1. Introduction
In recent years, the growth and productivity of crop plants have been greatly influenced by abiotic stresses. Periods of high temperature and drought are becoming more frequent in regions with extensively crop production, such as Central Europe, South-Central Asia, south-eastern South America and the south-eastern United States [
1]. Under climate change conditions, biostimulants play an important role in sustainable crop production. These natural products (seaweed extracts, humic substances, hydrolysed proteins, and amino acids containing products or microorganism) contain a bioactive substance which enhances nutrition efficiency, abiotic stress tolerance, and/or crop quality traits, regardless of its nutrients content [
2,
3,
4,
5]. In recent years, the use of seaweed extracts and humic substances as plant growth stimulants has been increasing. Seaweed extracts and humic acids can promote plant growth, enhance abiotic stress tolerance as well as increase nutrient use efficiency [
6,
7,
8,
9,
10].
Many plant growth-stimulating compounds (auxins, cytokinins, gibberellins, betaines, polysaccharides, polyamines, abscisic acids, brassinosteroids, and minerals) have been identified from seaweed. The chemical composition of seaweed extracts depends on the algae species and on the method of extraction. Brown algae (
Phaeophyta) are most commonly used for the manufacture of extracts used as biostimulants of plant growth, including
Ascophyllum nodosum and
Ecklonia maxima [
7,
8,
11]. An increase in leaf area and chlorophyll content are common plant responses to seaweed extract treatment. Cytokinins present in the seaweed extracts stimulate cell division, resulting in enlarged leaf area, and also stimulate chlorophyll biosynthesis, whereas betaines slow chlorophyll degradation and delay leaf senescence [
7,
8].
Ascophyllum nodosum extracts applied on foliage or to soil caused an increase in the leaf chlorophyll content of French bean, tomato, barley, maize, wheat, pepper, and strawberry [
8,
11,
12]. A one-year study carried out in Iraq showed an increase in chlorophyll content in potato following the application of brown seaweed
Sargassum extracts [
13]. Foliar application of seaweed extracts
Ascophyllum nodosum and
Ecklonia maxima increased potato yield [
14,
15,
16]. Biostimulants based on seaweed extracts improved plant growth and yield of wheat, barley, maize, potato, tomato, pepper, onion, and carrot [
7,
8,
10,
11].
The biological activity of humic substances depends on their source, chemical structure, and concentration. Humic substances may influence both respiration and photosynthesis. One of the effects of humic substances applied to growing plants was an increase in chlorophyll content, which can affect photosynthesis [
17]. Leonardite is the most common commercial source of humic substances. Leonardite humic acids stimulate melon and soybean growth and chlorophyll synthesis [
6]. A one-year study carried out in Iraq showed an increase in chlorophyll content in potato following the application of humic and fulvic acids in HumiMax [
13]. A one-year study carried out in Egypt showed that the application of humic acid under water stress conditions enhanced the leaf chlorophyll content of very early potato cultivars [
18]. Application of humic substances originating from leonardite increased potato yield and nutrient uptake [
19]. In most experiments, foliar or soil application of humic and fulvic acids increased potato yield [
13,
20,
21], but one study showed no clear effect of humic and fulvic acids on the potato yield [
22]. Humic and fulvic acids improved plant growth and yield quality of wheat, maize, tomato, pepper and cucumber [
2,
22,
23,
24]. The effect of humic acids on plant growth depends of their source and concentration, and on the date and method (foliar or soil) of application, as well as the plant species and environmental conditions [
9,
17].
There is a relationship between leaf chlorophyll content and Soil Plant Analysis Development (SPAD) index [
25]. Leaf SPAD values is related to nutrient plant status, especially nitrogen [
26,
27]. There was a relationship found between SPAD value and potato yield. A higher SPAD does not always guarantee a higher potato yield [
28,
29,
30,
31]. Plant-based biostimulants increased SPAD index and marketable yield of tomato and rocket [
32,
33,
34].
To date, few studies have been focused on the effect of seaweed extract and humic acid application in early crop potato culture. The aim of the study was to determine the effect of foliar application of brown seaweed extracts and humic acids on the asssimilation area and chlorophyll content of very early potato cultivars. In the current study, it was hypothesised that seaweed extracts and humic acids could contribute to increasing assimilation area and chlorophyll content and, as a result, increase the early crop potato yield. The assumption was also made that the response to the application of these biostimulants depends on the cultivar and environmental conditions.
