Effect of Natural Aging of Biochar on Soil Enzymatic Activity and Physicochemical Properties in Long-Term Field Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Characteristics
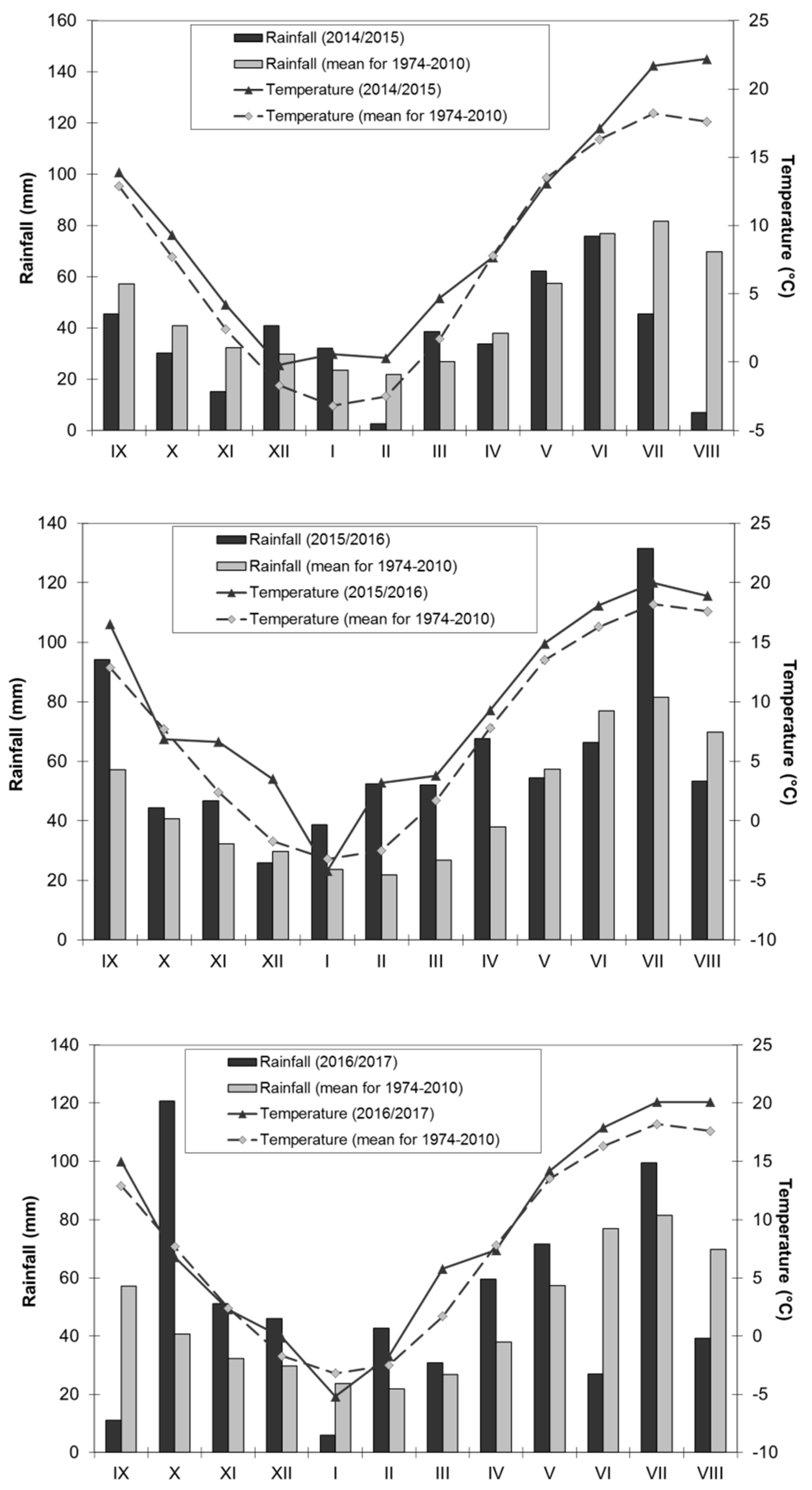
2.2. Weather Conditions
2.3. Experimental Site
2.4. Sample Collection
2.5. Enzymatic Activity and Physicochemical Properties
2.6. Data Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Dehydrogenase Activity
3.3. Phosphatase Activity
3.4. Urease Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard-Nielsen, H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012, 46, 73–79. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Das, K.C.; Kanagaratnam, J.; De Neve, S. Biochar amendment to soils with contrasting organic matter level: Effects on N mineralization and biological soil properties. GCB Bioenergy 2015, 7, 135–144. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Baronti, S.; Lugato, E.; Genesio, L.; Castaldi, S.; Fornasier, F.; Miglietta, F. Biochar as a strategy to sequester carbon and increase yield in durum wheat. Eur. J. Agron. 2011, 34, 231–238. [Google Scholar] [CrossRef]
- Karer, J.; Wimmer, B.; Zehetner, F.; Kloss, S.; Soja, G. Biochar application to temperate soils: Effects on nutrient uptake and crop yield under field conditions. Agric. Food Sci. 2013, 22, 390–403. [Google Scholar] [CrossRef]
- Farrell, M.; Macdonald, L.M.; Butler, G.; Chirino-Valle, I.; Condron, L.M. Biochar and fertiliser applications influence phosphorus fractionation and wheat yield. Biol. Fertil. Soils 2014, 50, 169–178. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Smebye, A.; Alling, V.; Vogt, R.D.; Gadmar, T.C.; Mulder, J.; Cornelissen, G.; Hale, S.E. Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 2016, 142, 100–105. [Google Scholar] [CrossRef]
- Cabrera, A.; Cox, L.; Spokas, K.; Hermosín, M.C.; Cornejo, J.; Koskinen, W.C. Influence of biochar amendments on the sorption–desorption of aminocyclopyrachlor, bentazone and pyraclostrobin pesticides to an agricultural soil. Sci. Total Environ. 2014, 470–471, 438–443. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Futa, B.; Pasieczna-Patkowska, S.; Pałys, E.; Kraska, P. Effect of pesticides on microorganisms, enzymatic activity and plant in biochar amended soil. Geoderma 2014, 214–215, 10–18. [Google Scholar] [CrossRef]
- Vithanage, M.; Bandara, T.; Al-Wabel, M.I.; Abduljabbar, A.; Usman, A.R.A.; Ahmad, M.; Sik, O.Y. Soil enzyme activities in waste biochar amended multi-metal contaminated soil; effect of different pyrolysis temperatures and application rates. Commun. Soil Sci. Plan 2018, 49, 635–643. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.H. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- McHenry, M.P. Agricultural bio-char production, renewable energy generation and farm carbon sequestration in Western Australia: Certainty, uncertainty and risk. Agric. Ecosyst. Environ. 2009, 129, 1–7. [Google Scholar] [CrossRef]
