Target Site Resistance to Acetolactate Synthase Inhibitors in Diplotaxis erucoides and Erucaria hispanica–Mechanism of Resistance and Response to Alternative Herbicides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Responses to ALS Herbicides
2.3. Plant Responses to Auxinic Herbicides
2.4. DNA Extraction and Molecular Studies
2.5. Statistical Analyses
3. Results and Discussion
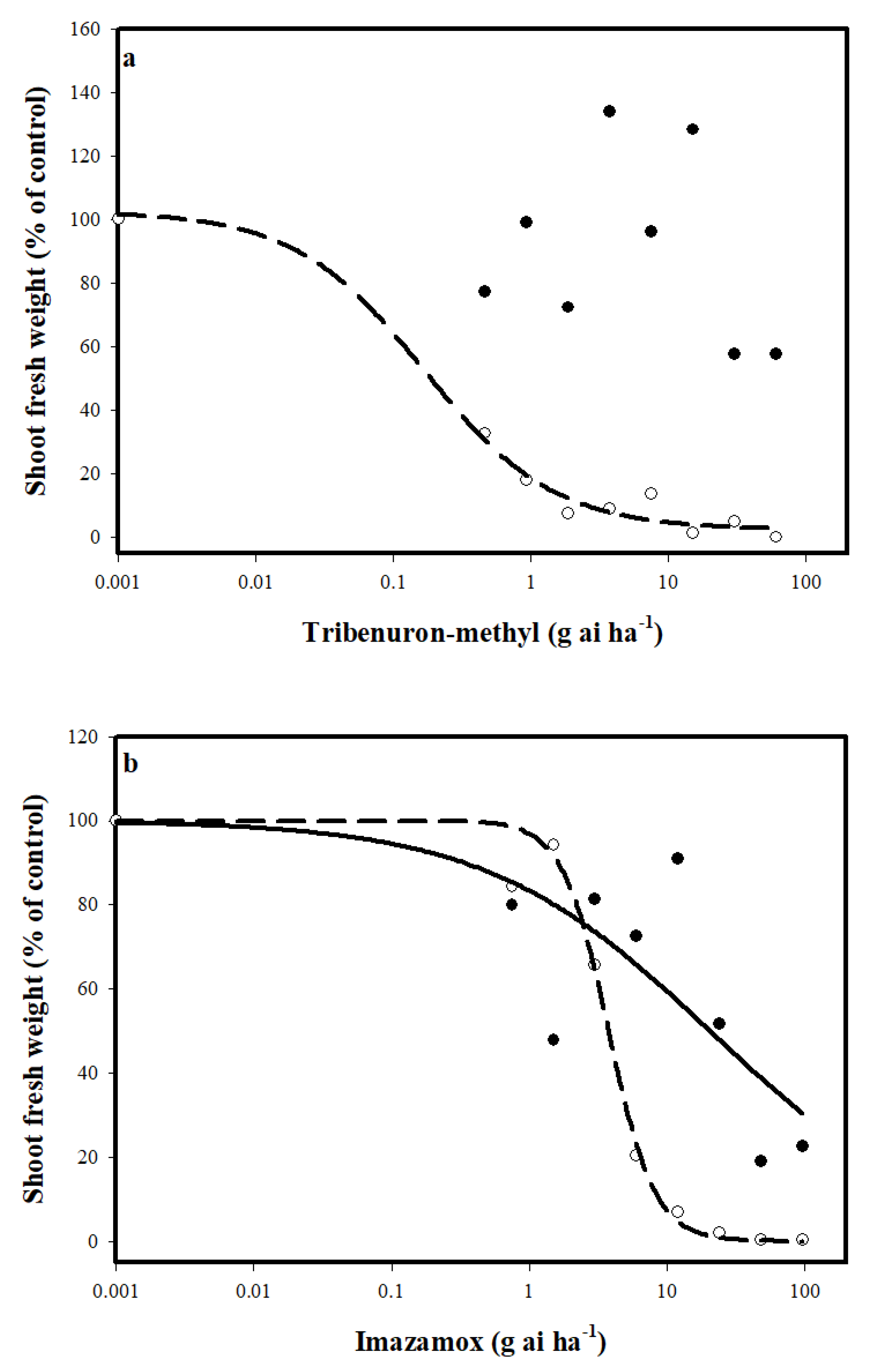
3.1. Response to ALS Iinhibitors
3.2. Mechanism of Resistance to ALS Inhibitors