2. Materials and Methods
2.1. Experimental Site and Season
The study was carried out in central-eastern Poland (52°03′N, 22°33′E), over three growing season 2012–2014, on Luvisol with a low total nitrogen content, a high content of available phosphorus, a medium-to-high content of potassium and a low-to-medium content of magnesium, with an acidic-to-slightly-acid reaction. Spring triticale was grown as a potato forecrop. Farmyard manure was applied in autumn, at rate of 25 t·ha−1, and mineral fertilizers were applied at rates of 80 kg N (ammonium nitrate), 35 kg P (superphosphate) and 100 kg K (potassium sulphate) per hectare in spring. Potato cultivation was carried out according to common agronomical practice.
The thermal and moisture conditions during the potato growth period were different (
Table 1). The mean air temperatures were above or similar to the long-term average. In 2012, total precipitation was similar and, in 2013 and 2014, above the long-term average, although it was unevenly distributed during the potato growth period. The most favourable hydrothermal conditions for early crop potato culture were in the warm and moderately wet growing season of 2012. The next year, 2013 was warm and with heavy rainfall, whereas 2014 was cool with heavy rainfall after plant emergence and a drought in the period of tuber growth.
2.2. Plant Material and Experimental Design
The field experiment was established in a split-plot design with three replications. The experimental factors were: (1) plant biostimulant; and (2) cultivar. The potato plants were treated with three biostimulants: Bio-algeen S90 and Keplak SL containing seaweed extracts, and HumiPlant based on humic and fulvic acids. Bio-algeen S90 is an extract from Ascophyllum nodosum which contains amino acids, vitamins, alginic acids and other active components of seaweeds, as well as macronutrients (N, P, K, Ca, Mg) and micronutrients (B, Fe, Cu, Mn, Zn, Se, Co). Kelpak SL is an extract from Ecklonia maxima containing auxin (11 mg·dm–3) and cytokinin (0.031 mg·dm–3). HumiPlant is an extract from leonardite which contains humic acid (12%) and fulvic acid (6%) as well as macronutrients (K, Ca, Mg, S) and micronutrients (Fe, Mn, B, Mo, Zn, Cu). The biostimulants were applied according to the manufacturers’ recommendations: Bio-algeen S90–2 dm3·ha−1 at the beginning of leaf development stage (BBCH 10–11) and 2 dm3·ha−1 two weeks after the first treatment, Kelpak SL–2 dm3·ha−1 at the leaf development stage (BBCH 14–16) and 2 dm3·ha−1 two weeks after the first treatment, HumiPlant–2 dm3·ha−1 at the leaf development stage (BBCH 14–16) and 2 dm3·ha−1 one week after the first treatment. Potato plants sprayed with water were used as a control without a biostimulant.
The most popular very early potato cultivars (Denar, Lord and Miłek) in the research area were grown. In successive years, 6-weeks pre-sprouted seed potatoes were planted on April 12, April 18 and April 7 with a row spacing of 0.25 m and 0.675 m between rows. The plots were six rows wide and 4 m long (96 plants per plot). Potatoes were harvested 75 days after planting (the end of June).
2.3. Determination of Assimilation Area, Chlorophyll Content and Tuber Yield
At the tuber formation stage (BBCH 41–43), the assimilation area, leaf area index (LAI), specific leaf area (SLA), and chlorophyll content (SPAD value) were determined. The measurements were made on four successive randomized plants per plot. The assimilation area was measured by the weight method [
36]. SLA was calculated as the ratio of assimilation area/weight of leaves [
37].
The chlorophyll content was estimated with non-destructive methods using a portable SPAD-502 chlorophyll meter (Minolta, Osaka, Japan). The measurements were made on the youngest fully expanded leaf, i.e., the fourth or fifth leaf from the top.
The total and marketable tuber yield were determined. The marketable tuber yield constituted tubers with a transverse diameter above 30 mm, excluding cracked and deformed tubers. The marketable tuber yield was determined on the basis of the total tuber yield of ten successive plants per plot using a hand calibrator with a square hole.
