Variation in Anther Extrusion and Its Impact on Fusarium Head Blight and Deoxynivalenol Content in Oat (Avena sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Variation in Anther Extrusion in Two Populations of Oat Accessions
2.1.1. The North American AFRI CORE Oat Panel
2.1.2. The Nordic Screening Population
2.2. The Variation and Inheritance of Anther Extrusion in Two Recombinant Inbred Line Populations
2.3. Statistical Analysis
2.4. The Effect of Anther Extrusion on Fusarium Infection and DON Content
2.4.1. Spawn-Inoculated Field Experiments
2.4.2. Spray-Inoculated Greenhouse Experiment
3. Results
3.1. The Variation in Anther Extrusion
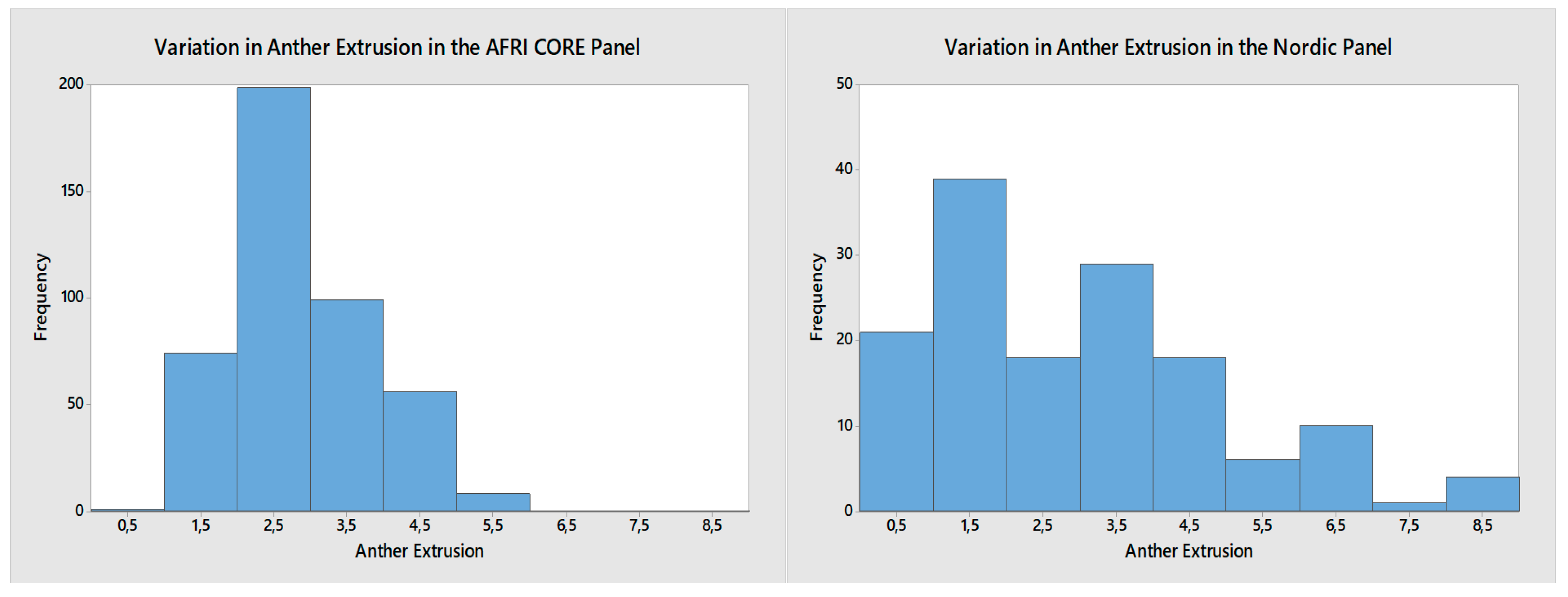
3.1.1. The AFRI CORE Screening Population
3.1.2. The Nordic Screening Population
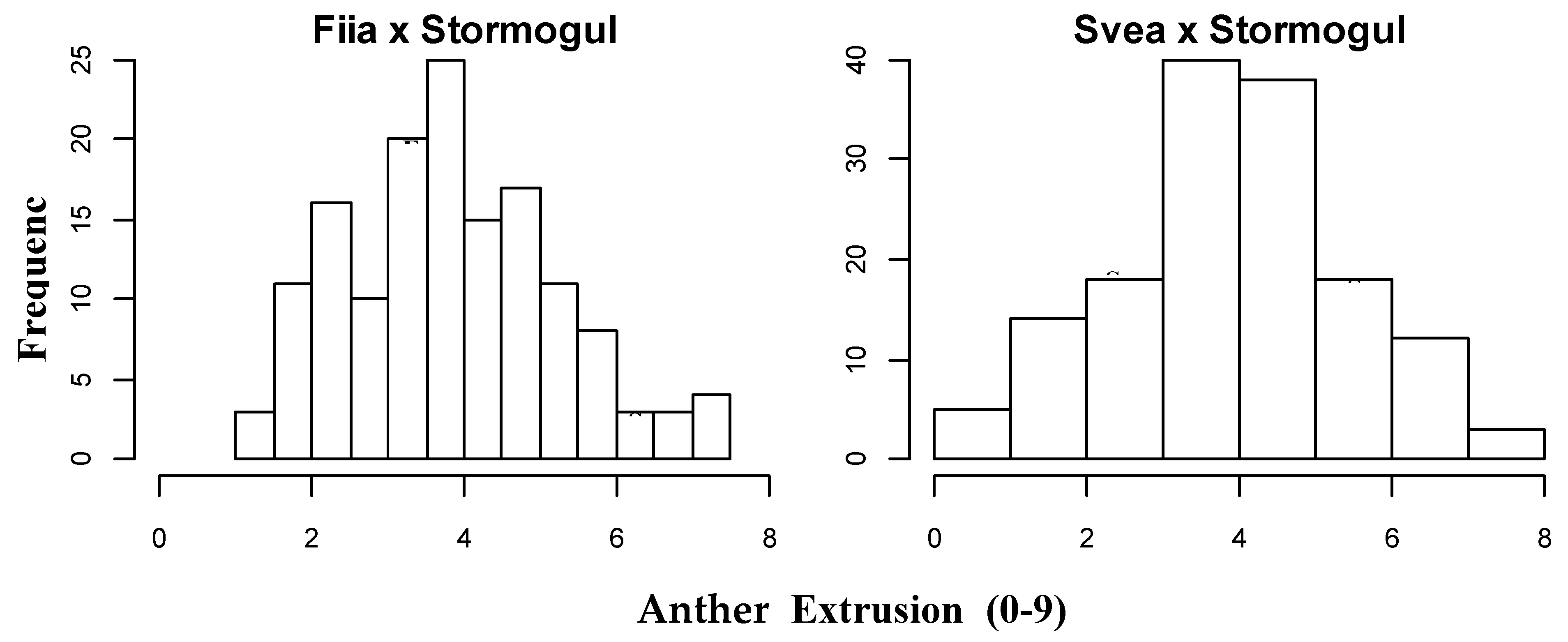
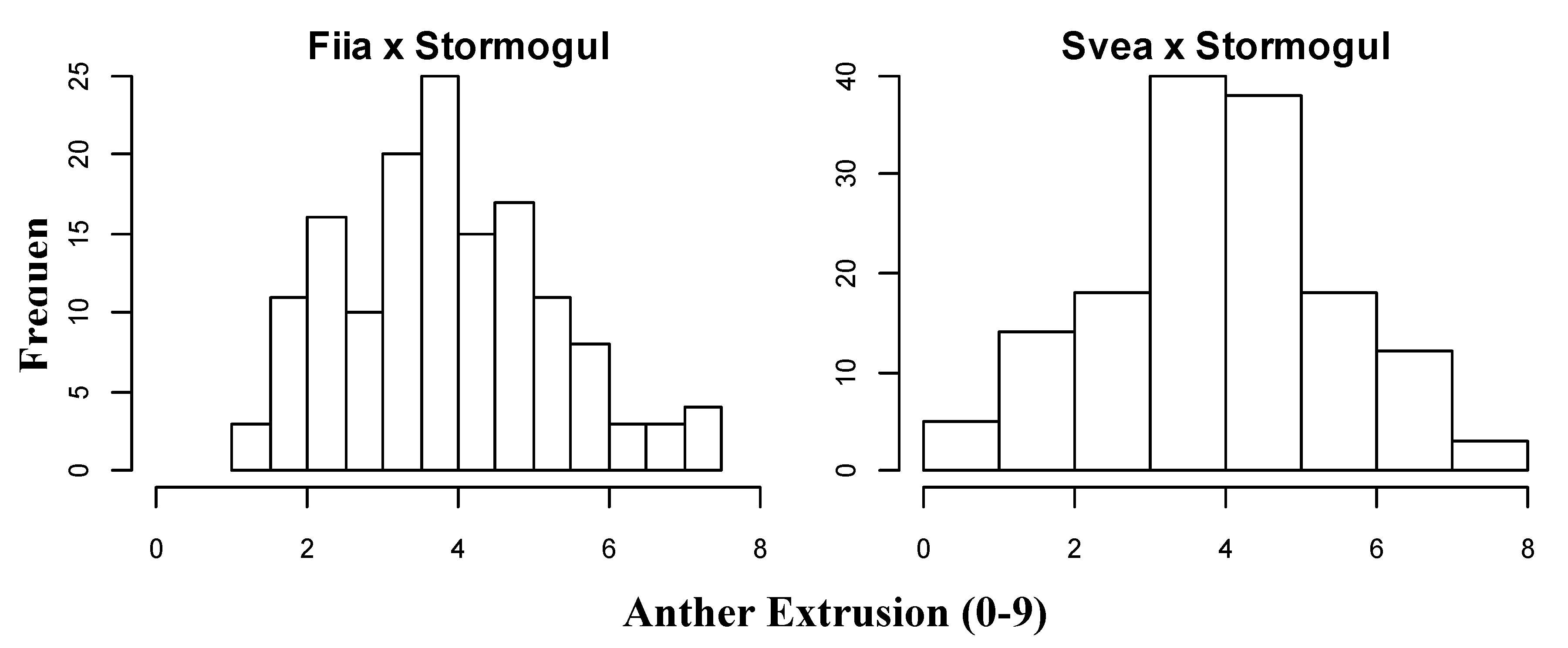
3.2. The Variation and Inheritance in Anther Extrusion and Days to Flowering in the Recombinant Inbred Line Populations
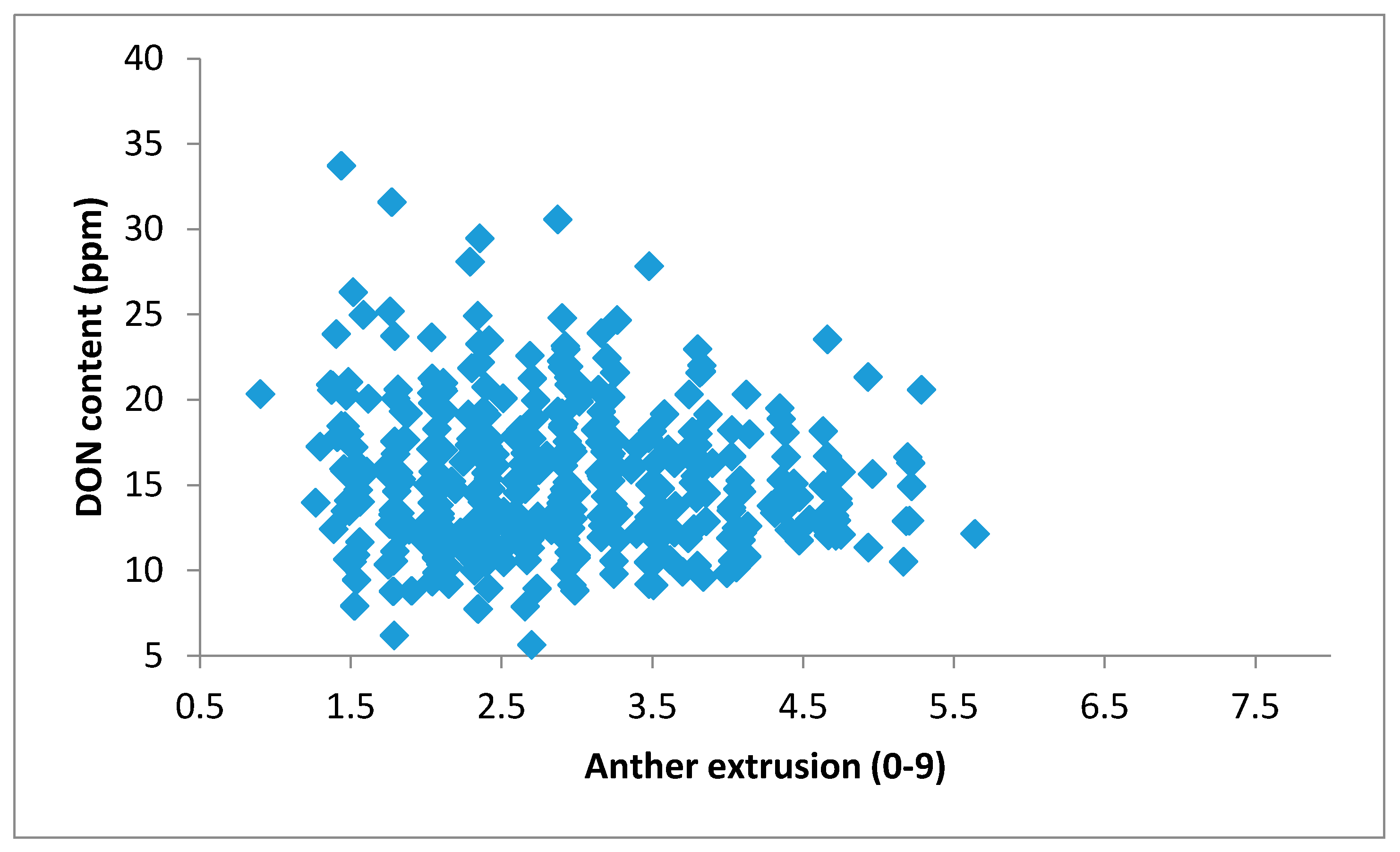
3.3. The Effect of Anther Extrusion on Fusarium Head Blight
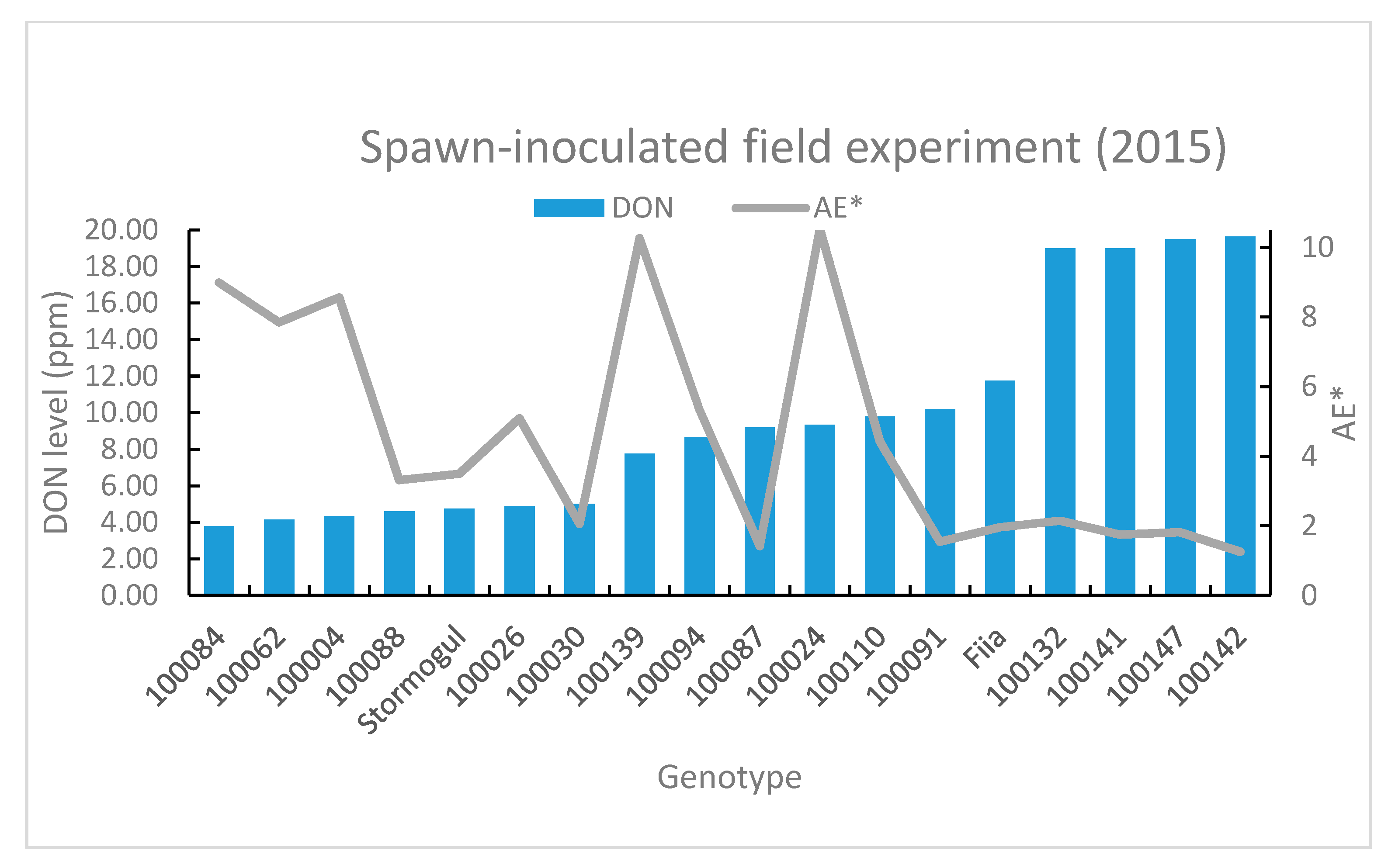
3.3.1. Spawn-Inoculated Field Experiments
3.3.2. Spray-Inoculated Greenhouse Experiment
4. Discussion
4.1. Variation and Inheritance of Anther Extrusion in Oat
4.2. Effect of Anther Extrusion on Fusarium Head Blight
4.3. Diversity of Nordic Oat
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marshall, A.; Cowan, S.; Edwards, S.; Griffiths, I.; Howarth, C.; Langdon, T.; White, E. Crops that feed the world 9. Oats- a cereal crop for human and livestock feed with industrial applications. Food Secur. 2013, 5, 13–33. [Google Scholar] [CrossRef]