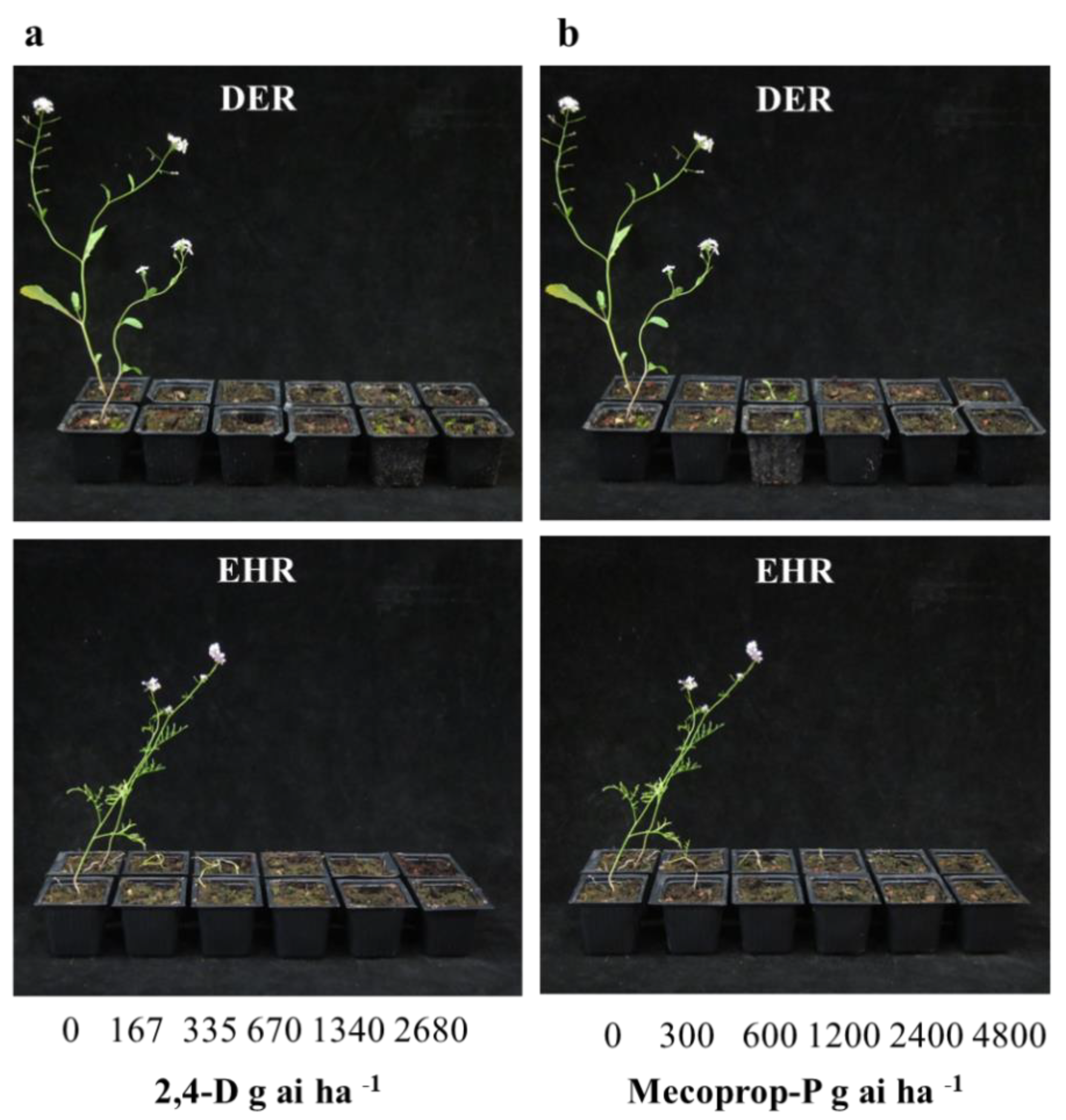
3.3. Alternative Management Using Auxinic Herbicides
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Danin, A. Flora of Israel Online. Available online: http://www.flora.huji.ac.il (accessed on 1 December 2019).
- Marhold, K. Brassicaceae. Euro + Med Plantbase—the Information Resource for Euro-Mediterranean Plant Diversity. 2014. Available online: http://www.bgbm.org/EuroPlusMed/query.asp (accessed on 1 December 2019).
