Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setting, Leafy Vegetables Tested and Cultural Practices
2.2. Experimental Design, Nitrogen Fertilization and Biostimulant Application
2.3. Marketable Yield and Sampling
2.4. Nitrogen Determination, N-use Efficiency and Uptake Efficiency
2.5. Leaf Quality: Antioxidant Activity and Compounds, Chlorophyll Content and SPAD Index
2.6. Statistical Processing
3. Results
3.1. Marketable Yield and SPAD Index
3.2. N-Use and Uptake Efficiency
3.3. Total Chlorophyll, Chlorophyll a and b and Nitrate content
3.4. Leaf Quality: Antioxidant Activity and Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ruiz, J.M.; Rivero, R.M.; Cervilla, L.M.; Castellano, R.; Romero, L. Grafting to improve nitrogen-use efficiency traits in tobacco plants. J. Sci. Food Agric. 2006, 86, 1014–1021. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, A.B.; Gupta, R. Pathophysiology of nitrate toxicity in humans in view of the changing trends of the global nitrogen cycle with special reference to India. In The Indian Nitrogen Assessment; Abrol, Y.P., Adhya, T.K., Aneja, V.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 459–468. [Google Scholar]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.M.; Bekunda, M.; Grizzetti, B.; de Vries, W.; van Grinsven, H.J.M.; Abrol, Y.P.; Adhya, T.K.; Billen, G.; et al. Our Nutrient World: The challenge to produce more food and energy with less pollution. Cent. Ecol. Hydrol. 2013, 8, 95–108. [Google Scholar]
- Aires, A.; Carvalho, R.; Rosa, E.A.S.; Saavedra, M.J. Effects of agriculture production systems on nitrate and nitrite accumulation on baby-leaf salads. Food Sci. Nutr. 2013, 1, 3–7. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 231–238. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Hawkesford, M.; Kopriva, S.; De Kok, L. Nutrient use efficiency in plants–Concepts and approaches. In Plant Ecophysiol; Springer International Publishing: Basel, Switzerland, 2014. [Google Scholar] [CrossRef]
- Sisson, V.A.; Rufty, T.W.; Williamson, R.E. Nitrogen-use efficiency among flue-cured tobacco genotypes. Crop Sci. 1991, 31, 1615–1620. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Mirabelli, C.; Cardarelli, M. Nitrogen-use efficiency traits of mini-watermelon in response to grafting and nitrogen-fertilization doses. J. Plant Nutr. Soil Sci. 2011, 174, 933–941. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Maynard, D.N.; Baker, A.V.; Minotti, P.L.; Peck, N.H. Nitrate accumulation in vegetables. Adv. Agron. 1976, 28, 71–118. [Google Scholar] [CrossRef]
- Blom-Zandstra, M.; Eenink, A.H. Nitrate content and reduction in different genotypes of lettuce. J. Am. Soc. Hortic. Sci. 1986, 111, 908–911. [Google Scholar]
- Pate, J.S. Uptake, assimilation and transport of nitrogen compounds by plants. Soil Biol. Biochem. 1973, 5, 109–119. [Google Scholar] [CrossRef]