2.4. Statistical Analysis
The results of the study were analysed statistically with an analysis of variance (ANOVA) for the split-pot design. The significance of differences between the compared averages was verified using Tukey’s test at the significance level p ≤ 0.05.
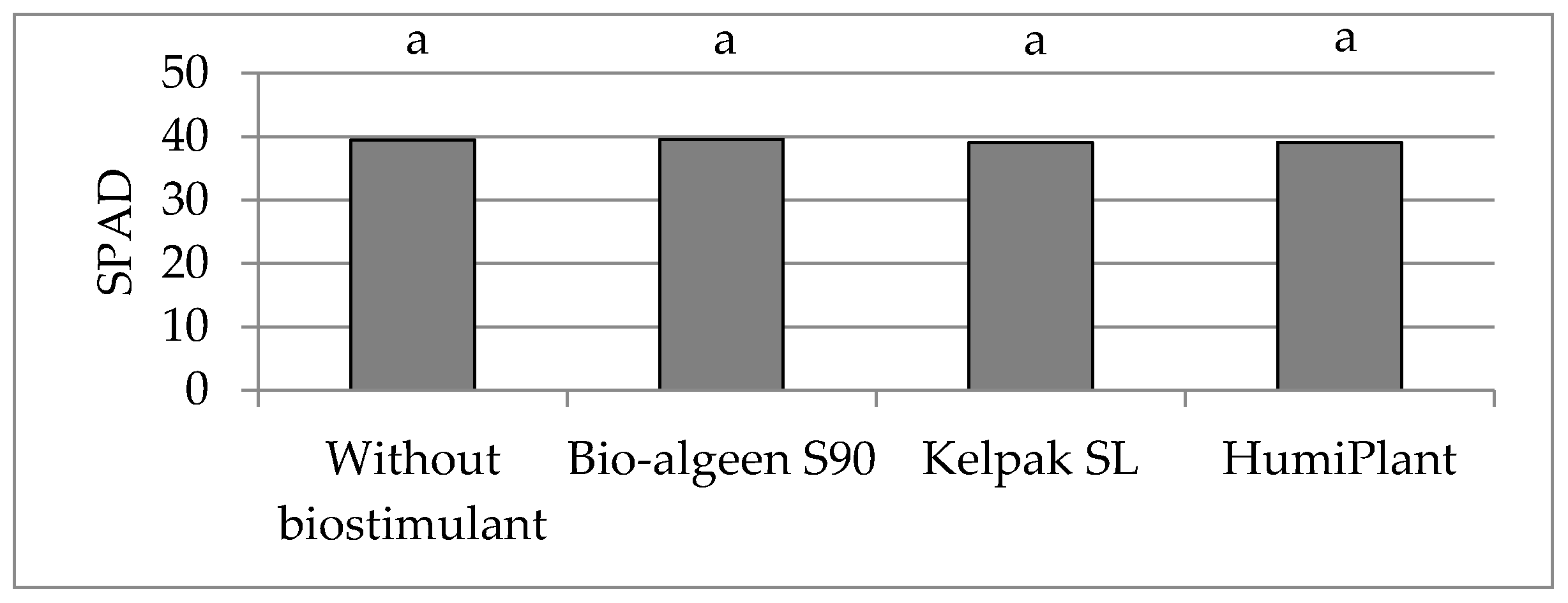
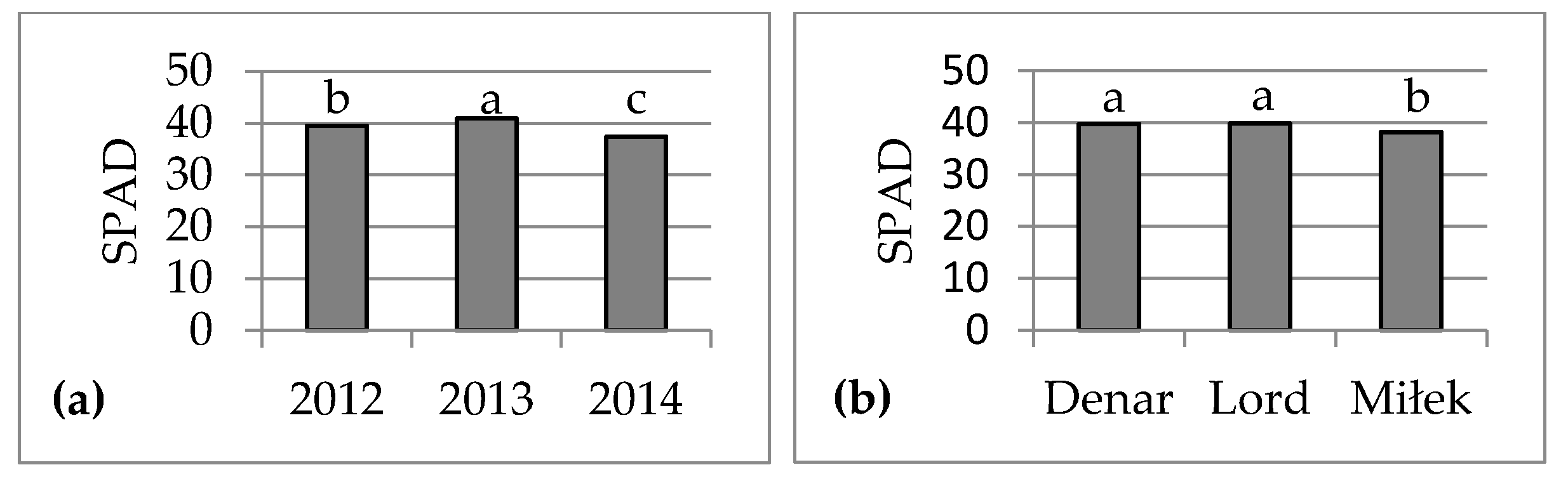
4. Discussion
In sustainable crop production, biostimulants play an important role in improving plant growth and crop quality. Assimilation area and chlorophyll content are important parameters of assessment plant growth. The biostimulants used in the experiment caused enlargement of assimilation area, but had no effect on the chlorophyll content (SPAD value) in leaves of very early potato cultivars. SPAD value depended on the cultivar and weather or soil conditions to a greater extent. The effect of foliar application of seaweed extracts on potato assimilation area was comparable to humic and fulvic acids. In the three years of the study, following biostimulant application, the average leaf area index (LAI) was 4.64, being higher by 0.30 compared to the average for the untreated control group. Potato cultivars showed different responses to the applied biostimulants. Studies have shown the highest light absorption efficiency values at the LAI value of 3, which corresponded to maximum ground cover. If potato LAI exceeds 3, the intercepted photosynthetically active radiation value changes very little [
39,
40]. According to Howlader and Hoque [
41], irrespective of potato cultivars, LAI increased progressively over time, reaching a peak at 60 days after planting and thereafter declining. The rate of assimilation area expansion showed the interaction between genotype and environment and varied by year [