- Aronson, J.; Kigel, J.; Shmida, A.; Klein, J. Adaptive phenology of desert and Mediterranean populations of annual plants grown with and without water stress. Oecologia 1992, 89, 17–26. [Google Scholar] [CrossRef]
- Perez-Garcia, F.; Iriondo, J.M.; Martinezn-Laborde, J.B. Germination behaviour in seeds of Diplotaxis erucoides and D. virgata. Weed Res. 1995, 35, 495–502. [Google Scholar] [CrossRef]
- Kunin, W.E. Density and reproductive success in wild populations of Diplotaxis erucoides (Brassicaceae). Oecologia 1992, 91, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Boaz, M.; Piltman, U.; Heyn, C.C. Reproductive effort in desert versus mediterranean crucifers: The allogamous Erucaria rostrata and E. hispanica and the autogamous Erophila minima. Oecologia 1994, 100, 286–292. [Google Scholar] [CrossRef]
- Délye, C.; Jasieniuk, M.; Le Corre, V. Deciphering the evolution of herbicide resistance in weeds. Trends Genet. 2013, 29, 649–658. [Google Scholar] [CrossRef]
- Brown, H.M. Mode of action, crop selectivity, and soil relations of the sulfonylurea herbicides. Pestic. Sci. 1990, 29, 263–281. [Google Scholar] [CrossRef]
- Duggleby, R.G.; McCourt, J.A.; Guddat, L.W. Structure and mechanism of inhibition of plant acetohydroxyacid synthase. Plant Physiol. Biochem. 2008, 46, 309–324. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.com (accessed on 1 December 2019).
- Rubin, B.; Yaacoby, T.; Schonfeld, M. Triazine resistant grass weeds: Cross resistance with wheat herbicide, a possible threat to cereal crops. In Proceedings of the British Crop Protection Conference—Weeds, Brighton, UK, 18–21 November 1985; pp. 1171–1178. [Google Scholar]
- Sibony, M.; Rubin, B. Molecular basis for multiple resistance to acetolactate synthase-inhibiting herbicides and atrazine in Amaranthus blitoides (prostrate pigweed). Planta 2003, 216, 1022–1027. [Google Scholar] [CrossRef]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Tranel, P.J.; Wright, T.R.; Heap, I. ALS mutations from herbicide-resistant weeds. Available online: http://www.weedscience.com (accessed on 1 December 2019).
- Rubin, B.; Tal, A.; Yasuor, H. The significance and impact of herbicide resistance weeds—A global overview. Acta Herbol. 2004, 13, 277–288. [Google Scholar]
- Yu, Q.; Han, H.; Vila-aiub, M.M.; Powles, S.B. AHAS herbicide resistance endowing mutations: Effect on AHAS functionality and plant growth. J. Exp. Bot. 2010, 61, 3925–3934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldhuis, L.J.; Hall, L.M.; O’Donovan, J.T.; Dyer, W.; Hall, J.C. Metabolism-based resistance of a wild mustard (Sinapis arvensis L.) biotype to ethametsulfuron-methyl. J. Agric. Food Chem. 2000, 48, 2986–2990. [Google Scholar] [CrossRef] [PubMed]
- Rey-Caballero, J.; Menéndez, J.; Osuna, M.; Salas, M.; Torra, J. Target-site and non-target-site resistance mechanisms to ALS inhibiting herbicides in Papaver rhoeas. Pestic. Biochem. Physiol. 2017, 138, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Abdallah, I.; Han, H.; Owen, M.; Powles, S. Distinct non-target site mechanisms endow resistance to glyphosate, ACCase and ALS-inhibiting herbicides in multiple herbicide-resistant Lolium rigidum. Planta 2009, 230, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Serious 1999, 41, 95–98. [Google Scholar]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Feature log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Korboulewsky, N.; Bonin, G.; Massiani, C. Biological and ecophysiological reactions of white wall rocket (Diplotaxis erucoides L.) grown on sewage sludge compost. Environ. Pollut. 2002, 117, 365–370. [Google Scholar] [CrossRef]
