim, H.L.; Lee, J.H.; Chung, K.H.; Chae, W.B. ‘Goldone’, a Yellow—Fleshed Kiwifruit Cultivar with Large Fruit Size. Korean J. Hortic. Sci. 2017, 35, 142–146. [Google Scholar]
- Ferguson, A. Kiwifruit: Evolution of a crop. Acta Hortic. 2011, 913, 31–42. [Google Scholar] [CrossRef]
- Kim, G.H.; Jung, J.S.; Koh, Y.J. Occurrence and Epidemics of Bacterial Canker of Kiwifruit in Korea. Plant Pathol. J. 2017, 33, 351–361. [Google Scholar] [CrossRef]
- Choi, H.R.; Tilahun, S.; Park, D.S.; Lee, Y.M.; Choi, J.H.; Baek, M.W.; Jeong, C.S. Harvest time affects quality and storability of kiwifruit (Actinidia spp.). Sci. Hortic. 2019, 256, 108523. [Google Scholar] [CrossRef]
- Tilahun, S.; Choi, H.R.; Park, D.S.; Lee, Y.M.; Choi, J.H.; Baek, M.W.; Hyok, K.; Park, S.M.; Jeong, C.S. Ripening quality of kiwifruit cultivars is affected by harvest time. Sci. Hortic. 2020, 261, 108936. [Google Scholar] [CrossRef]
- Petkou, I.T.; Pritsa, T.S.; Sfakiotakis, E.M. Effects of polyamines on ethylene production, respiration and ripening of kiwifruit. J. Hortic. Sci. Biotechnol. 2004, 79, 977–980. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Zhang, S.; Ferguson, I. The role of salicylic acid in postharvest ripening of kiwifruit. Postharvest Boil. Technol. 2003, 28, 67–74. [Google Scholar] [CrossRef]
- Antunes, M.D.; Sfakiotakis, E. Effect of high temperature stress on ethylene biosynthesis, respiration and ripening of ‘Hayward’ kiwifruit. Postharvest Boil. Technol. 2000, 20, 251–259. [Google Scholar] [CrossRef]
- Richardson, A.; Boldingh, H.; A McAtee, P.; Gunaseelan, K.; Luo, Z.; Atkinson, R.G.; David, K.; Burdon, J.; Schaffer, R. Fruit development of the diploid kiwifruit, Actinidia chinensis ’Hort16A’. BMC Plant Boil. 2011, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Mitalo, O.W.; Asiche, W.O.; Kasahara, Y.; Tosa, Y.; Owino, W.; Mworia, E.G.; Ushijima, K.; Nakano, R.; Kubo, Y. Characterization of Ripening-related Genes Involved in Ethylene-independent Low Temperature-modulated Ripening in ‘Rainbow Red’ Kiwifruit during Storage and On-vine. Hortic. J. 2018, 87, 421–429. [Google Scholar] [CrossRef]
- Fei, Z.; Tang, X.; Alba, R.M.; White, J.A.; Ronning, C.M.; Martin, G.B.; Tanksley, S.D.; Giovannoni, J.J. Comprehensive EST analysis of tomato and comparative genomics of fruit ripening. Plant J. 2004, 40, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, R.; Crowhurst, R.; Gleave, A.; Rikkerink, E.H.; Allan, A.C.; Beuning, L.L.; Bowen, J.H.; Gera, E.; Jamieson, K.R.; Janssen, B.J.; et al. Analyses of Expressed Sequence Tags from Apple1. Plant Physiol. 2006, 141, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.H.; Lakhwani, D.; Pathak, S.; Gupta, P.; Bag, S.; Nath, P.; Trivedi, P.K. Transcriptome analysis of ripe and unripe fruit tissue of banana identifies major metabolic networks involved in fruit ripening process. BMC Plant Boil. 2014, 14, 316. [Google Scholar] [CrossRef]
- Park, D.S.; Tilahun, S.; Park, K.C.; Choi, I.Y.; Jeong, C.S. Transcriptome analysis of astringent ‘Cheongdo-Bansi’ persimmon fruit treated with ethylene for removal of astringency. Postharvest Boil. Technol. 2019, 150, 52–59. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zeng, S.; Xiao, G.; Wang, G.; Wang, Y.; Peng, M.; Huang, H. Gene expression profiling of development and anthocyanin accumulation in kiwifruit (Actinidia chinensis) based on transcriptome sequencing. PLoS One 2015, 10, e0138743. [Google Scholar]