42], which was confirmed in the present study. The effect of seaweed extracts on potato assimilation area depended on the weather conditions after plant emergence. In the year with the highest air temperature and heavy rainfall after plant emergence, the assimilation area was larger after the application of Kelpak SL (
Ecklonia maxima), whereas in the year with the lowest air temperature and with heavy rainfall after plant emergence, the assimilation area was larger after the application of Bio-algeen S90 (
Ascophyllum nodosum). Potato plants are very sensitive to heat stress. In general, heat stress increases plant height, reduces leaf size, increases leaf chlorophyll content, and severely reduces tuber mass [
43]. Kelpak SL contains auxins and cytokinins in a ratio of 350/1. Exogenous auxin plays an important role in plant stress resistance. The action of auxin depends on its concentration, the light conditions and carbohydrate content in the plant [
44]. Exogenous cytokinins also play an important role in plant adaptation to environmental stresses [
45]. Cytokinins present in the seaweed extracts stimulate cell division, resulting in enlarged leaf area [
7,
8], which was confirmed in the present study.
The leaf area index describes the growth of lowland fields, whereas the growth of individual plants is characterized by the specific leaf area (SLA). Biostimulants caused enlargement of the assimilation area, but had no effect on the SLA. The SLA for potato depends on the cultivar and growth stage, and temperature [
42], which was confirmed in the present study. Early foliar expansion of potato is associated with a strong increase in SLA [
41].