- Han, H.; Yu, Q.; Purba, E.; Li, M.; Walsh, M.; Friesen, S.; Powles, S.B. A novel amino acid substitution Ala-122-Tyr in ALS confers high-level and broad resistance across ALS-inhibiting herbicides. Pest Manag. Sci. 2012, 1164–1170. [Google Scholar] [CrossRef]
- Boutsalis, P.; Karotam, J.; Powles, S.B. Molecular basis of resistance to acetolactate synthase-inhibiting herbicides in Sisymbrium orientale and Brassica tournefortii. Pestic. Sci. 1999, 516, 507–516. [Google Scholar] [CrossRef]
- Scarabel, L.; Varotto, S.; Sattin, M. A European biotype of Amaranthus retroflexus cross-resistant to ALS inhibitors and response to alternative herbicides. Weed Res. 2007, 47, 527–533. [Google Scholar] [CrossRef]
- Matzrafi, M.; Lazar, T.W.; Sibony, M.; Rubin, B. Conyza species: Distribution and evolution of multiple target-site herbicide resistances. Planta 2015, 242, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Medd, R. Characterisation of the acetolactate synthase (ALS) gene of Raphanus raphanistrum L. and the molecular assay of mutations associated with herbicide resistance. Plant Sci. 2002, 163, 195–205. [Google Scholar] [CrossRef]
- Kaloumenos, N.S.; Eleftherohorinos, I.G. Identification of a johnsongrass (Sorghum halepense) biotype resistant to ACCase-inhibiting herbicides in Northern Greece. Weed Technol. 2009, 23, 470–476. [Google Scholar] [CrossRef]
- Cruz-Hipolito, H.; Rosario, J.; Ioli, G.; Osuna, M.D.; Smeda, R.J.; González-Torralva, F.; De Prado, R. Resistance mechanism to tribenuron-methyl in white mustard (Sinapis alba) from Southern Spain. Weed Sci. 2013, 61, 341–347. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef]
- Watahiki, M.K.; Yamamoto, K.T. The massugu1 mutation of Arabidopsis identified with failure of auxin-induced growth curvature of hypocotyl confers auxin insensitivity to hypocotyl and leaf. Plant Physiol. 1997, 115, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Goss, G.A.; Dyer, W.E. Physiological characterization of auxinic herbicide-resistant biotypes of kochia (Kochia scoparia). Weed Sci. 2003, 51, 839–844. [Google Scholar] [CrossRef]
- Walsh, M.J.; Owen, M.J.; Powles, S.B. Frequency and distribution of herbicide resistance in Raphanus raphanistrum populations randomly collected across the Western Australian wheatbelt. Weed Res. 2007, 47, 542–550. [Google Scholar] [CrossRef]
- Beckie, H.J.; Blackshaw, R.E.; Low, R.; Hall, L.M.; Sauder, C.A.; Martin, S.; Brandt, R.N.; Shirriff, S.W. Glyphosate- and acetolactate synthase inhibitor–resistant Kochia (Kochia scoparia) in Western Canada. Weed Sci. 2013, 61, 310–318. [Google Scholar] [CrossRef]
- Tranel, P.J.; Riggins, C.W.; Bell, M.S.; Hager, A.G. Herbicide resistances in Amaranthus tuberculatus: A call for new options. J. Agric. Food Chem. 2011, 59, 5808–5812. [Google Scholar] [CrossRef]
- Busi, R.; Powles, S.B. Cross-resistance to prosulfocarb and triallate in pyroxasulfone-resistant Lolium rigidum. Pest Manag. Sci. 2013, 69, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Bay, G.; Pernin, F.; Délye, C. Prevalence of cross- or multiple resistance to the acetyl-coenzyme A carboxylase inhibitors fenoxaprop, clodinafop and pinoxaden in black-grass (Alopecurus myosuroides Huds.) in France. Pest Manag. Sci. 2010, 66, 168–177. [Google Scholar] [CrossRef] [PubMed]


| MOA | Common Name | ALS Chemical Family | Trade Name | Label Field Rate (g ai ha−1) | Manufacturer |