- Blom-Zandstra, M. Nitrate accumulation in vegetables and its relationship to quality. Ann. Appl. Biol. 1989, 115, 553–561. [Google Scholar] [CrossRef]
- Steingröver, E.; Ratering, P.; Siesling, J. Daily changes in uptake, reduction and storage of nitrate in spinach grown at low light intensity. Physiol. Plant. 1986, 66, 550–556. [Google Scholar] [CrossRef]
- Steingröver, E.; Siesling, J.; Ratering, P. Effect on one nigth with “low light” on uptake, reduction and storage of nitrate in spinach. Physiol. Plant. 1986, 66, 557–562. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]
- Biemond, H.; Vos, J.; Struik, P.C. Effects of nitrogen on accumulation and partitioning of dry matter and nitrogen of vegetables. 3. Spinach. Neth. J. Agric. Sci. Wagening. J. Sci. 1996, 44, 227–239. [Google Scholar]
- Smolders, E.; Buysse, J.; Merckx, R. Growth analysis of soil-grown spinach plants at different N-regimes. Plant Soil 1993, 154, 73–80. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 64, 145–158. [Google Scholar] [CrossRef]
- Botta, A. Enhancing plant tolerance to temperature stress with amino acids: An approach to their mode of action. Acta Hortic. 2013, 1009, 29–35. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front. Plant Sci. 2018, 9, 472. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.I.; Kyriacou, M.C.; et al. Biostimulant application with a tropical plant extract enhances Corchorus olitorius adaptation to sub-optimal nutrient regimens by improving physiological parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Caruso, G.; Stoleru, V.; De Pascale, S.; Cozzolino, E.; Pannico, A.; Giordano, M.; Teliban, G.; Cuciniello, A.; Rouphael, Y. Production, leaf quality and antioxidants of perennial wall rocket as affected by crop cycle and mulching type. Agronomy 2019, 9, 194. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and nutritional quality of Vesuvian Piennolo tomato PDO as affected by farming system and biostimulant application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Sady, W.; Smole’n, S. The Influence of Pentakeep V and Nitrogen Fertilization on the Yield and Biological Quality of Carrot and Spinach Crop; Final Report for Cosmo Oil Co., Ltd.; Cosmo Oil Co., Ltd.: Tokyo, Japan, 2007. [Google Scholar]
- Smole’n, S.; Sady, W.; Wierzbi’nska, J. The effect of plant biostimulation with ‘Pentakeep V’ and nitrogen fertilization on yield, nitrogen metabolism and quality of spinach. Acta Sci. Pol. Hortorum Cultus 2010, 9, 25–36. [Google Scholar] [CrossRef]