- Park, D.S.; Tilahun, S.; Heo, J.Y.; Jeong, C.S. Quality and expression of ethylene response genes of ‘Daebong’ persimmon fruit during ripening at different temperatures. Postharvest Boil. Technol. 2017, 133, 57–63. [Google Scholar] [CrossRef]
- Johnson, M.T.J.; Carpenter, E.J.; Tian, Z.; Bruskiewich, R.; Burris, J.N.; Carrigan, C.T.; Chase, M.W.; Clarke, N.D.; Covshoff, S.; Depamphilis, C.W.; et al. Evaluating Methods for Isolating Total RNA and Predicting the Success of Sequencing Phylogenetically Diverse Plant Transcriptomes. PLoS ONE 2012, 7, e50226. [Google Scholar] [CrossRef]
- Kim, S.H. Study on CEACAM1 Mediated Cell Death and Antitumor Effects of Metformin in 5-Fluorouracil Resistant Gastrointestinal Cancer Cells. Ph.D Thesis, Seoul National University, Seoul, Korea, 2017. [Google Scholar]
- Tilahun, S.; Seo, M.H.; Park, D.S.; Jeong, C.S. Effect of cultivar and growing medium on the fruit quality attributes and antioxidant properties of tomato (Solanum lycopersicum L.). Hortic. Environ. Biotechnol. 2018, 59, 215–223. [Google Scholar] [CrossRef]
- Belew, D.; Park, D.S.; Tilahun, S.; Jeong, C.S. The Effects of Treatment with Ethylene-Producing Tablets on the Quality and Storability of Banana (Musa sp.). Korean J. Hortic. Sci. 2016, 34, 746–754. [Google Scholar]
- Tilahun, S.; Park, D.S.; Taye, A.M.; Jeong, C.S. Effects of Storage Duration on Physicochemical and Antioxidant Properties of Tomato (Lycopersicon esculentum Mill.). Korean J. Hortic. Sci. 2017, 35, 88–97. [Google Scholar]
- Tilahun, S.; Park, D.S.; Taye, A.M.; Jeong, C.S. Effect of ripening conditions on the physicochemical and antioxidant properties of tomato (Lycopersicon esculentum Mill.). Food Sci. Biotechnol. 2017, 26, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.H.; Tilahun, S.; Park, D.S.; Melaku, A.; Jeong, C.S. Effect of Ripening Conditions on the Quality and Storability of Muskmelon (Cucumis melo L.) Fruits. Korean J. Hortic. Sci. 2018, 36, 741–755. [Google Scholar]
- Chang, Y.-Y.; Chu, Y.-W.; Chen, C.-W.; Leu, W.-M.; Hsu, H.-F.; Yang, C.-H. Characterization of Oncidium ‘Gower Ramsey’ Transcriptomes using 454 GS-FLX Pyrosequencing and Their Application to the Identification of Genes Associated with Flowering Time. Plant Cell Physiol. 2011, 52, 1532–1545. [Google Scholar] [CrossRef]
- Parchman, T.L.; Geist, K.; Grahnen, J.A.; Benkman, C.W.; Buerkle, C.A. Transcriptome sequencing in an ecologically important tree species: Assembly, annotation, and marker discovery. BMC Genom. 2010, 11, 180. [Google Scholar] [CrossRef]
- Sui, C.; Zhang, J.; Wei, J.-H.; Chen, S.; Li, Y.; Xu, J.; Jin, Y.; Xie, C.-X.; Gao, Z.; Chen, H.; et al. Transcriptome analysis of Bupleurum chinense focusing on genes involved in the biosynthesis of saikosaponins. BMC Genom. 2011, 12, 539. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Zhao, L.; Larson-Rabin, Z.; Li, D.-Z.; Guo, Z.-H. De Novo Sequencing and Characterization of the Floral Transcriptome of Dendrocalamus latiflorus (Poaceae: Bambusoideae). PLoS ONE 2012, 7, e42082. [Google Scholar] [CrossRef]
- Goulao, L.; Oliveira, C. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Bennett, A.B.; Labavitch, J.M. Ethylene and ripening-regulated expression and function of fruit cell wall modifying proteins. Plant Sci. 2008, 175, 130–136. [Google Scholar] [CrossRef]
- Glissant, D.; Dédaldéchamp, F.; Delrot, S. Transcriptomic analysis of grape berry softening during ripening. OENO ONE 2008, 42, 1. [Google Scholar] [CrossRef]