- Lehmann, J.; Rilling, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar- mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Romano, M.; Marzaioli, R.; Baglivo, I.; Baronti, S.; Miglietta, F.; Castaldi, S. Effect of biochar addition on soil microbial community in a wheat crop. Eur. J. Soil Biol. 2014, 60, 9–15. [Google Scholar] [CrossRef]
- Ouyang, L.; Tang, Q.; Yu, L.; Zhang, R. Effects of amendment of different biochars on soil enzyme activities related to carbon mineralization. Soil Res. 2014, 52, 706–716. [Google Scholar] [CrossRef]
- Hairani, A.; Osaki, M.; Watanabe, T. Effect of biochar application on mineral and microbial properties of soils growing different plant species. Soil Sci. Plant Nutr. 2016, 62, 519–525. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Kuśmierz, M. Biochar properties regarding to contaminants content and ecotoxicological assessment. J. Hazard. Mater. 2013, 260, 375–382. [Google Scholar] [CrossRef]
- Shukla, G.; Varma, A. (Eds.) Soil Enzymology. In Soil Biology; Springer Science & Business Media: Berlin, Germany, 2011; Volume 22. [Google Scholar]
- Gianfreda, L.; Rao, M.A. (Eds.) Enzymes in Agricultural Sciences; OMICS International: Hyderabad, India, 2014. [Google Scholar]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia (Jena) 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Makoi, J.H.J.R.; Ndakidemi, P.A. Selected soil enzymes: Examples of their potential roles in the ecosystem. Afr. J. Biotechnol. 2008, 7, 181–191. [Google Scholar]
- Piotrowska, A.; Wilczewski, E. Effects of catch crops cultivated for green manure and mineral nitrogen fertilization on soil enzyme activities and chemical properties. Geoderma 2012, 189, 72–80. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutiérrez, B.; Méndez, A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Majchrzak, L.; Sawinska, Z.; Natywa, M.; Skrzypczak, G.; Głowicka-Wołoszyn, R. Impact of different tillage systems on soil dehydrogenase activity and spring wheat infection. J. Agric. Sci. Technol. 2016, 18, 1871–1881. [Google Scholar]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Kraska, P.; Oleszczuk, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Różyło, K.; Pałys, E.; Gierasimiuk, P.; Michałojć, Z. Effect of various biochar rates on winter rye yield and the concentration of available nutrients in the soil. Plant Soil Environ. 2016, 62, 483–489. [Google Scholar] [CrossRef]
- Kraska, P.; Andruszczak, S.; Oleszczuk, P.; Świeca, M.; Kwiecińska-Poppe, E.; Gierasimiuk, P.; Różyło, K.; Pałys, E. The content of elements and quality parameters of winter rye grain as influenced by biochar-amended soil. Zemdirbyste 2018, 105, 11–20. [Google Scholar] [CrossRef]
- Pranagal, J.; Oleszczuk, P.; Tomaszewska-Krojańska, D.; Kraska, P.; Różyło, K. Effect of biochar application on the physical properties of Haplic Podzol. Soil Tillage Res. 2017, 174, 92–103. [Google Scholar] [CrossRef]
