New Eco-Friendly Polymeric-Coated Urea Fertilizers Enhanced Crop Yield in Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Fertilizer Treatments
2.3. Soil Fertility Characterization
2.4. Plant Growth
2.5. Leaf Greenness and Effective Quantum Yield of Photosystem II
2.6. Gas Exchange Analysis
2.7. Foliar Nutrient Analysis
2.8. Growth, Yield, and Cereal Grain Composition
2.9. Statistics
3. Results
3.1. Plant Growth, Leaf Greenness and Effective Quantum Yield of Photosystem II
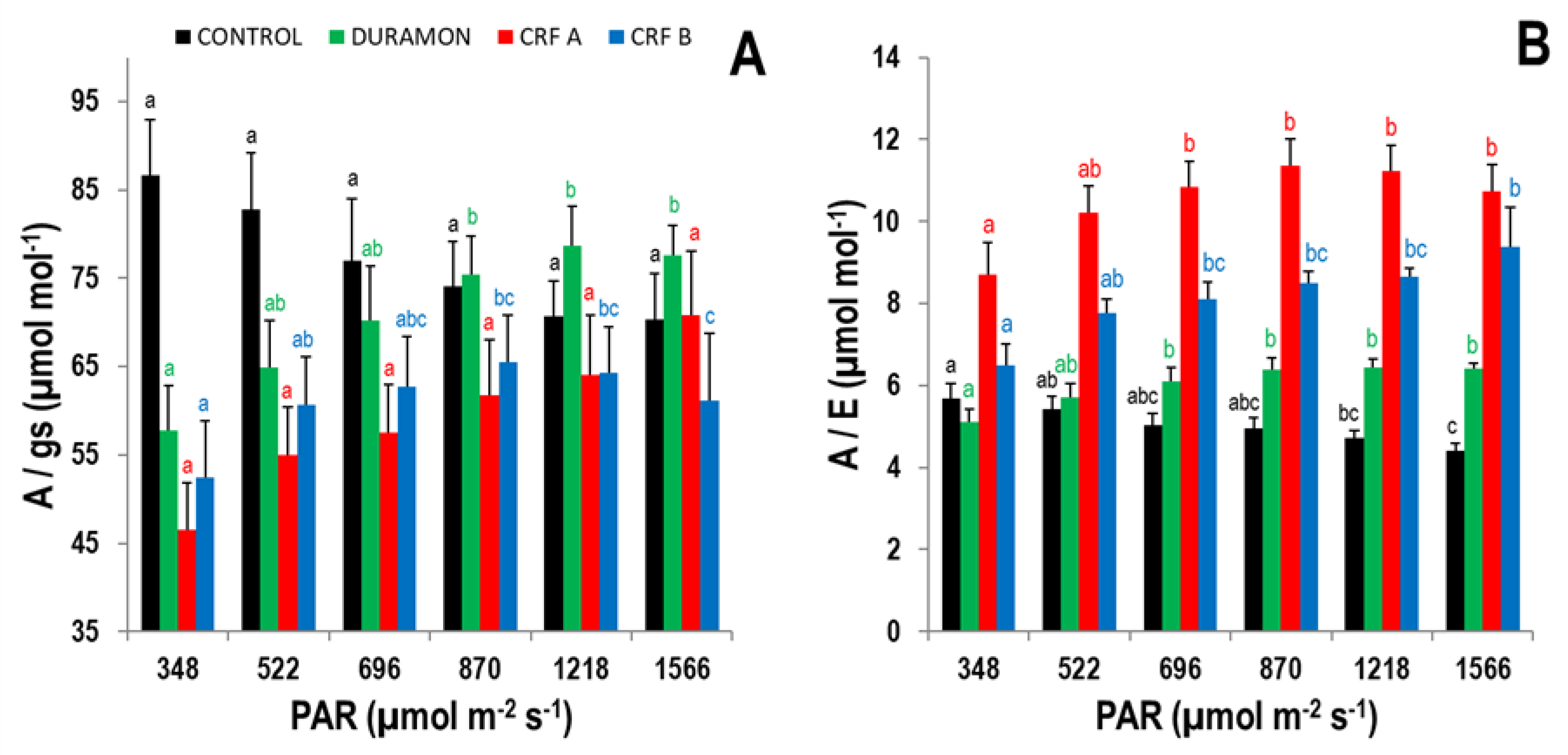
3.2. Gas Exchange Analysis
3.3. Foliar Nutrient Content
3.4. Growth, Yield, and Cereal Grain Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 23 February 2020).
- Feng, J.F.; Li, F.B.; Deng, A.X.; Feng, X.M.; Fang, F.P.; Zhang, W.J. Integrated assessment of the impact of enhanced-efficiency nitrogen fertilizer on N2O emission and crop yield. Agric. Ecosyst. Environ. 2016, 231, 218–228. [Google Scholar] [CrossRef]
- Zuk-Golaszewska, K.; Zeranskal, A.; Krukowska, A.; Bojarczuk, J. Biofortification of the nutritional value of foods from the grain of Triticum durum desf. by an agrotechnical method: A scientific review. J. Elem. 2016, 21, 963–975. [Google Scholar]
- Barakat, M.; Cheviron, B.; Angulo-Jaramillo, R. Influence of the irrigation technique and strategies on the nitrogen cycle and budget: A review. Agric. Water Manage. 2016, 178, 225–238. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; MacDonald, N.W.; Pregitzer, K.S. Soil temperature, matric potential, and the kinetics of microbial respiration and nitrogen mineralization. Soil Sci. Soc. Am. J. 1999, 63, 575–584. [Google Scholar] [CrossRef]
- Achat, D.L.; Augusto, L.; Gallet-Budynek, A.; Loustau, D. Future challenges in coupled C–N–P cycle models for terrestrial ecosystems under global change: A review. Biogeochemistry 2016, 131, 173–202. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: A review. J. Soils Sediments 2016, 16, 1401–1420. [Google Scholar] [CrossRef]
- Akelah, A. Novel utilizations of conventional agrochemicals by controlled release formulations. Mater. Sci. Eng. C-Biomimetic Mater. Sens. Syst. 1996, 4, 83–98. [Google Scholar] [CrossRef]
- Shaviv, A.; Mikkelsen, R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation - A review. Fertil. Res. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Ni, X.Y.; Wu, Y.J.; Wu, Z.Y.; Wu, L.; Qiu, G.N.; Yu, L.X. A novel slow-release urea fertiliser: Physical and chemical analysis of its structure and study of its release mechanism. Biosyst. Eng. 2013, 115, 274–282. [Google Scholar]
- Prasad R, R.G.B.; Lakhdive, B.A. Nitrification retarders and slow-release nitrogen fertilizers. Adv. Agron. 1971, 23, 337–383. [Google Scholar]
- Wang, Z.H.; Miao, Y.F.; Li, S.X. Wheat responses to ammonium and nitrate N applied at different sown and input times. Field Crops Res. 2016, 199, 10–20. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Academic Press Inc: San Diego, CA, USA, 2009; Volume 102, pp. 267–322. [Google Scholar]