- Figueroa, C.; Pimentel, P.; Gaete, C.; Moya, M.; Herrera, R.; Caligari, P.D.; Moya-León, M.A. Softening rate of the Chilean strawberry (Fragaria chiloensis) fruit reflects the expression of polygalacturonase and pectate lyase genes. Postharvest Boil. Technol. 2008, 49, 210–220. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Polygalacturonase and β-galactosidase activities in Hayward kiwifruit as affected by light exposure, maturity stage and storage time. Sci. Hortic. 2009, 120, 342–347. [Google Scholar] [CrossRef]
- Schroder, R.; Atkinson, R.G. Kiwifruit cell walls: Towards an understanding of softening? NZ. J. For. Sci. 2006, 36, 112–129. [Google Scholar]
- Lubkowitz, M. The Oligopeptide Transporters: A Small Gene Family with a Diverse Group of Substrates and Functions? Mol. Plant 2011, 4, 407–415. [Google Scholar] [CrossRef]
- Sharma, R.; Tan, F.; Jung, K.-H.; Sharma, M.K.; Peng, Z.; Ronald, P.C. Transcriptional dynamics during cell wall removal and regeneration reveals key genes involved in cell wall development in rice. Plant Mol. Boil. 2011, 77, 391–406. [Google Scholar] [CrossRef]
- Li, H.; Suo, J.; Han, Y.; Liang, C.; Jin, M.; Zhang, Z.; Rao, J. The effect of 1-methylcyclopropene, methyl jasmonate and methyl salicylate on lignin accumulation and gene expression in postharvest ‘Xuxiang’ kiwifruit during cold storage. Postharvest Boil. Technol. 2017, 124, 107–118. [Google Scholar] [CrossRef]
- Li, X.-J.; Nakagawa, N.; Sakurai, N. Purification and Biochemical Characterization of Cell Wall-bound Trehalase from Pericarp Tissues of Actinidia deliciosa. J. Jpn. Soc. Hortic. Sci. 2008, 77, 229–235. [Google Scholar] [CrossRef][Green Version]
- Liu, C.-W.; Hsu, Y.-K.; Cheng, Y.-H.; Yen, H.-C.; Wu, Y.-P.; Wang, C.-S.; Lai, C. Proteomic analysis of salt-responsive ubiquitin-related proteins in rice roots. Rapid Commun. Mass Spectrom. 2012, 26, 1649–1660. [Google Scholar] [CrossRef]
- Zenoni, S.; Fasoli, M.; Tornielli, G.B.; Santo, S.D.; Sanson, A.; De Groot, P.; Sordo, S.; Citterio, S.; Monti, F.; Pezzotti, M. Overexpression of PhEXPA1 increases cell size, modifies cell wall polymer composition and affects the timing of axillary meristem development in Petunia hybrida. New Phytol. 2011, 191, 662–677. [Google Scholar] [CrossRef]
- Schröder, R.; Atkinson, R.G.; Redgwell, R.J. Re-interpreting the role of endo-β-mannanases as mannan endotransglycosylase/hydrolases in the plant cell wall. Ann. Bot. 2009, 104, 197–204. [Google Scholar] [CrossRef]
- Saleme, M.D.L.S.; Cesarino, I.; Vargas, L.; Kim, H.; Vanholme, R.; Goeminne, G.; Van Acker, R.; Fonseca, F.; Pallidis, A.; Voorend, W.; et al. Silencing Caffeoyl Shikimate Esterase Affects Lignification and Improves Saccharification in Poplar. Plant Physiol. 2017, 175, 1040–1057. [Google Scholar] [CrossRef]
- Choat, B.; Gambetta, G.; Shackel, K.A.; Matthews, M.A. Vascular Function in Grape Berries across Development and Its Relevance to Apparent Hydraulic Isolation. Plant Physiol. 2009, 151, 1677–1687. [Google Scholar] [CrossRef]
- Gao, F.; Niu, Y.-D.; Hao, J.-F.; Bade, R.; Zhang, L.-Q.; Hasi, A. Identification of Differentially Expressed Genes During Ethylene Climacteric of Melon Fruit by Suppression Subtractive Hybridization. J. Integr. Agric. 2013, 12, 1431–1440. [Google Scholar] [CrossRef]
- Xu, S.; Rahman, A.; Baskin, T.I.; Kieber, J.J. Two Leucine-Rich Repeat Receptor Kinases Mediate Signaling, Linking Cell Wall Biosynthesis and ACC Synthase in Arabidopsis. Plant Cell 2008, 20, 3065–3079. [Google Scholar] [CrossRef]