- Peterson, D.M. Oat antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Welch, R.W. Nutrient composition and nutritional quality of oats and comparisons with other cereals. In Oats Chemistry and Technology, 2nd ed.; Webster, F.H., Wood, P.J., Eds.; AACC International: St. Paul, MN, USA, 2011; pp. 95–107. [Google Scholar]
- Tekle, S.; Dill-Macky, R.; Skinnes, H.; Tronsmo, A.M.; Bjørnstad, Å. Infection process of Fusarium graminearum in oats (Avena sativa L.). Eur. J. Plant. Pathol. 2012, 132, 431–442. [Google Scholar] [CrossRef]
- Bjørnstad, Å.; He, X.; Tekle, S.; Klos, K.; Huang, Y.F.; Tinker, N.A.; Skinnes, H. Genetic variation and associations involving Fusarium head blight and deoxynivalenol accumulation in cultivated oat (Avena sativa L.). Plant. Breed. 2017, 136, 620–636. [Google Scholar] [CrossRef]
- He, X.; Skinnes, H.; Oliver, R.E.; Jackson, E.W.; Bjørnstad, Å. Linkage mapping and identification of QTL affecting deoxynivalenol (DON) content (Fusarium resistance) in oats (Avena sativa L.). Theor. Appl. Genet. 2013, 126, 2655–2670. [Google Scholar] [CrossRef]
- Tekle, S.; Lillemo, M.; Skinnes, H.; Reitan, L.; Buraas, T.; Bjørnstad, Å. Screening of oat accessions for Fusarium head blight resistance using spawn-inoculated field experiments. Crop. Sci. 2018, 58, 143–151. [Google Scholar] [CrossRef]
- Misonoo, G. Ecological and Physiological Studies on the Blooming of Oat Flowers. Ph.D. Thesis, Hokkaido University, Saporo, Japan, 1936. [Google Scholar]
- Rajala, A.; Peltonen-Sainio, P. Pollination dynamics, grain weight and grain cell number within the inflorescence and spikelet in oat and wheat. Agric. Sci. 2011, 2, 283–290. [Google Scholar] [CrossRef]