- Diez, J.A.; Caballero, R.; Bustos, A.; Roman, R.; Cartagena, M.C.; Vallejo, A. Control of nitrate pollution by application of controlled release fertilizer (CRF), compost and an optimized irrigation system. Fertil. Res. 1996, 43, 191–195. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Snyder, C.S.; Blaylock, A.D.; Del Grosso, S.J. Enhanced-Efficiency Nitrogen Fertilizers: Potential role in nitrous oxide emission mitigation. Agron. J. 2014, 106, 715–722. [Google Scholar] [CrossRef]
- Carson, L.C.; Ozores-Hampton, M. Factors affecting nutrient availability, placement, rate, and application timing of controlled-release fertilizers for Florida vegetable production using seepage irrigation. Horttechnology 2013, 23, 553–562. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [PubMed]
- Herrera, J.M.; Rubio, G.; Haner, L.L.; Delgado, J.A.; Lucho-Constantino, C.A.; Islas-Valdez, S.; Pellet, D. Emerging and Established Technologies to Increase Nitrogen Use Efficiency of Cereals. Agronomy-Basel 2016, 6, 25. [Google Scholar] [CrossRef]
- Dou, H.; Alva, A.K. Nitrogen uptake and growth of two citrus rootstock seedlings in a sandy soil receiving different controlled-release fertilizer sources. Biol. Fert. Soils 1998, 26, 169–172. [Google Scholar] [CrossRef]
- Razali, R.; Daud, H.; Nor, S.M. Modelling and Simulation of Nutrient Dispersion from Coated Fertilizer Granules. In Proceedings of the 3rd International Conference on Fundamental and Applied Sciences, Kuala Lumpur, Malaysia, 3–5 June 2014; pp. 442–448. [Google Scholar]
- Feng, C.; Lu, S.Y.; Gao, C.M.; Wang, X.G.; Xu, X.B.; Bai, X.; Gao, N.N.; Liu, M.Z.; Wu, L. "Smart" fertilizer with temperature- and pH-responsive behavior via surface-initiated polymerization for controlled release of nutrients. ACS Sustain. Chem. Eng. 2015, 3, 3157–3166. [Google Scholar] [CrossRef]
- Kenawy, E.R. Recent advances in controlled release of argochemicals. J. Macromol. Sci.-Rev. Macromol. Chem. Phys. 1998, C38, 365–390. [Google Scholar] [CrossRef]
- Dubey, S.; Jhelum, V.; Patanjali, P.K. Controlled release agrochemicals formulations: A review. J. Sci. Ind. Res. India 2011, 70, 105–112. [Google Scholar]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–96. [Google Scholar] [CrossRef]
- Chowdhury, M.A. The controlled release of bioactive compounds from lignin and lignin-based biopolymer matrices. Int. J. Biol. Macromol. 2014, 65, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Perez, M.; Garrido-Herrera, F.J.; Gonzalez-Pradas, E.; Villafranca-Sanchez, M.; Flores-Cespedes, F. Lignin and ethylcellulose as polymers in controlled release formulations of urea. J. Appl. Polym. Sci. 2008, 108, 3796–3803. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Birrenkott, B.A.; Craig, J.L.; McVey, G.R. A leach collection system to track the release of nitrogen from controlled-release fertilizers in container ornamentals. Hortscience 2005, 40, 1887–1891. [Google Scholar] [CrossRef]
