Effect of Wood Waste and Sunflower Husk Biochar on Tensile Strength and Porosity of Dystric Cambisol Artificial Aggregates
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Biochar Physicochemical Characterization
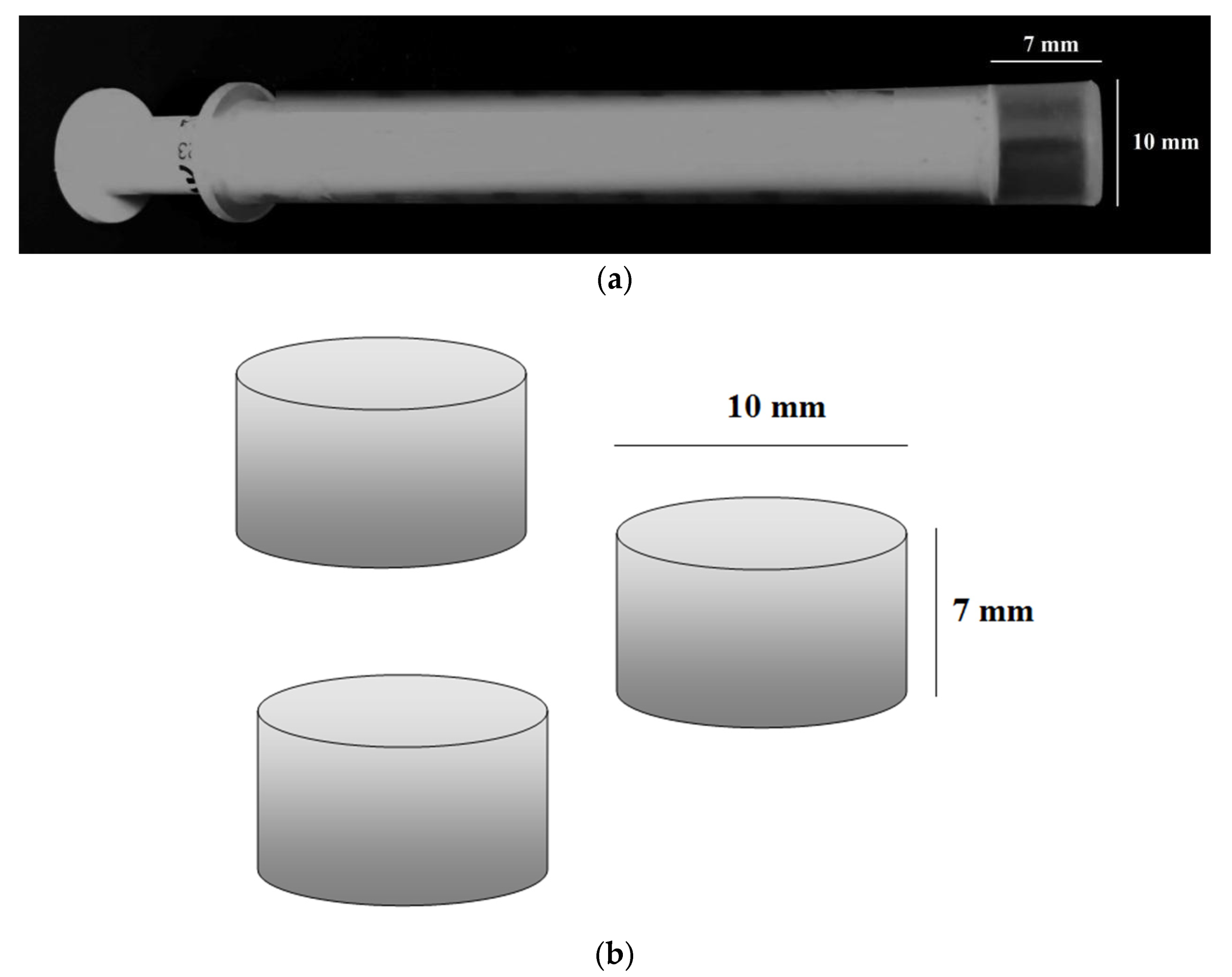