|---|---|---|---|---|---|
| ALS Inhibitors | Tribenuron-methyl | SU | Express® | 15 | DuPont |
| Imazamox | IMI | Pulsar® | 24 | BASF | |
| Florasulam | TP | Darbuka® | 4 | ADAMA-Agan | |
| Propoxycarbazone-sodium | SCT | Olympus® | 45.5 | Bayer | |
| Auxinic Herbicides | 2,4-D | Albar Super® | 670 | ADAMA-Makhteshim | |
| Mecoprop-P | Duplosan® | 1200 | Nufarm |
| Primers | Sequence (5’–3’) | Position | Product | Reference |
|---|---|---|---|---|
| Size (bp) | ||||
| ALS-A F | GCTGATATCCTCGTCGAAGC | 122, 197, 205 | 490 | Arabidopsis thaliana (X51514) and Lolium rigidum (DQ184640.1) |
| ALS-A R | GAATCGGAAGCTGTTGA | |||
| ALS-B F | CGCTGTTGATAAGGCTGACC | 376, 377, 574 | 800 | |
| ALS-B R | ACAAGTATGGCCCAGGAGTC | |||
| ALS-C F | AAGTACTGGTGTCGGGCAAC | 574, 653, 654 | 500 | |
| ALS-C R | GGCAACACATGTTCTGGTG |
| Pop’ | Shoot FW (% of untreated control) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imazamox a | Propoxycarbazone-Sodium b | Tribenuron-methyl c | Florasulam d | |||||||||||||
| X | 4X | ED50 | RI | X | 4X | ED50 | RI | X | 4X | ED50 | RI | X | 4X | ED50 | RI | |
| DER | 21 ± 40 | 16 ± 31 | 23.7 | 14 | 24 ± 10 * | 17 ± 7 * | 14.5 | 72.5 | 47 ± 19 * | 25 ± 17 | 3.4 | 85 | 20 ± 24 | 31 ± 31 | 1 | 5 |
| DES | 0.2 ± 0.1 | 0.2 ± 0.1 | 1.7 | 1.7 ± 0.3 | 0.9 ± 0.4 | 0.2 | 1.5 ± 1 | 2.6 ± 0.7 | 0.04 | 0.6 ± 0.9 | 0.16 ± 0.1 | 0.2 | ||||
| EHR | 51 ± 12 ** | 22 ± 31 | 28.5 | 7 | 55 ± 33 * | 31 ± 27 * | 55.9 | 69.8 | 128 ± 35 ** | 57 ± 64 | >60 | >60 | 106 ± 31 ** | 47 ± 55 | 14.4 | 24 |
| EHS | 2 ± 1.3 | 0.2 ± 0.2 | 4.1 | 0.9 ± 1.1 | 0.5 ± 0.3 | 0.8 | 1.3 ± 0.3 | 0.1 ± 0.1 | 0.4 | 2.6 ± 2.7 | 0.2 ± 0.1 | 0.6 | ||||
| 9 | Survival (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imazamox a | Propoxycarbazone-sodium b | Tribenuron-methyl c | Florasulam d | |||||||||||||
| X | 4X | LD50 | RI | X | 4X | LD50 | RI | X | 4X | LD50 | RI | X | 4X | LD50 | RI | |
| DER | 50 | 25 | 70.30 | 19.42 | 50 | 50 | 69.93 | >182 | 100 | 75 | >60 | >60 | 50 | 75 | 132.17 | >16 |
| DES | 0 | 0 | 3.62 | 0 | 0 | 0.002 | 0 | 0 | 0.01 | 0 | 0 | 1.02 | ||||
| EHR | 100 | 33 | 93.82 | 36.67 | 100 | 50 | 50.00 | >182 | 100 | 50 | 62.45 | >60 | 100 | 75 | >16 | >16 |
| EHS | 0 | 0 | 2.85 | 0 | 0 | 0.002 | 0 | 0 | 0.70 | 0 | 0 | 0.69 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matzrafi, M.; Gerson, O.; Sibony, M.; Rubin, B. Target Site Resistance to Acetolactate Synthase Inhibitors in Diplotaxis erucoides and Erucaria hispanica–Mechanism of Resistance and Response to Alternative Herbicides. Agronomy 2020, 10, 471. https://doi.org/10.3390/agronomy10040471
Matzrafi M, Gerson O, Sibony M, Rubin B. Target Site Resistance to Acetolactate Synthase Inhibitors in Diplotaxis erucoides and Erucaria hispanica–Mechanism of Resistance and Response to Alternative Herbicides. Agronomy. 2020; 10(4):471. https://doi.org/10.3390/agronomy10040471
Chicago/Turabian StyleMatzrafi, Maor, Ofri Gerson, Moshe Sibony, and Baruch Rubin. 2020. "Target Site Resistance to Acetolactate Synthase Inhibitors in Diplotaxis erucoides and Erucaria hispanica–Mechanism of Resistance and Response to Alternative Herbicides" Agronomy 10, no. 4: 471. https://doi.org/10.3390/agronomy10040471
APA StyleMatzrafi, M., Gerson, O., Sibony, M., & Rubin, B. (2020). Target Site Resistance to Acetolactate Synthase Inhibitors in Diplotaxis erucoides and Erucaria hispanica–Mechanism of Resistance and Response to Alternative Herbicides. Agronomy, 10(4), 471. https://doi.org/10.3390/agronomy10040471