- De Vries, A.P. Flowering biology of wheat, particularly in view of hybrid seed production—A review. Euphytica 1971, 20, 152–170. [Google Scholar] [CrossRef]
- Nair, S.K.; Wang, N.; Turuspekov, Y.; Pourkheirandish, M.; Sinsuwongwat, S.; Chen, G.; Komatsuda, T. Cleistogamous flowering in barley arises from the suppression of microRNA-guided HvAP2 mRNA cleavage. Proc. Natl. Acad. Sci. USA 2010, 107, 490–495. [Google Scholar] [CrossRef]
- Abdel-Ghani, A.H.; Parzies, H.K.; Ceccarelli, S.; Grando, S.; Geiger, H.H. Estimation of quantitative genetic parameters for outcrossing-related traits in barley. Crop. Sci. 2005, 45, 98–105. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Buerstmayr, H. Comparative mapping of quantitative trait loci for Fusarium head blight resistance and anther retention in the winter wheat population Capo× Arina. Theor. Appl. Genet. 2015, 128, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Buerstmayr, M.; Michel, S.; Schweiger, W.; Lemmens, M.; Buerstmayr, H. Breeding strategies and advances in line selection for Fusarium head blight resistance in wheat. Trop. Plant. Pathol. 2017, 42, 165–174. [Google Scholar] [CrossRef]
- Gilsinger, J.; Kong, L.; Shen, X.; Ohm, H. DNA markers associated with low Fusarium head blight incidence and narrow flower opening in wheat. Theor. Appl. Genet. 2005, 110, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Skinnes, H.; Semagn, K.; Tarkegne, Y.; Marøy, A.G.; Bjørnstad, Å. The inheritance of anther extrusion in hexaploid wheat and its relationship to Fusarium head blight resistance and deoxynivalenol content. Plant. Breed. 2010, 129, 149–155. [Google Scholar] [CrossRef]