2.2. Aggregate Preparation
2.3. Porosity Measurement
2.4. Structural Stability Measurements
2.5. Statistical Analysis
3. Results and Discussion
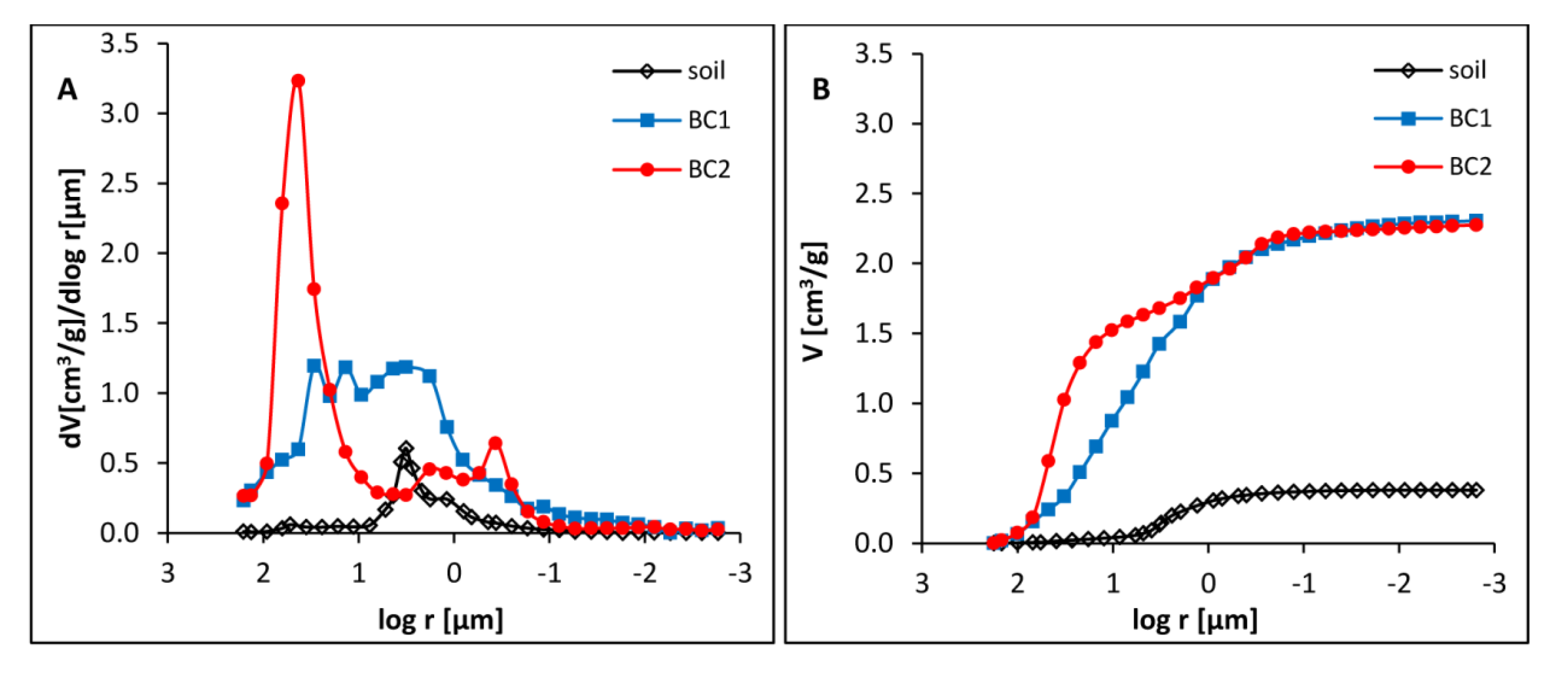
3.1. Physicochemical Properties of Dystric Cambisol and Biochars