- Clark, M.J.; Zheng, Y.B. Species-specific fertilization can benefit container nursery crop production. Can. J. Plant Sci. 2015, 95, 251–262. [Google Scholar] [CrossRef]
- Cox, D.A. Reducing nitrogen leaching-losses from containerized plants - the effectiveness of controlled-release fertilizers. J. Plant Nutr. 1993, 16, 533–545. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. The effects of biochar, compost and their mixture and nitrogen fertilizer on yield and nitrogen use efficiency of barley grown on a Nitisol in the highlands of Ethiopia. Sci. Total Environ. 2016, 569–570, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Huett, D.O.; Gogel, B.J. Longevities and nitrogen, phosphorus, and potassium release patterns of polymer-coated controlled-release fertilizers at 30 degrees C and 40 degrees C. Commun. Soil Sci. Plan. 2000, 31, 959–973. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.G.; Li, H.; Qiu, J.J.; Liu, H.Y. Impacts of fertilization alternatives and crop straw incorporation on N2O emissions from a spring maize field in Northeastern China. J. Integr. Agric. 2014, 13, 881–892. [Google Scholar] [CrossRef]
- Harrison, R.; Webb, J. A review of the effect of N fertilizer type on gaseous emissions. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Academic Press Inc: San Diego, CA, USA, 2001; Volume 73, pp. 65–108. [Google Scholar]
- Obreza, T.A.; Rouse, R.E.; Sherrod, J.B. Economics of controlled-release fertilizer use on young citrus trees. J. Prod. Agric. 1999, 12, 69–73. [Google Scholar] [CrossRef]
- Garcia, C.; Vallejo, A.; Diez, J.A.; Garcia, L.; Cartagena, M.C. Nitrogen use efficiency with the application of controlled release fertilizers coated with kraft pine lignin. Soil Sci. Plant Nutr. 1997, 43, 443–449. [Google Scholar] [CrossRef]
- Treinyte, J.; Grazuleviciene, V.; Ostrauskaite, J. Biodegradable polymer composites with nitrogen- and phosphorus-containing waste materials as the fillers. Ecol. Chem. Eng. S. 2014, 21, 515–528. [Google Scholar]
- Medina, L.C.; Sartain, J.B.; Obreza, T.A.; Hall, W.L.; Thiex, N.J. Evaluation of a soil incubation method to characterize nitrogen release patterns of slow- and controlled-release fertilizers. J. AOAC Int. 2014, 97, 643–660. [Google Scholar] [CrossRef]
- Gasparin, E.; Araujo, M.M.; Saldanha, C.W.; Tolfo, C.V. Controlled release fertilizer and container volumes in the production of Parapiptadenia rigida (Benth.) Brenan seedlings. Acta Sci.-Agron. 2015, 37, 473–481. [Google Scholar] [CrossRef]
- Haver, D.L.; Schuch, U.K. Production and postproduction performance of two New Guinea Impatiens cultivars grown with controlled-release fertilizer and no leaching. J. Am. Soc. Hortic. Sci. 1996, 121, 820–825. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Salifu, K.F.; Seifert, J.R. Growth and nutritional response of hardwood seedlings to controlled-release fertilization at outplanting. For. Ecol. Manage. 2005, 214, 28–39. [Google Scholar] [CrossRef]