- Tanaka, H.; Takebayashi, S.I.; Sakamoto, A.; Igata, T.; Nakatsu, Y.; Saitoh, N.; Hino, S.; Nakao, M. The SETD8/PR-Set7 methyltransferase functions as a barrier to prevent senescence-associated metabolic remodeling. Cell Rep. 2017, 18, 2148–2161. [Google Scholar] [CrossRef]
- Weng, L.; Zhao, F.; Li, R.; Xu, C.; Chen, K.; Xiao, H. The Zinc Finger Transcription Factor SlZFP2 Negatively Regulates Abscisic Acid Biosynthesis and Fruit Ripening in Tomato1. Plant Physiol. 2015, 167, 931–949. [Google Scholar] [CrossRef]
- Roy, M.; Wu, R. Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci. 2002, 163, 987–992. [Google Scholar] [CrossRef]
- Apelbaum, A.; Burgoon, A.C.; Anderson, J.D.; Lieberman, M.; Ben-Arie, R.; Mattoo, A.K. Polyamines Inhibit Biosynthesis of Ethylene in Higher Plant Tissue and Fruit Protoplasts. Plant Physiol. 1981, 68, 453–456. [Google Scholar] [CrossRef]
- Fujimoto, S.Y.; Ohta, M.; Usui, A.; Shinshi, H.; Ohme-Takagi, M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 2000, 12, 393–404. [Google Scholar] [PubMed]
- Shi, Y.; Jiang, L.; Zhang, L.; Kang, R.; Yu, Z. Dynamic changes in proteins during apple (Malus x domestica) fruit ripening and storage. Hortic. Res. 2014, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Moing, A.; Rothan, C.; Svanella, L.; Just, D.; Diakou, P.; Raymond, P.; Gaudillère, J.P.; Monet, R. Role of phosphoenolpyruvate carboxylase in organic acid accumulation during peach fruit development. Physiol. Plant. 2000, 108, 1–10. [Google Scholar] [CrossRef]
- Bumee, S.; Ingkasuwan, P.; Kalapanulak, S.; Meechai, A.; Cheevadhanarak, S.; Saithong, T. Transcriptional Regulatory Network of Arabidopsis Starch Metabolism under Extensive Light Condition: A Potential Model of Transcription-modulated Starch Metabolism in Roots of Starchy Crops. Procedia Comput. Sci. 2013, 23, 113–121. [Google Scholar] [CrossRef][Green Version]
- Duque, P.; Barreiro, M.G.; Arrabaça, J.D. Respiratory metabolism during cold storage of apple fruit. I. Sucrose metabolism and glycolysis. Physiol. Plant. 1999, 107, 14–23. [Google Scholar] [CrossRef]
- Wang, J.; De Luca, V. The biosynthesis and regulation of biosynthesis of Concord grape fruit esters, including ‘foxy’ methylanthranilate. Plant J. 2005, 44, 606–619. [Google Scholar] [CrossRef]
- Rippert, P.; Matringe, M. Purification and kinetic analysis of the two recombinant arogenate dehydrogenase isoforms of Arabidopsis thaliana. JBIC J. Boil. Inorg. Chem. 2002, 269, 4753–4761. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, X.-R.; Li, X.; Yang, S.-L.; Ferguson, I.B.; Chen, K.-S. Lipoxygenase Gene Expression in Ripening Kiwifruit in Relation to Ethylene and Aroma Production. J. Agric. Food Chem. 2009, 57, 2875–2881. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, S.; Yu, J.; Zhan, Z.; Li, M.; Liu, G.; Wang, X.; Huang, L. Predicting the Function of 4-Coumarate:CoA Ligase (LJ4CL1) in Lonicera japonica. Int. J. Mol. Sci. 2014, 15, 2386–2399. [Google Scholar] [CrossRef]
- Gershater, M.C.; Edwards, R. Regulating biological activity in plants with carboxylesterases. Encycl. Appl. Plant Sci. 2007, 173, 579–588. [Google Scholar] [CrossRef]
- Yauk, Y.-K.; Ged, C.; Wang, M.Y.; Matich, A.J.; Tessarotto, L.; Cooney, J.; Chervin, C.; Atkinson, R.G. Manipulation of flavour and aroma compound sequestration and release using a glycosyltransferase with specificity for terpene alcohols. Plant J. 2014, 80, 317–330. [Google Scholar] [CrossRef]