- Bushnell, W.R.; Hazen, B.E.; Pritsch, C. Histology and physiology of Fusarium head blight. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2003; pp. 44–83. [Google Scholar]
- Lewandowski, S.M.; Bushnell, W.R.; Evans, C.K. Distribution of mycelial colonies and lesions in field grown barley inoculated with Fusarium graminearum. Phytopathology 2006, 96, 567–581. [Google Scholar] [CrossRef]
- Yoshida, M.; Kawada, N.; Nakajima, T. Effect of infection timing on Fusarium head blight and mycotoxin accumulation in open- and closed-flowering barley. Phytopathology 2007, 97, 1054–1062. [Google Scholar] [CrossRef][Green Version]
- Divon, H.H.; Bøe, L.; Tveit, M.M.N.; Klemsdal, S.S. Histological studies of F. langsethiae infection on oats and wheat. In Proceedings of the Nordic Baltic Fusarium Seminar, Uppsala, Sweden, 13–15 November 2012. [Google Scholar]
- He, X.; Bjørnstad, Å. Diversity of north European oat analyzed by SSR, AFLP, and DArT markers. Theor. Appl. Genet. 2012, 125, 57–70. [Google Scholar] [CrossRef]
- Mattsson, B. Svensk växtförädling av havre. In Den Svenska Växtförädlingens Historia: Jordbruksväxternas Utveckling Senda 1880-Talet; Olsson, G., Ed.; Kungliga skogs- och lantbruksakademien: Stockholm, Sweden, 1997; p. 320. [Google Scholar]