Foliar or soil application of
Ascophyllum nodosum extracts caused an increase in the chlorophyll content of some agriculture (barley, wheat, maize) and horticulture (French bean, tomato, pepper, strawberry) plants [
8,
11,
12], which was not confirmed in the present study. A study carried out in Egypt showed that the application of humic acid under water stress conditions enhanced the chlorophyll content of very early potato ‘Spunta’ grown on sandy soil [
18], which was not confirmed in the present study with very early potato cultivars grown on loamy soil (Luvisol). A one-year study carried out in Iraq showed that foliar application of humic and fulvic acids caused an increase in the chlorophyll content of medium-early potato cultivar [
13]. The effect of humic acids depends on their source and concentration, and on the date and method of application, as well as the plant species and cultivar [
9]. The increase in chlorophyll alone does not necessarily result in higher yields [
17,
26].
The biostimulants used in the experiment enhanced tolerance to abiotic stress and improved crop quality. In the three years of the study, the marketable tuber yield (diameter above 30 mm) was higher, on average, by 2.15 t·ha−1. Bio-algeen S90 and Keplak SL containing seaweed extracts produced better results in a warm and very wet growing season, whereas HumiPlant based on humic and fulvic acids produced better results in a year with lower air temperature and with drought periods during potato growth.
A correlation between the tuber yield and assimilation area was not found. Li et al. [
46] found a significant positive correlation between LAI and tuber yield, which suggests that the enlargement of leaf area could enhance the export of photosynthetic products and cause an increase in tuber yield. According to Ascione et al. [
47], the tuber growth rate is only slightly correlated with LAI, and still less so with SLA, which was not confirmed in the present study. A significant negative correlation was found between the total and marketable (diameter above 30 mm) tuber yield and SLA.
No correlation was found between the tuber yield of three very early potato cultivars and SPAD value measured on the fourth or fifth leaf from the top at the tuber formation stage (BBCH 41-43), which suggest that the biostimulants used in the experiment had no effect on the plant nitrogen status. Bărăscu et al. [
30] found a significant negative correlation between SPAD measured on the fourth and fifth leaves from the top and the tuber weight of two mid-early potato cultivars, which could have been associated with oxidative stress [
29]. SPAD index as an indicator of crop nitrogen status may be used for the prediction of the potato yield, however a higher SPAD does not always guarantee a higher tuber yield [
26,
28,
31]. SPAD value is a useful indicator for selecting the high yield cultivars in the early period, however, no single threshold leaf SPAD value can be used for all potato cultivars. The SPAD value can predict the level of tuber yield if the value is calibrated for a particular potato cultivar [
28,
31]. Establishing threshold SPAD value is quite difficult due to the influence of climate and technical factors. SPAD values can be affected by leaf age and position, as well as, time of the day [
26,
27]. As a rule SPAD measurements are carried out on the third-fifth leaf from the top. Recently it was demonstrated that there is a significance difference in SPAD values between the upper and lower leaves among potato cultivars. It was shown that cultivar affects the SPAD values of the fourth and eighth leaf, but does not affect SPAD value of the fourth-eighth leaves and the difference between SPAD of the fourth and eighth leaf. Therefore the SPAD values of the fourth-eighth leaves could be applied as a general index of nitrogen status across different potato cultivars [
27].
5. Conclusions
In conclusion, the foliar application of seaweed extracts Ascophyllum nodosum (Bio-algeen S90) and Ecklonia maxima (Kelpak SL), as well as humic and fulvic acids from leonardite (HumiPlant), resulted in enlargement of the assimilation area of very early potato cultivars, but had no effect on the SLA or chlorophyll content (SPAD value). The assimilation area was larger, on average, by 0.0505 m2 (7%), and LAI was higher by 0.30 compared with the plants from the control group without a biostimulant. The SLA and SPAD depend on the cultivar and weather conditions, or nitrogen and magnesium content, in soil to a greater extent. These biostimulants enhanced abiotic stress tolerance and increased marketable tuber yield (diameter above 30 mm) 75 days after planting (the end of June), on average, by 2.15 t·ha−1. Bio-algeen S90 and Keplak SL containing seaweed extracts produced better results in a warm and very wet growing season, whereas HumiPlant based on humic and fulvic acids produced better results in a year with lower air temperature and with drought periods during potato growth. No correlation was found between the tuber yield and assimilation area or between the tuber yield and SPAD value, although a significant negative correlation was found between the tuber yield and SLA.