- Kumar, V.; Prakash, O.; Kumar, S.; Narwal, S. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef]
- Nishiyama, I.; Yamashita, Y.; Yamanaka, M.; Shimohashi, A.; Fukuda, T.; Oota, T. Varietal Difference in Vitamin C Content in the Fruit of Kiwifruit and OtherActinidiaSpecies. J. Agric. Food Chem. 2004, 52, 5472–5475. [Google Scholar] [CrossRef]
- Wilson, J.X. The physiological role of dehydroascorbic acid. FEBS Lett. 2002, 527, 5–9. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Carr, A.C.; Pullar, J.M.; Bozonet, S.M. The Bioavailability of Vitamin C from Kiwifruit. Adv. Food Nutr. Res. 2013, 68, 125–147. [Google Scholar]
- Shin, N.; Moon, S.-J.; Han, S.; Kim, B.-G.; Park, S.R.; Lee, S.-K.; Yoon, H.-J.; Lee, H.E.; Kwon, H.-B.; Baek, N.; et al. Expression of StMYB1R-1, a Novel Potato Single MYB-Like Domain Transcription Factor, Increases Drought Tolerance. Plant Physiol. 2010, 155, 421–432. [Google Scholar] [CrossRef]
- Lopez, A.P.; Portales, R.B.; Muñoz-Blanco, J.; Rodríguez-Franco, A.; López-Ráez, J.A.; Medina-Escobar, N. Characterization of a strawberry late-expressed and fruit-specific peptide methionine sulphoxide reductase. Physiol. Plant. 2006, 126, 129–139. [Google Scholar] [CrossRef]
- Tkaczuk, K.L.; Shumilin, I.A.; Chruszcz, M.; Evdokimova, E.; Savchenko, A.; Minor, W. Structural and functional insight into the universal stress protein family. Evol. Appl. 2013, 6, 434–449. [Google Scholar] [CrossRef]
- Bansal, N.; Kanwar, S.S. Peroxidase(s) in Environment Protection. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Contreras-Vergara, C.; Muhlia-Almazan, A.; Islas-Osuna, M.; González-Aguilar, G. Expression and enzymatic activity of phenylalanine ammonia-lyase and p-coumarate 3-hydroxylase in mango (Mangifera indica ‘Ataulfo’) during ripening. Genet. Mol. Res. 2014, 13, 3850–3858. [Google Scholar] [CrossRef]
- Boudet, A.-M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Han, X.; Lu, W.; Wei, X.; Li, L.; Mao, L.; Zhao, Y. Proteomics analysis to understand the ABA stimulation of wound suberization in kiwifruit. J. Proteom. 2018, 173, 42–51. [Google Scholar] [CrossRef]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef]
- Queiroz, C.; Mendes Lopes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Polyphenoloxidase: Characteristics and mechanisms of browning control. Food Rev. Int. 2008, 24, 361–375. [Google Scholar] [CrossRef]
- Khanna-Chopra, R.; Sabarinath, S. Heat-stable chloroplastic Cu/Zn superoxide dismutase in Chenopodium murale. Biochem. Biophys. Res. Commun. 2004, 320, 1187–1192. [Google Scholar] [CrossRef]
- Figueiredo, J.; Silva, M.S.; Figueiredo, A. Subtilisin-like proteases in plant defence: The past, the present and beyond. Mol. Plant Pathol. 2017, 19, 1017–1028. [Google Scholar] [CrossRef]
- Martinoia, E.; Klein, M.; Geisler, M.; Bovet, L.; Forestier, C.; Kolukisaoglu, U.; Müller-Röber, B.; Schulz, B. Multifunctionality of plant ABC transporter—More than just detoxifiers. Planta 2002, 214, 345–355. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Krokida, A.; Karagiannis, E.; Belghazi, M.; Vasilakakis, M.; Papadopoulou, K.K.; Molassiotis, A. Ozone-induced inhibition of kiwifruit ripening is amplified by 1-methylcyclopropene and reversed by exogenous ethylene. BMC Plant Boil. 2018, 18, 358. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, R.; Guo, Z.; Yang, S.; Deng, X.-P. Genome-wide identification and characterization of Glyceraldehyde-3-phosphate dehydrogenase genes family in wheat (Triticum aestivum). BMC Genom. 2016, 17, 240. [Google Scholar] [CrossRef]