- Scott, R.A.; Milliken, G.A. A SAS program for analyzing augmented randomized complete-block designs. Crop. Sci. 1993, 33, 865–867. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Tekle, S.; Skinnes, H.; Bjørnstad, Å. The germination problem of oat seed lots affected by Fusarium head blight. Eur. J. Plant. Pathol. 2013, 135, 147–158. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Kolaczkowski, E.; Xie, W.P.; Yu, H.; Jelen, H. Analysis of deoxynivalenol and its derivatives (batch and single kernel) using gas chromatography mass spectrometry. J. Agric. Food Chem. 1998, 46, 1414–1418. [Google Scholar] [CrossRef]
- Fuentes, R.G.; Mickelson, H.R.; Busch, R.H.; Dill-Macky, R.; Evans, C.K.; Thompson, W.G.; Anderson, J.A. Resource allocation and cultivar stability in breeding for Fusarium head blight resistance in spring wheat. Crop. Sci. 2005, 45, 1965–1972. [Google Scholar] [CrossRef]
- Choo, T.M.; Vigier, B.; Savard, M.E.; Blackwell, B.; Martin, R.; Wang, J.; Abdel-Aal, E.-S.M. Black barley as a means of mitigating deoxynivalenol contamination. Crop. Sci. 2015, 55, 1096–1103. [Google Scholar] [CrossRef]
- Choo, T.M.; Vigier, B.; Shen, Q.Q.; Martin, R.A.; Ho, K.M.; Savard, M. Barley traits associated with resistance to Fusarium head blight and deoxynivalenol accumulation. Phytopathology 2004, 94, 1145–1150. [Google Scholar] [CrossRef]
- Kubo, K.; Fujita, M.; Kawada, N.; Nakajima, T.; Nakamura, K.; Maejima, H.; Matsunaka, H. Minor differences in anther extrusion affect resistance to Fusarium head blight in wheat. J. Phytopathol. 2013, 161, 308–314. [Google Scholar] [CrossRef]
- Lu, Q.; Lillemo, M.; Skinnes, H.; He, X.; Shi, J.; Ji, F.; Bjørnstad, Å. Anther extrusion and plant height are associated with Type I resistance to Fusarium head blight in bread wheat line ‘Shanghai-3/Catbird’. Theor. Appl. Genet. 2013, 126, 317–334. [Google Scholar] [CrossRef]