- Kaplan, L.; Tlustos, P.; Szakova, J.; Najmanova, J. The influence of slow-release fertilizers on potted chrysanthemum growth and nutrient consumption. Plant Soil Environ. 2013, 59, 385–391. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yano, T.; Sugiura, M.; Nagasaki, Y. Effects of controlled-release fertilizer on leaf area index and fruit yield in high-density soilless tomato culture using low node-order pinching. PLoS ONE 2014, 9, 10. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yamazaki, H.; Inamoto, K.; Yamazaki, H. Analysis of yield components and dry matter production in a simplified soilless tomato culture system by using controlled-release fertilizers during summer-winter greenhouse production. Sci. Hortic. 2016, 202, 17–24. [Google Scholar] [CrossRef]
- Oliet, J.; Planelles, R.; Segura, M.L.; Artero, F.; Jacobs, D.F. Mineral nutrition and growth of containerized Pinus halepensis seedlings under controlled-release fertilizer. Sci. Hortic. 2004, 103, 113–129. [Google Scholar] [CrossRef]
- Pack, J.E.; Hutchinson, C.M.; Simonne, E.H. Evaluation of controlled-release fertilizers for northeast Florida chip potato production. J. Plant Nutr. 2006, 29, 1301–1313. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar]
- Cacco, G.; Attina, E.; Gelsomino, A.; Sidari, M. Effect of nitrate and humic substances of different molecular size on kinetic parameters of nitrate uptake in wheat seedlings. J. Plant Nutr. Soil Sci. 2000, 163, 313–320. [Google Scholar] [CrossRef]
- Nardi, S.; Ertani, A.; Francioso, O. Soil-root cross-talking: The role of humic substances. J. Plant Nutr. Soil Sci. 2017, 180, 5–13. [Google Scholar] [CrossRef]
- Michalak, I.; Gorka, B.; Wieczorek, P.P.; Roj, E.; Lipok, J.; Leska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzynska-Inger, A.; et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Lotze, E.; Hoffman, E.W. Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 2016, 28, 1379–1386. [Google Scholar] [CrossRef]
- Gaju, O.; DeSilva, J.; Carvalho, P.; Hawkesford, M.J.; Griffiths, S.; Greenland, A.; Foulkes, M.J. Leaf photosynthesis and associations with grain yield, biomass and nitrogen-use efficiency in landraces, synthetic-derived lines and cultivars in wheat. Field Crops Res. 2016, 193, 1–15. [Google Scholar] [CrossRef]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liu, G.; Ma, J.; Xu, H.; Yagi, K. Effect of controlled-release fertilizer on nitrous oxide emission from a winter wheat field. Nutr. Cycl. Agroecosyst. 2012, 94, 111–122. [Google Scholar] [CrossRef]
- Zhang, J.S.; Wang, C.Q.; Li, B.; Liang, J.Y.; He, J.; Xiang, H.; Yin, B.; Luo, J. Effects of controlled release blend bulk urea on soil nitrogen and soil enzyme activity in wheat and rice fields. Chin. J. Appl. Ecol. 2017, 28, 1899–1908. [Google Scholar]