- Smole’n, S.; Sady, W. The influence of nitrogen fertilization and Pentakeep V application on contents of nitrates in carrot. Acta Hortic. Regiotect. 2009, 12, 221–223. [Google Scholar]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of vegetal- and seaweed extract-based biostimulants on agronomical and leaf quality traits of plastic tunnel-grown baby lettuce under four regimes of nitrogen fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis. Agronomy Monograph; Part 2; Black, C.A., Evans, D.D., White, I.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 1149–1178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 2011, 9, 1452–1485. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake e_ciency, yield and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Youssef, R. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, Y.; Kubo, M. Soybean peptide: Novel plant growth promoting peptide from soybean. In Soybean and Nutrition; El-Shemy, H., Ed.; In Tech Europe Publisher: Rijeka, Croatia, 2011; pp. 215–230. [Google Scholar]
- Colla, G.; Svecova, E.; Rouphael, Y.; Cardarelli, M.; Reynaud, H.; Canaguier, R.; Planques, B. Effectiveness of a plant—Derived protein hydrolysate to improve crop performances under different growing conditions. Acta Hortic. 2013, 1009, 175–179. [Google Scholar] [CrossRef]
- Abdelraouf, E.A.A. The Effects of Nitrogen Fertilization on Yield and Quality of Spinach Grown in High Tunnels. Alex. Sci. Exch. J. 2016, 37, 488–496. [Google Scholar]
- Canali, S.; Montemurro, F.; Tittarelli, F.; Masetti, O. Is it possible to reduce nitrogen fertilization in processing spinach? J. Plant Nutr. 2011, 34, 534–546. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, Y.; Sha, Z.; Kirumba, G.; Zhang, Y.; Bei, Z.; Cao, L. Spinach-irrigating and fertilizing for optimum quality, quantity, and economy. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 590–598. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Tognoni, F.; Serra, G.; Ferrante, A.; Piagessi, A. Use of biostimulants for reducing nutrient solution concentration in floating system. ISHM Acta Hortic. 2006, 718, 477–484. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.J.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Ugolini, L.; Cinti, S.; Righetti, L.; Stefan, A.; Matteo, R.; D’Avino, L.; Lazzeri, L. Production of an enzymatic protein hydrolyzate from defatted sunflower seed meal for potential application as a plant biostimulant. Ind. Crops Prod. 2015, 75, 15–23. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, S.X.; Malhi, S. Effects of fertilization and other agronomic measures on nutritional quality of crops. J. Sci. Food Agric. 2008, 88, 7–23. [Google Scholar] [CrossRef]
- Pereira, C.; Dias, M.I.; Petropoulos, S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R.C.; Ivanov, M.; Stojković, D.; Soković, M.; et al. The Effects of Biostimulants, Biofertilizers and Water-Stress on Nutritional Value and Chemical Composition of Two Spinach Genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef] [PubMed]
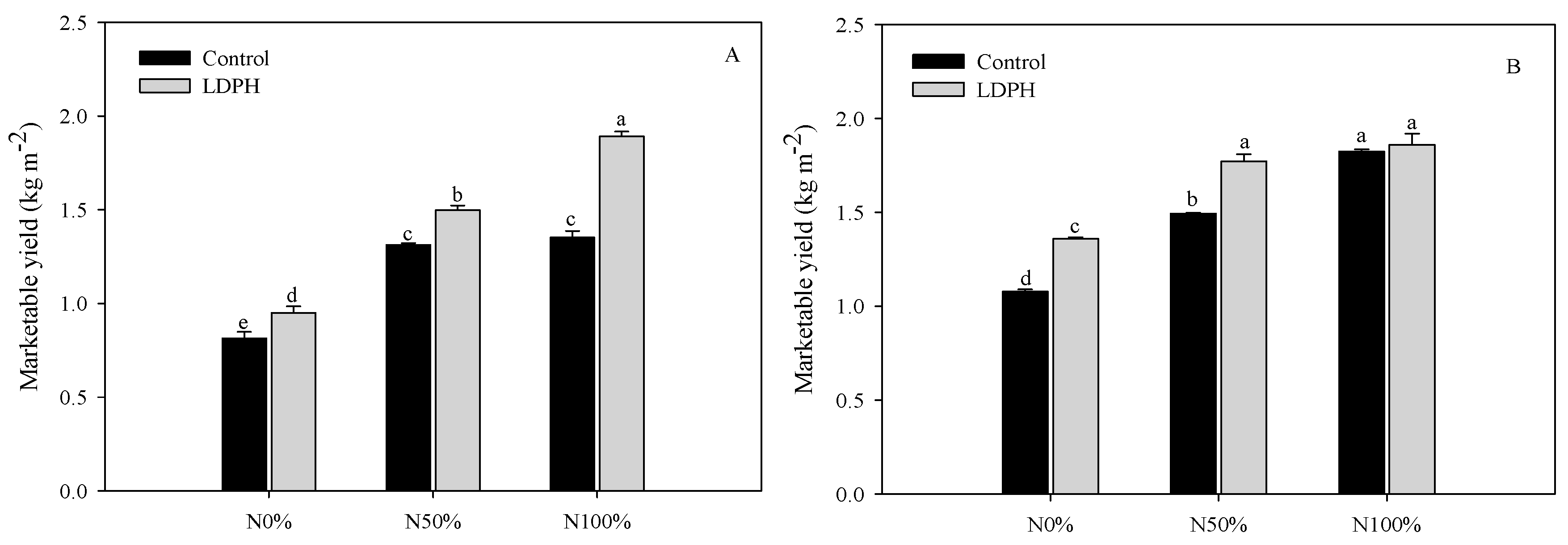