- Verheijen, F.G.A.; Jeffery, S.; Bastos, A.C.; van der Velde, M.; Diafas, I. Biochar Application to Soils—A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; EUR 24099 EN; Office for the Official Publications of the European Communities: Ispra, Italy, 2010; pp. 1–167. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis; International Soil Reference and Information Centre: Wageningen, The Netherlands, 1992. [Google Scholar]
- Skowera, B. Changes of hydrothermal conditions in the polish area (1971–2010). Fragm. Agron. 2014, 31, 74–87. [Google Scholar]
- WRB 2014. World Reference base for Soil Resources 2014; World Soil Resources Reports, 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Thalmann, A. Zur Methodik derestimmung der Dehydrogenase aktivit in Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Zantua, M.I.; Bremner, J.M. Comparison of methods of assaying urease activity in soils. Soil Biol. Biochem. 1975, 7, 291–295. [Google Scholar] [CrossRef]
- International Organization for Standardization. Soil Quality. Determination of Organic Carbon by Sulfochromic Oxidation; ISO, 14235; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- International Organization for Standardization. Soil Quality. Determination of Total Nitrogen Content by Dry Combustion; ISO, 13878; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- International Organization for Standardization. Soil Quality—Determination of Nitrate Nitrogen, Ammonium Nitrogen and Total Soluble Nitrogen in Air-Dry Soils Using Calcium Chloride Solution as Extractant; ISO 14255; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- International Organization for Standardization. Soil Quality. Determination of pH; ISO, 10390; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- Nigussie, A.; Kissi, E.; Misganaw, M.; Ambaw, G. Effect of biochar application on soil properties and nutrient uptake of lettuces (Lactuca sativa) grown in chromium polluted soils. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 369–376. [Google Scholar]
- Raison, R.J. Modification of the soil environment by vegetation fires, with particular reference to nitrogen transformation: A Review. Plant Soil 1979, 51, 73–108. [Google Scholar] [CrossRef]
- Solaiman, Z.N.; Anawar, H.M. Application of biochars for soil constraints: Challenge and solutions. Pedosphere 2015, 25, 631–638. [Google Scholar] [CrossRef]
- Curaqueo, G.; Meier, S.; Khan, N.; Cea, M.; Navia, R. Use of biochar on two volcanic soils: Effects on soil properties and barley yield. J. Soil Sci. Plant Nutr. 2014, 14, 911–924. [Google Scholar] [CrossRef]
- Abrishamkesh, S.; Gorji, M.; Asadi, H.; Bagheri-Marandi, G.H.; Pourbabaee, A.A. Effects of rice husk biochar application on the properties of alkaline soil and lentil growth. Plant Soil Environ. 2015, 61, 475–482. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Clough, T.; Condron, L. Biochar and the Nitrogen Cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef]