- Jones, J.B. Plant tissue analysis in micronutrients. In Micronutrients in Agriculture, 2nd ed.; Mortvedt, J.J., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1991; pp. 477–521. [Google Scholar]
- Grunes, D.L. Effect of nitrogen on the availability of soil and fertilizer phosphorus to plants. Adv. Agron. 1959, 11, 369–396. [Google Scholar]
- Zhao, B.; Dong, S.T.; Zhang, J.W.; Liu, P. Effects of controlled-release fertiliser on nitrogen use efficiency in summer maize. PLoS ONE 2013, 8, 8. [Google Scholar] [CrossRef]
- Dong, Y.J.; He, M.R.; Wang, Z.L.; Chen, W.F.; Hou, J.; Qiu, X.K.; Zhang, J.W. Effects of new coated release fertilizer on the growth of maize. J. Soil Sci. Plant Nutr. 2016, 16, 637–649. [Google Scholar] [CrossRef][Green Version]
- Mi, W.H.; Yang, X.; Wu, L.H.; Ma, Q.X.; Liu, Y.L.; Zhang, X. Evaluation of nitrogen fertilizer and cultivation methods for agronomic performance of rice. Agron. J. 2016, 108, 1907–1916. [Google Scholar] [CrossRef]
- Roshanravan, B.; Soltani, S.M.; Mahdavi, F.; Rashid, S.A.; Yusop, M.K. Preparation of encapsulated urea-kaolinite controlled release fertiliser and their effect on rice productivity. Chem. Speciation Bioavailab. 2014, 26, 249–256. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M.; Nakanishi, H.; Nishizawa, N.K.; Hasegawa, K.; Mori, S. Co-situs application of controlled-release fertilizers to alleviate iron chlorosis of Paddy rice grown in calcareous soil. Soil Sci. Plant Nutr. 2004, 50, 1013–1021. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M.; Nishizawa, N.K.; Mori, S. Importance of contact between rice roots and co-situs applied fertilizer granules on iron absorption by paddy rice in a calcareous paddy soil. Soil Sci. Plant Nutr. 2008, 54, 467–472. [Google Scholar] [CrossRef]



| Parameters | Mean ± SD (%) | |
|---|---|---|
| Microscale | Field | |
| Total nitrogen (g 100 g−1) | 0.09 ± 0.02 | 0.19 ± 0.03 |
| Total carbon (g 100 g−1) | 2.06 ± 0.28 | 6.76 ± 0.34 |
| Organic carbon (g 100 g−1) | 0.66 ± 0.07 | 1.84 ± 0.28 |
| pH | 8.75 ± 0.095 | 8.26 ± 0.11 |
| EC (µS cm−1) | 120.7± 27.99 | 109.12 ± 47.40 |
| P (g 100 g−1) | 0.064 ± 0.001 | 0.03 ± 0.01 |
| K (g 100 g−1) | 0.339 ± 0.14 | 0.74 ± 0.08 |
| Mg (g 100 g−1) | 0.285 ± 0.04 | 0.26 ± 0.04 |
| Ca (g 100 g−1) | 3.561 ± 0.42 | 1.35 ± 0.61 |
| Fe (g 100 g−1) | 11.113 ± 1.77 | 15.94 ± 6.27 |
| Cu (mg kg−1) | 15.929 ± 1.45 | 7.46 ± 1.81 |
| Mn (mg kg−1) | 191.99 ± 11.68 | 216.75 ± 73.57 |
| Zn (mg kg−1) | 26.951 ± 2.50 | 24.66 ± 4.61 |
| Parameters | CRFA | CRFB | DURAMON® | CONTROL |
|---|---|---|---|---|
| ΦPSII | 0.69 ± 0.03 b | 0.69 ± 0.03 b | 0.68 ± 0.03 b | 0.58 ± 0.07 a |
| Leaf greenness content (SPAD units) | 54.5 ± 2.3 b | 52.5 ± 2.3 b | 54 ± 1.5 b | 40.2 ± 6.8 a |
| N content (%) | 5.5 ± 0.4 b | 5 ± 0.3 ab | 5.3 ± 0.4 ab | 3.5 ± 0.9 a |
| Total fresh weight (aerial part) (g) | 58.9 ± 9.3 b | 46.9 ± 20.9 ab | 79.6 ± 15.9 c | 38.5 ± 10.5 a |