- Pilkington, S.; Montefiori, M.; Galer, A.L.; Emery, R.J.N.; Allan, A.C.; Jameson, P.E. Endogenous cytokinin in developing kiwifruit is implicated in maintaining fruit flesh chlorophyll levels. Ann. Bot. 2013, 112, 57–68. [Google Scholar] [CrossRef]
- Ha, S.; Vanková, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1819, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kesari, R.; Trivedi, P.K.; Nath, P. Ethylene-induced ripening in banana evokes expression of defense and stress related genes in fruit tissue. Postharvest Boil. Technol. 2007, 46, 136–143. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Li, S.; Zhang, L.; Zou, C.; Yu, D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1819, 120–128. [Google Scholar] [CrossRef]
- Zhu, X.; Pan, L.; Xiao, T.; Ren, X.; Liu, Z. Exogenous niacin treatment increases NADPH oxidase in kiwifruit. Braz. J. Boil. 2018, 78, 686–690. [Google Scholar] [CrossRef]
- Horie, Y.; Ito, H.; Kusaba, M.; Tanaka, R.; Tanaka, A. Participation of Chlorophyll b Reductase in the Initial Step of the Degradation of Light-harvesting Chlorophyll a/b-Protein Complexes in Arabidopsis. J. Boil. Chem. 2009, 284, 17449–17456. [Google Scholar] [CrossRef]
- Wang, X.; Huang, R.; Quan, R. Mutation in Mg-Protoporphyrin IX Monomethyl Ester Cyclase Decreases Photosynthesis Capacity in Rice. PLoS ONE 2017, 12, e0171118. [Google Scholar] [CrossRef]
- Lee, Y.; Nishizawa, T.; Takemoto, M.; Kumazaki, K.; Yamashita, K.; Hirata, K.; Minoda, A.; Nagatoishi, S.; Tsumoto, K.; Ishitani, R.; et al. Structure of the triose-phosphate/phosphate translocator reveals the basis of substrate specificity. Nat. Plants 2017, 3, 825–832. [Google Scholar] [CrossRef]
- Pilkington, S.; Montefiori, M.; Jameson, P.E.; Allan, A.C. The control of chlorophyll levels in maturing kiwifruit. Planta 2012, 236, 1615–1628. [Google Scholar] [CrossRef]
- Keown, J.; Griffin, M.D.W.; Mertens, H.D.T.; Pearce, F. Small Oligomers of Ribulose-bisphosphate Carboxylase/Oxygenase (Rubisco) Activase Are Required for Biological Activity. J. Boil. Chem. 2013, 288, 20607–20615. [Google Scholar] [CrossRef]
- Boggio, S.B.; Palatnik, J.F.; Heldt, H.W.; Valle, E. Changes in amino acid composition and nitrogen metabolizing enzymes in ripening fruits of Lycopersicon esculentum Mill. Plant Sci. 2000, 159, 125–133. [Google Scholar] [CrossRef]
- Binder, S. Branched-Chain Amino Acid Metabolism in Arabidopsis thaliana. Arab. Book 2010, 8, e0137. [Google Scholar] [CrossRef]
- Ringer, K.L.; Davis, E.M.; Croteau, R. Monoterpene metabolism. Cloning, expression, and characterization of (−)-isopiperitenol/(−)-carveol dehydrogenase of peppermint and spearmint. Plant Physiol. 2005, 137, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin-Wang, K.; Deng, C.; Warran, B.; Wang, L.; Yu, B.; Yang, H.; Wang, J.; Espley, R.; Zhang, J.; et al. Comparative Transcriptome Analysis of White and Purple Potato to Identify Genes Involved in Anthocyanin Biosynthesis. PLoS ONE 2015, 10, 0129148. [Google Scholar] [CrossRef] [PubMed]
- Onik, J.C.; Hu, X.; Lin, Q.; Wang, Z. Comparative Transcriptomic Profiling to Understand Pre- and Post-Ripening Hormonal Regulations and Anthocyanin Biosynthesis in Early Ripening Apple Fruit. Molecules 2018, 23, 1908. [Google Scholar] [CrossRef]
- Li, S.-B.; Xie, Z.-Z.; Hu, C.-G.; Zhang, J.-Z. A Review of Auxin Response Factors (ARFs) in Plants. Front. Plant Sci. 2016, 7, 137. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).