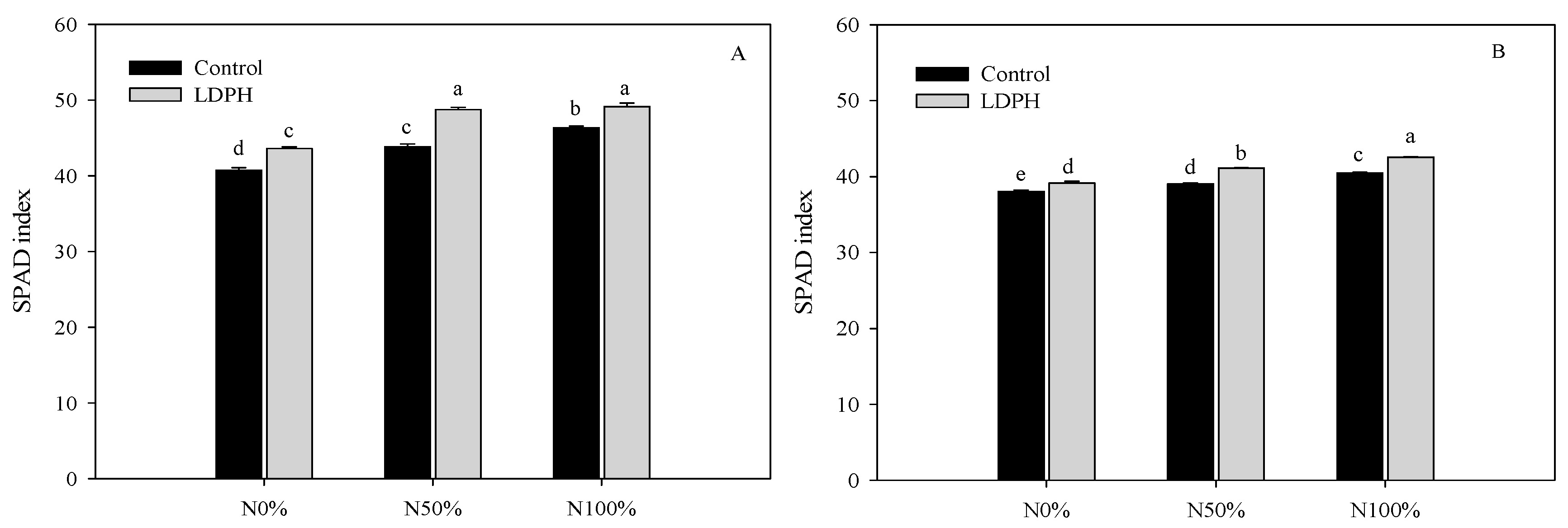
| Spinach | Lamb’s Lettuce | |||
|---|---|---|---|---|
| Yield | SPAD Index | Yield | SPAD Index | |
| Nitrogen × Biostimulant | ||||
| f value | 25.198 | 9.580 | 6.195 | 7.554 |
| Degrees of freedom | 17 | 17 | 17 | 17 |
| p value | 0.001 | 0.01 | 0.05 | 0.01 |
| Treatments | Spinach | Lamb’s Lettuce | ||
|---|---|---|---|---|
| N-Use Efficiency | N-Uptake Efficiency | N-Use Efficiency | N-Uptake Efficiency | |
| (t kg−1) | (kg kg−1) | (t kg−1) | (kg kg−1) | |
| Fertilization | ||||
| N0% | 0.35 a | 0.14 | 0.49 a | 0.20 |
| (0.29–0.40) | (0.29–0.45) | (0.48–0.56) | (0.15–0.26) | |
| N50% | 0.31 ab | 0.17 | 0.33 b | 0.21 |
| (0.25–0.36) | (0.25–0.36) | (0.25–0.34) | (0.12–0.22) | |
| N100% | 0.25 b | 0.14 | 0.25 c | 0.20 |
| (0.19–0.30) | (0.19–0.30) | (0.17–0.26) | (0.12–0.23) | |
| Biostimulant | ||||
| Control | 0.28 b | 0.12 b | 0.32 b | 0.15 b |
| (0.23–0.31) | (0.09–0.14) | (0.28–0.35) | (0.07–0.16) | |
| LDPH | 0.33 a | 0.18 a | 0.38 a | 0.26 a |
| (0.29–0.37) | (0.15–0.21) | (0.34–0.41) | (0.21–0.30) | |
| Significance | ||||
| Fertilization (F) | * | NS | ** | NS |
| Biostimulant (B) | * | ** | * | ** |
| F × B | NS | NS | NS | NS |
| Treatments | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Nitrate |
|---|---|---|---|---|
| (mg g−1 fw) | (mg g−1 fw) | (mg g−1 fw) | (mg kg−1 fw) | |
| Fertilization | ||||
| N0% | 0.905 b | 0.547 b | 1.452 b | 84.9 c |
| (0.846–0.965) | (0.418–0.675) | (1.268–1.639) | (−209.1–379.0) | |
| N50% | 0.976 ab | 0.716 ab | 1.692 ab | 2932.8 b |
| (0.917–1.035) | (0.587–0.844) | (1.508–1.876) | (20638.7–3226.8) | |
| N100% | 1.015 a | 0.786 a | 1.801 a | 3867.5 a |
| (0.955–1.074) | (0.657–0.914) | (1.616–1.984) | (3573.4–4161.6) | |
| Biostimulant | ||||
| Control | 0.957 | 0.681 | 1.637 | 476.5 b |
| (0.908–1.005) | (0.576–0.786) | (1.487-1.787) | (236.3–716.6) | |
| LDPH | 0.974 | 0.685 | 1.659 | 4113.7 a |
| (0.925–1.022) | (0.58–0.790) | (1.509–1.809) | (3873.6–4353.8) | |
| Significance | ||||
| Fertilization (F) | * | * | * | ** |
| Biostimulants (B) | NS | NS | NS | ** |
| F × B | NS | NS | NS | NS |
| Treatments | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Nitrate |
|---|---|---|---|---|
| (mg g−1 fw) | (mg g−1 fw) | (mg g−1 fw) | (mg kg−1 fw) | |
| Fertilization | ||||
| N0% | 0.673 b | 0.347 c | 1.020 c | 102.8 b |
| (0.621–0.726) | (0.312–0.372) | (0.954–1.067) | (−925.1–1130.7) | |
| N50% | 0.722 b | 0.397 b | 1.120 b | 3191.8 a |
| (0.672–0.777) | (0.373–0.429) | (1.075–1.189) | (2163.8–4219.7) | |
| N100% | 0.831 a | 0.464 a | 1.295 a | 3210.0 a |
| (0.780–0.885) | (0.430–0.498) | (1.228–1.362) | (2182.0–4237.9) | |
| Biostimulant | ||||
| Control | 0.655 b | 0.330 b | 0.985 b | 562.4 b |
| (0.613–0.698) | (0.302–0.359) | (0.931–1.040) | (−315.4–1363.1) | |
| LDPH | 0.829 a | 0.475 a | 1.304 a | 3774.0 a |