| Dry weight (aerial part) (%) | 29 ± 8 a | 33.3 ± 7.6 ab | 35.4 ± 3.2 ab | 32.9 ± 3.5 b |
| PAFL fresh weight (g) | 24.5 ± 12.7 b | 26.6 ± 25.5 b | 27.2 ± 9.2 b | 2.8 ± 3.8 a |
| PAFL dry weight (%) | 6.13 ± 3.02 b | 6.67 ± 5.81 b | 7.23 ± 2.09 b | 0.89 ± 1.13 a |
| PALF area (cm2) | 200.2 ± 69.8 b | 156.9 ± 96.7 b | 229.5 ± 72.7 b | 67.6 ± 43.1 a |
| LAI | 1 ± 0.3 b | 0.8 ± 0.5 b | 1.1 ± 0.4 b | 0.3 ± 0.2 a |
| Parameters | CRFA | CRFB | DURAMON® | CONTROL |
|---|---|---|---|---|
| Total dry weight (aerial part) (g) | 40.2 ± 3.4 b | 36.7 ± 6.0 ab | 41.4 ± 7.7 b | 31.7 ± 3.9 a |
| Primary stem length (cm) | 60.8 ± 2.1 a | 61.1 ± 3.0 a | 53.3 ± 7.9 a | 53.6 ± 10.0 a |
| Tillers number | 10.0 ± 0.9 a | 10.2 ± 1.6 a | 9.8 ± 2.8 a | 11.5 ± 2.4 a |
| Ears number | 10.7 ± 1.3 a | 9.8 ± 1.1 a | 9.7 ± 2.3 a | 9.2 ± 2.1 a |
| Ear weight (g) | 2.7 ± 0.2 ab | 2.4 ± 0.2 ab | 3.0 ± 0.3 b | 2.0 ± 0.2 a |
| Ear length (cm) | 13.4 ± 0.4 ab | 13.2 ± 0.9 ab | 13.9 ± 0.3 b | 12.9 ± 0.9 a |
| Total dry grain weight (g) | 22.6 ± 2.0 c | 18.6 ± 2.7 b | 20.4 ± 3.2 bc | 15.9 ± 3.0 a |
| Grain weight (n = 100) | 4.8 ± 0.2 b | 4.8 ± 0.4 b | 4.8 ± 0.3 b | 4.4 ± 0.3 a |
| Total grain number | 473.7 ± 46.3 c | 392.7 ± 67.4 ab | 428.4 ± 79.8 bc | 364.7 ± 52.8 a |
| Parameters | CRFA 100% | CRFA 80% | NSA | CONTROL |
|---|---|---|---|---|
| Biomass of the aerial part (t ha−1) | 9.32 ± 1.34 b | 9.61 ± 1.86 b | 8.78 ± 1.41 b | 4.91 ± 1.82 a |
| Ear weight (t ha−1) | 3.58 ± 0.54 b | 3.67 ± 0.54 b | 3.76 ± 0.67 b | 1.91 ± 0.46 a |
| Grain weight (t ha−1) | 2.35 ± 0.45 b | 2.49 ± 0.12 b | 2.19 ± 0.37 b | 1.16 ± 0.43 a |
| Nitrogen Use Efficiency (kg kg−1 N) | 14.93 ± 5.58 a | 20.76 ± 1.88 b | 12.89 ± 4.66 a | - |
| Harvest Index | 0.31 ± 0.05 a | 0.33 ± 0.08 a | 0.3 ± 0.01 a | 0.29 ± 0.02 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Caballero-Molada, M.; Atares, S.; García, C.; Vicente, O. New Eco-Friendly Polymeric-Coated Urea Fertilizers Enhanced Crop Yield in Wheat. Agronomy 2020, 10, 438. https://doi.org/10.3390/agronomy10030438
Gil-Ortiz R, Naranjo MÁ, Ruiz-Navarro A, Caballero-Molada M, Atares S, García C, Vicente O. New Eco-Friendly Polymeric-Coated Urea Fertilizers Enhanced Crop Yield in Wheat. Agronomy. 2020; 10(3):438. https://doi.org/10.3390/agronomy10030438
Chicago/Turabian StyleGil-Ortiz, Ricardo, Miguel Ángel Naranjo, Antonio Ruiz-Navarro, Marcos Caballero-Molada, Sergio Atares, Carlos García, and Oscar Vicente. 2020. "New Eco-Friendly Polymeric-Coated Urea Fertilizers Enhanced Crop Yield in Wheat" Agronomy 10, no. 3: 438. https://doi.org/10.3390/agronomy10030438
APA StyleGil-Ortiz, R., Naranjo, M. Á., Ruiz-Navarro, A., Caballero-Molada, M., Atares, S., García, C., & Vicente, O. (2020). New Eco-Friendly Polymeric-Coated Urea Fertilizers Enhanced Crop Yield in Wheat. Agronomy, 10(3), 438. https://doi.org/10.3390/agronomy10030438