- Clough, T.; Condron, L.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Zwydak, M. The relationship between soil properties, enzyme activity and land use. For. Res. Pap. 2017, 78, 39–44. [Google Scholar] [CrossRef]
- Gałązka, A.; Gawryjołek, K.; Perzyński, A.; Gałązka, R.; Księżak, J. Changes in Enzymatic Activities and Microbial Communities in Soil under Long-Term Maize Monoculture and Crop Rotation. Pol. J. Environ. Stud. 2017, 26, 39–46. [Google Scholar] [CrossRef]
- Sopeña, F.; Bending, G.D. Impacts of biochar on bioavailability of the fungicide azoxystrobin: A comparison of the effect on biodegradation rate and toxicity to the fungal community. Chemosphere 2013, 91, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska, M.; Włodarczyk, T. Enzymes of intracellular redox trasformations (Oxidoreductases). Acta Agrophysica Rozprawy i Monografie 2005, 3, 11–26. [Google Scholar]
- Bielińska, E.J. Methods of determination of photosphatase activity. Acta Agrophysica Rozprawy i Monografie 2005, 3, 63–74. [Google Scholar]
- Nannipieri, P.; Grego, S.; Ceccanti, B. Ecological Significance of the Biological Activity in Soil. In Soil Biochemistry; Bollag, J.M., Stotzky, G., Eds.; Marcek Dekker: New York, NY, USA, 1990; Volume 6, pp. 293–355. [Google Scholar]
- Dick, W.A.; Tabatabai, M.A. Significance and Potential Uses o Soil Enzymes. In Soil Microbial Ecology: Application in Agricultural and Environmental Management; Metting, F.B., Ed.; Marcel Dekker: New York, NY, USA, 1993; pp. 95–125. [Google Scholar]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Chen, S.; Wang, R.; Xu, P.; Cheng, M.; Zhang, C.; Xue, W. Biochar facilitated the phytoremediation of cadmium contaminated sediments: Metal behavior, plant toxicity, and microbial activity. Sci. Total Environ. 2019, 666, 1126–1133. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Caster, M.R.; Augers, D.A.; Monreal, C.M.; Ellest, B.H. Towards a minimum data set to asses soil organic matter quality in agricultural soils. Can. J. Soil Sci. 1994, 74, 367–385. [Google Scholar] [CrossRef]
- Bremner, J.M.; Douglas, L.A. Inhibition of urease activity in soils. Soil Biol. Biochem. 1971, 3, 297–307. [Google Scholar] [CrossRef]
- Vahed, H.S.; Shahinrokhsar, P.; Rezaei, M. Influence of some soil properties and temperature on urease activity in Wetland Rice soils. Am. Eurasian J. Agric. Environ. Sci. 2011, 11, 310–313. [Google Scholar]
- Natywa, M.; Selwet, M.; Maciejewski, T. Effect of some agrotechnical factors on the number and activity soil microorganisms. Fragm. Agron. 2014, 31, 56–63. [Google Scholar]

| Year | III | IV | V | VI | VII | VIII |
|---|---|---|---|---|---|---|
| 2015 | 2.73 | 1.47 | 4.75 | 0.30 | 0.70 | 0.10 |
| 2016 | 4.49 | 2.40 | 1.23 | 1.23 | 2.20 | 0.94 |
| 2017 | 1.79 | 2.66 | 1.67 | 0.50 | 1.66 | 0.65 |
| Assessment Date/Year | Biochar Rate | pHKCl | TOC | Nt | C:N | N-NH4+ | N-NO3− |
|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | ||||||
| After 48 months | BC0 | 5.38 a | 5.95 a* | 0.47 a | 12.75 a | 16.17 a | 57.53 a |