| (0.788–0.874) | (0446–0.504) | (1.251–1.361) | (2973.2–4651.8) | |
| Significance | ||||
| Fertilization (F) | ** | ** | ** | ** |
| Biostimulants (B) | ** | ** | ** | ** |
| F × B | NS | NS | NS | NS |
| Treatments | LAA | HAA | Total Phenols | AsA |
|---|---|---|---|---|
| (mM Trolox eq. 100g−1 dw) | (mM AA eq. 100g−1 dw) | (mg Gallic Acid eq. g−1 dw) | (mg g−1 fw) | |
| Fertilization | ||||
| N0% | 22.65 a | 8.08 | 3.22 a | 33.49 a |
| (22.16–23.13) | (7.38–8.77) | (2.97–3.46) | (31.46–35.51) | |
| N50% | 22.02 ab | 8.11 | 2.88 ab | 27.45 b |
| (21.54–22.50) | (7.41–8.80) | (2.63–3.12) | (25.42–29.46) | |
| N100% | 21.80 b | 8.15 | 2.48 b | 23.62 c |
| (21.32–22.28) | (7.44–8.84) | (2.23–2.72) | (21.56–25.60) | |
| Biostimulant | ||||
| Control | 20.95 b | 8.05 | 2.36 | 28.55 |
| (20.55–21.34) | (7.48–8.62) | (2.69–3.09) | (16.86–40.23) | |
| LDPH | 23.37 a | 8.17 | 2.37 | 27.82 |
| (22.97–23.76) | (7.60–8.74) | (2.62–3.03) | (16.13–39.51) | |
| Significance | ||||
| Fertilization (F) | * | NS | ** | ** |
| Biostimulants (B) | ** | NS | NS | NS |
| F × B | NS | NS | NS | NS |
| Treatments | LAA | HAA | Total phenols | AsA |
|---|---|---|---|---|
| (mM Trolox eq. 100g−1 dw) | (mM AA eq. 100g−1 dw) | (mg Gallic Acid eq. g−1 dw) | (mg g−1 fw) | |
| Fertilization | ||||
| N0% | 30.08 a | 6.26 b | 10.16 a | 63.04 a |
| (28.91–31.25) | (5.920–6.60) | (9.520–10.80) | (56.543–69.54) | |
| N50% | 28.49 ab | 7.18 a | 8.55 b | 53.41 b |
| (27.31–29.66) | (6.840–7.52) | (7.913–9.19) | (46.911–59.91) | |
| N100% | 27.77 b | 7.65 a | 7.62 c | 49.68 b |
| (26.60–28.94) | (7.311–7.99) | (6.974–8.25) | (43.177–56.17) | |
| Biostimulant | ||||
| Control | 29.40 | 6.82 b | 8.96 | 56.85 |
| (28.44–30.36) | (6.537–7.09) | (8.439–9.48) | (51.537–62.15) | |
| LDPH | 28.16 | 7.25 a | 8.59 | 53.91 |
| (27.20–29.11) | (6.968–7.52) | (8.067–9.11) | (48.603–59.21) | |
| Significance | ||||
| Fertilization (F) | * | ** | ** | * |
| Biostimulants (B) | NS | * | NS | NS |
| F × B | NS | NS | NS | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. https://doi.org/10.3390/agronomy10020278
Di Mola I, Cozzolino E, Ottaiano L, Nocerino S, Rouphael Y, Colla G, El-Nakhel C, Mori M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy. 2020; 10(2):278. https://doi.org/10.3390/agronomy10020278
Chicago/Turabian StyleDi Mola, Ida, Eugenio Cozzolino, Lucia Ottaiano, Sabrina Nocerino, Youssef Rouphael, Giuseppe Colla, Christophe El-Nakhel, and Mauro Mori. 2020. "Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application" Agronomy 10, no. 2: 278. https://doi.org/10.3390/agronomy10020278
APA StyleDi Mola, I., Cozzolino, E., Ottaiano, L., Nocerino, S., Rouphael, Y., Colla, G., El-Nakhel, C., & Mori, M. (2020). Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy, 10(2), 278. https://doi.org/10.3390/agronomy10020278