- Atashi-Rang, G.; Lucken, K. Variability, combining ability, and interrelationships of anther length, anther extrusion, glume tenacity, and shattering in spring wheat. Crop. Sci. 1978, 18, 267–272. [Google Scholar] [CrossRef]
- Langer, S.M.; Longin, C.F.H.; Würschum, T. Phenotypic evaluation of floral and flowering traits with relevance for hybrid breeding in wheat (Triticum aestivum L.). Plant. Breed. 2014, 133, 433–441. [Google Scholar] [CrossRef]
- Sage, G.C.M.; Isturiz, M.J.D. The inheritance of anther extrusion in two spring wheat varieties. Theor. Appl. Genet. 1974, 45, 126–133. [Google Scholar] [CrossRef]
- Ladizinsky, G. Domestication via hybridization of the wild tetraploid oats Avena magna and A. murphyi. Theor. Appl. Genet. 1995, 91, 639–646. [Google Scholar] [CrossRef]
- Ladizinsky, G. A synthetic hexaploid (2n = 42) oat from the cross of Avena strigosa (2n = 14) and domesticated A. magna (2n = 28). Euphytica 2000, 116, 231–235. [Google Scholar] [CrossRef]
- Arya, R.; Sethi, S. Studies on floral traits influencing outcrossing in wheat (Triticum aestivum L.). Natl. J. Plant. Improv. 2005, 7, 73–76. [Google Scholar]
- Holland, J.; Portyanko, V.; Hoffman, D.; Lee, M. Genomic regions controlling vernalization and photoperiod responses in oat. Theor. Appl. Genet. 2002, 105, 113–126. [Google Scholar] [CrossRef] [PubMed]
| Traits | Source | DF | Mean Square | F Value | P Value |
|---|---|---|---|---|---|
| 2013 | Genotype | 430 | 3.28 | 2.35 | <0.0001 |
| Check | 6 | 4.98 | 3.57 | 0.0026 | |
| Block | 7 | 0.89 | 0.64 | 0.7239 | |
| Error | 130 | 1.40 | |||