| BC10 | 6.76 b | 6.32 b | 0.56 b | 11.35 b | 13.67 b | 69.24 b | |
| BC20 | 6.85 b | 7.41 c | 0.64 c | 11.52 b | 10.54 c | 80.81 c | |
| BC30 | 7.15 c | 7.59 d | 0.69 d | 11.05 c | 9.16 d | 77.62 d | |
| After 60 months | BC0 | 5.37 a | 5.94 a | 0.46 a | 12.91 a | 11.68 a | 74.81 a |
| BC10 | 6.38 b | 6.48 b | 0.63 b | 10.32 b | 9.30 b | 90.44 b | |
| BC20 | 6.65 b | 7.42 c | 0.68 c | 10.99 c | 4.59 c | 101.32 c | |
| BC30 | 6.73 b | 7.79 d | 0.71 d | 10.90 c | 3.01 d | 92.85 d | |
| After 72 months | BC0 | 5.40 a | 5.93 a | 0.47 a | 12.63 a | 10.86 a | 121.29 a |
| BC10 | 5.72 b | 6.56 b | 0.65 b | 10.02 b | 8.65 b | 131.31 b | |
| BC20 | 5.99 c | 7.78 c | 0.70 c | 11.17 c | 3.94 c | 190.89 c | |
| BC30 | 6.16 d | 7.88 c | 0.74 d | 10.68 d | 2.07 d | 142.89 d | |
| After 48 months | Average for biochar rates | 6.82 a | 0.59 a | 11.58 a | 12.38 a | 71.30 a | |
| After 60 months | 6.91 a | 0.62 b | 11.28 a | 7.14 b | 89.86 b | ||
| After 72 months | 7.04 a | 0.64 c | 11.00 a | 6.38 b | 146.60 c | ||
| Assessment Date/Year | Biochar Rate | ADh | APh | AU |
|---|---|---|---|---|
| After 48 months | BC0 | 1.99 a* | 81.27 a | 2.86 a |
| BC10 | 3.66 b | 92.63 b | 3.43 b | |
| BC20 | 4.12 c | 97.42 c | 4.42 c | |
| BC30 | 7.30 d | 95.38 d | 4.32 c | |
| After 60 months | BC0 | 1.66 a | 59.44 a | 2.36 a |
| BC10 | 3.07 b | 60.37 b | 2.88 b | |
| BC20 | 3.38 c | 81.01 c | 4.28 c | |
| BC30 | 5.17 d | 65.41 d | 3.43 d | |
| After 72 months | BC0 | 1.25 a | 43.03 a | 2.05 a |
| BC10 | 2.80 b | 49.21 b | 2.32 b | |
| BC20 | 3.29 c | 59.32 c | 2.98 c | |
| BC30 | 5.04 d | 53.81 d | 2.51 d | |
| After 48 months | Average for biochar rates | 4.27 a | 91.67 a | 3.76 a |
| After 60 months | 3.32 a | 66.56 b | 3.24 b | |
| After 72 months | 3.10 a | 51.34 c | 2.47 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Futa, B.; Oleszczuk, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Kraska, P. Effect of Natural Aging of Biochar on Soil Enzymatic Activity and Physicochemical Properties in Long-Term Field Experiment. Agronomy 2020, 10, 449. https://doi.org/10.3390/agronomy10030449
Futa B, Oleszczuk P, Andruszczak S, Kwiecińska-Poppe E, Kraska P. Effect of Natural Aging of Biochar on Soil Enzymatic Activity and Physicochemical Properties in Long-Term Field Experiment. Agronomy. 2020; 10(3):449. https://doi.org/10.3390/agronomy10030449
Chicago/Turabian StyleFuta, Barbara, Patryk Oleszczuk, Sylwia Andruszczak, Ewa Kwiecińska-Poppe, and Piotr Kraska. 2020. "Effect of Natural Aging of Biochar on Soil Enzymatic Activity and Physicochemical Properties in Long-Term Field Experiment" Agronomy 10, no. 3: 449. https://doi.org/10.3390/agronomy10030449
APA StyleFuta, B., Oleszczuk, P., Andruszczak, S., Kwiecińska-Poppe, E., & Kraska, P. (2020). Effect of Natural Aging of Biochar on Soil Enzymatic Activity and Physicochemical Properties in Long-Term Field Experiment. Agronomy, 10(3), 449. https://doi.org/10.3390/agronomy10030449