| 2014 | Genotype | 421 | 4.79 | 4.02 | <0.0001 |
| Check | 6 | 36.98 | 31.03 | <0.0001 | |
| Block | 7 | 2.24 | 1.88 | 0.1066 | |
| Error | 32 | 1.19 | |||
| 2013 + 2014 | Genotype | 430 | 5.47 | 2.46 | <0.0001 |
| Check | 8 | 30.93 | 13.94 | <0.0001 | |
| Year | 1 | 9.33 | 4.20 | 0.0408 | |
| Block (Year) | 14 | 2.80 | 1.26 | 0.2254 | |
| Error | 587 | 2.22 |
| Traits | Source | DF | Mean Square | F Value | P Value | |
|---|---|---|---|---|---|---|
| F × S | AE | Genotype (Check) | 145 | 7.56 | 6.83 | <0.0001 |
| Check | 5 | 25.83 | 23.36 | <0.0001 | ||
| Year | 1 | 1.52 | 1.37 | 0.2430 | ||
| Block (Year) | 4 | 0.85 | 0.77 | 0.5475 | ||
| Column (Year) | 23 | 0.81 | 0.73 | 0.8036 | ||
| Check*Year | 3 | 2.07 | 1.87 | 0.1374 | ||
| Error | 134 | 1.11 | ||||
| DTF | Genotype (Check) | 145 | 69.24 | 4.01 | <0.0001 | |
| Check | 5 | 423.18 | 24.50 | <0.0001 | ||
| Year | 1 | 67.54 | 3.91 | 0.0501 | ||
| Block (Year) | 4 | 26.66 | 1.54 | 0.1932 | ||
| Column (Year) | 23 | 7.16 | 0.41 | 0.9917 | ||
| Check*Year | 3 | 9.88 | 0.57 | 0.6344 | ||
| Error | 133 | 17.27 | ||||
| S × S | AE | Genotype (Check) | 138 | 5.75 | 6.01 | <0.0001 |
| Check | 5 | 30.03 | 31.40 | <0.0001 | ||
| Year | 1 | 1.37 | 1.44 | 0.2328 | ||
| Block (Year) | 4 | 2.49 | 2.60 | 0.0393 | ||
| Column (Year) | 23 | 1.1124 | 1.16 | 0.2905 | ||
| Check*Year | 3 | 1.59 | 1.66 | 0.1779 | ||
| Error | 128 | 0.96 | ||||
| DTF | Genotype (Check) | 147 | 131.67 | 3.50 | <0.0001 | |
| Check | 5 | 498.13 | 13.25 | <0.0001 | ||
| Year | 1 | 276.71 | 7.36 | 0.0075 | ||
| Block (Year) | 4 | 19.13 | 0.51 | 0.7295 | ||
| Column (Year) | 23 | 27.93 | 0.74 | 0.7941 | ||
| Check*Year | 3 | 98.72 | 2.63 | 0.0530 |
| AE12 | AE13 | DTF12 | DTF13 | |||
|---|---|---|---|---|---|---|
| F × S | AE13 | 0.74 | DTF13 | 0.78 | ||
| AE* | 0.58 | 0.73 | DTF15 | 0.79 | 0.89 | |
| S × S | AE13 | 0.70 | DTF13 | 0.73 | ||
| F × S | DON | FHB | DTF | S × S | DON | FHB | DTF |
|---|---|---|---|---|---|---|---|
| FHB | 0.57 ** | FHB | 0.03 ns | ||||
| DTF | −0.16 ns | −0.34 ns | DTF | −0.42 * | −0.29 ns | ||
| AE* | −0.51 ** | −0.58 ** | 0.19 ns | AE * | −0.21 ns | 0.01 ns | 0.40 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekle, S.; Stråbø Schofer, S.; He, X.; Dong, Y.; Bjørnstad, Å. Variation in Anther Extrusion and Its Impact on Fusarium Head Blight and Deoxynivalenol Content in Oat (Avena sativa L.). Agronomy 2020, 10, 354. https://doi.org/10.3390/agronomy10030354
Tekle S, Stråbø Schofer S, He X, Dong Y, Bjørnstad Å. Variation in Anther Extrusion and Its Impact on Fusarium Head Blight and Deoxynivalenol Content in Oat (Avena sativa L.). Agronomy. 2020; 10(3):354. https://doi.org/10.3390/agronomy10030354
Chicago/Turabian StyleTekle, Selamawit, Sissela Stråbø Schofer, Xinyao He, Yanhong Dong, and Åsmund Bjørnstad. 2020. "Variation in Anther Extrusion and Its Impact on Fusarium Head Blight and Deoxynivalenol Content in Oat (Avena sativa L.)" Agronomy 10, no. 3: 354. https://doi.org/10.3390/agronomy10030354
APA StyleTekle, S., Stråbø Schofer, S., He, X., Dong, Y., & Bjørnstad, Å. (2020). Variation in Anther Extrusion and Its Impact on Fusarium Head Blight and Deoxynivalenol Content in Oat (Avena sativa L.). Agronomy, 10(3), 354. https://doi.org/10.3390/agronomy10030354