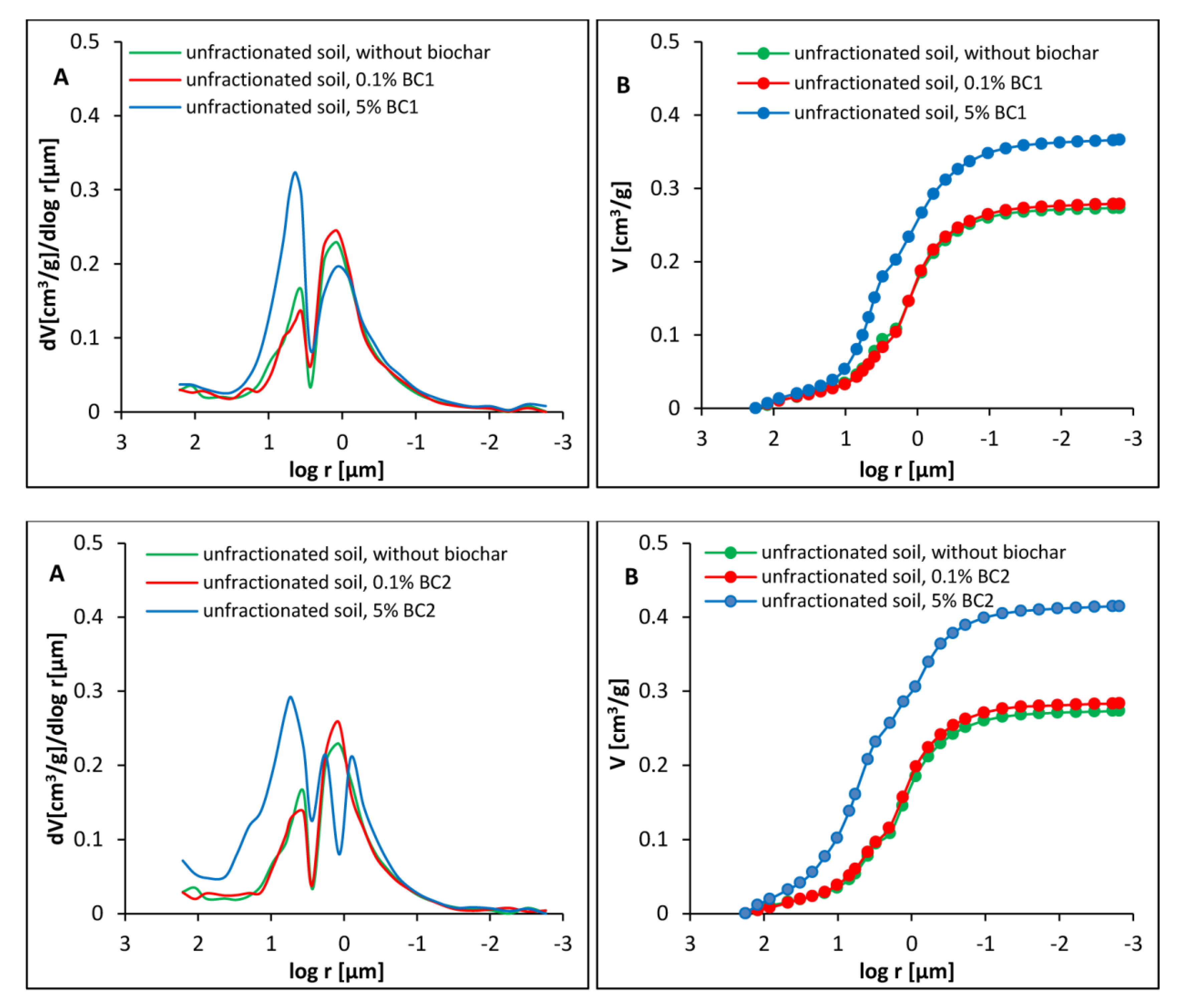
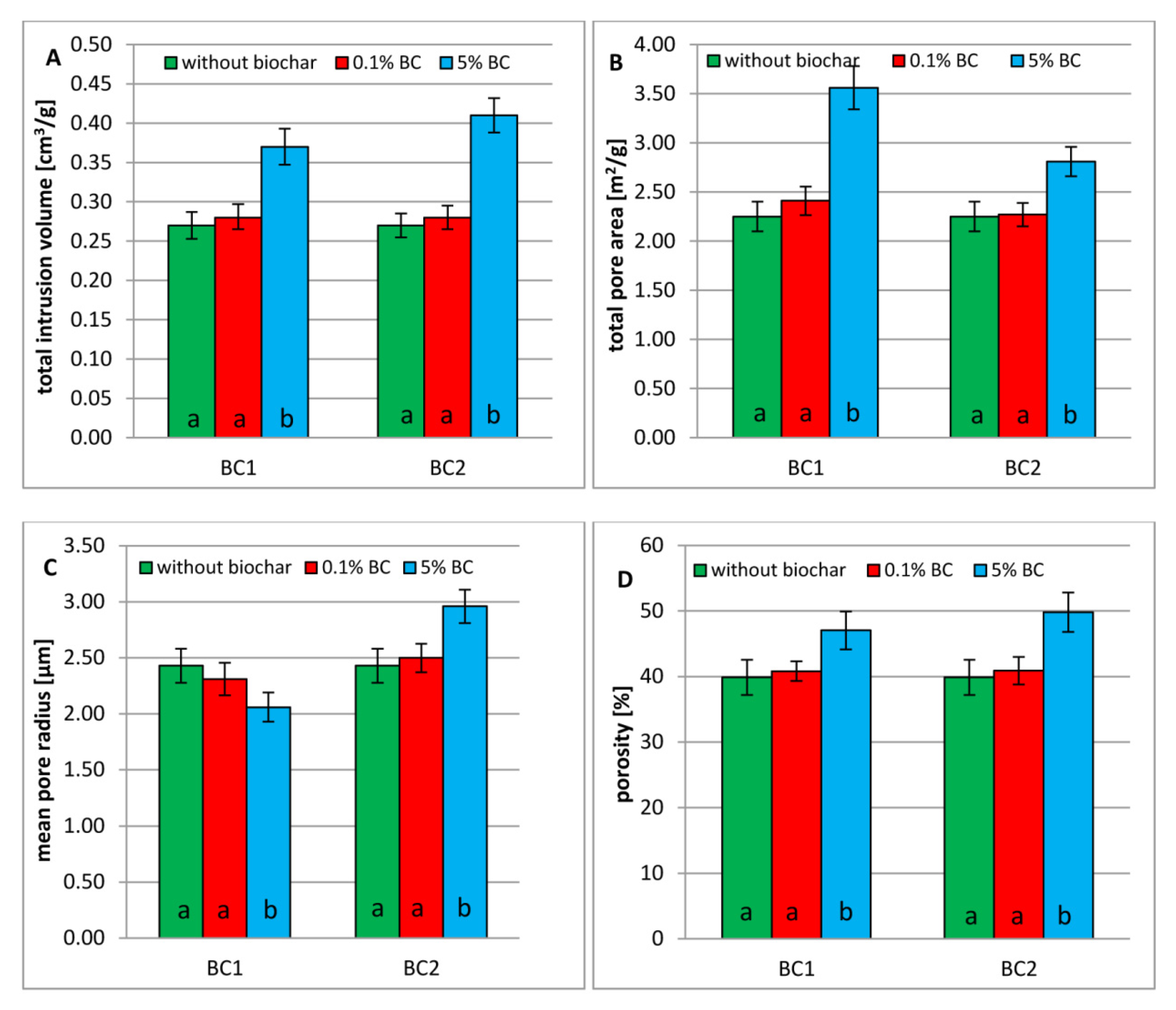
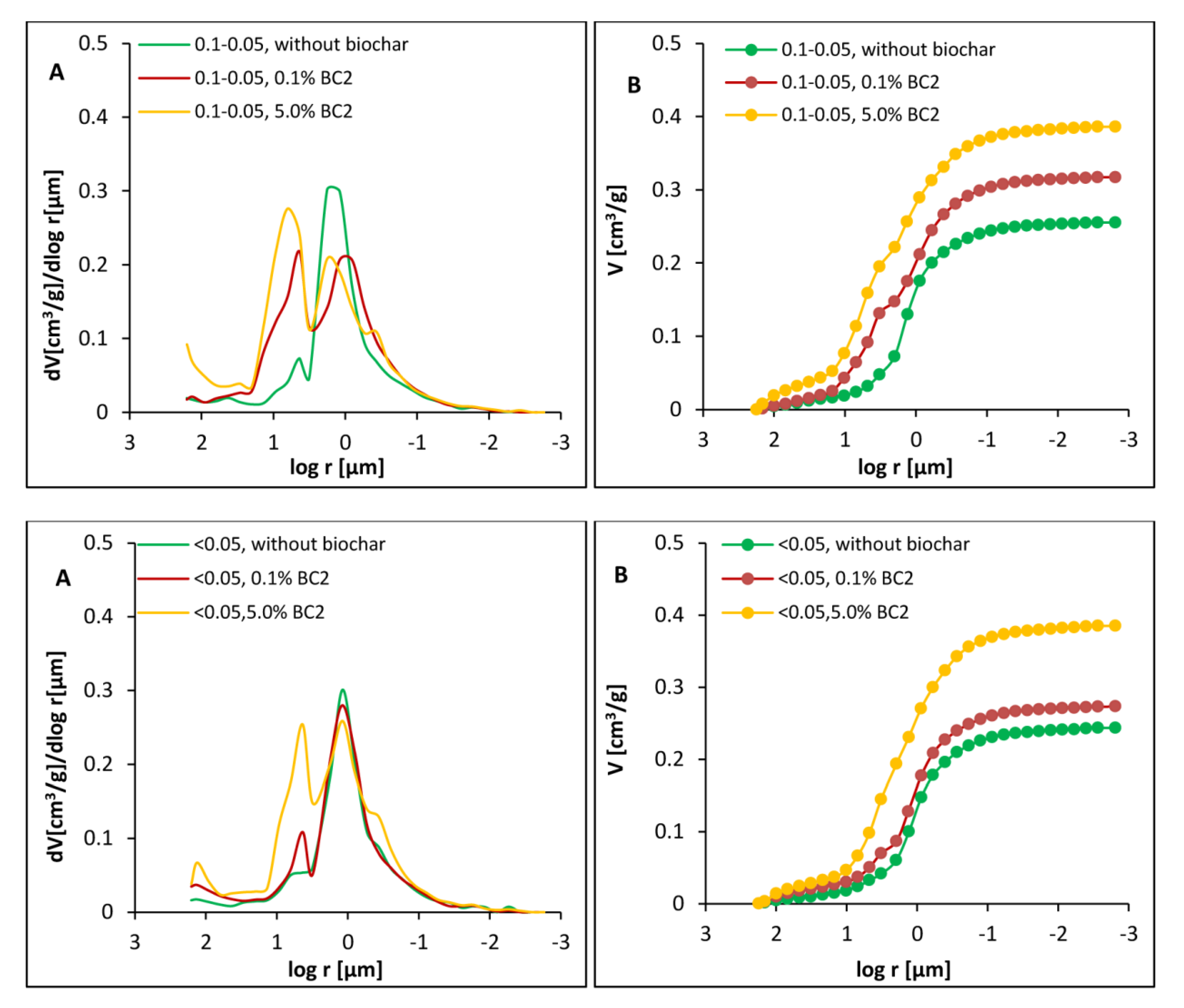
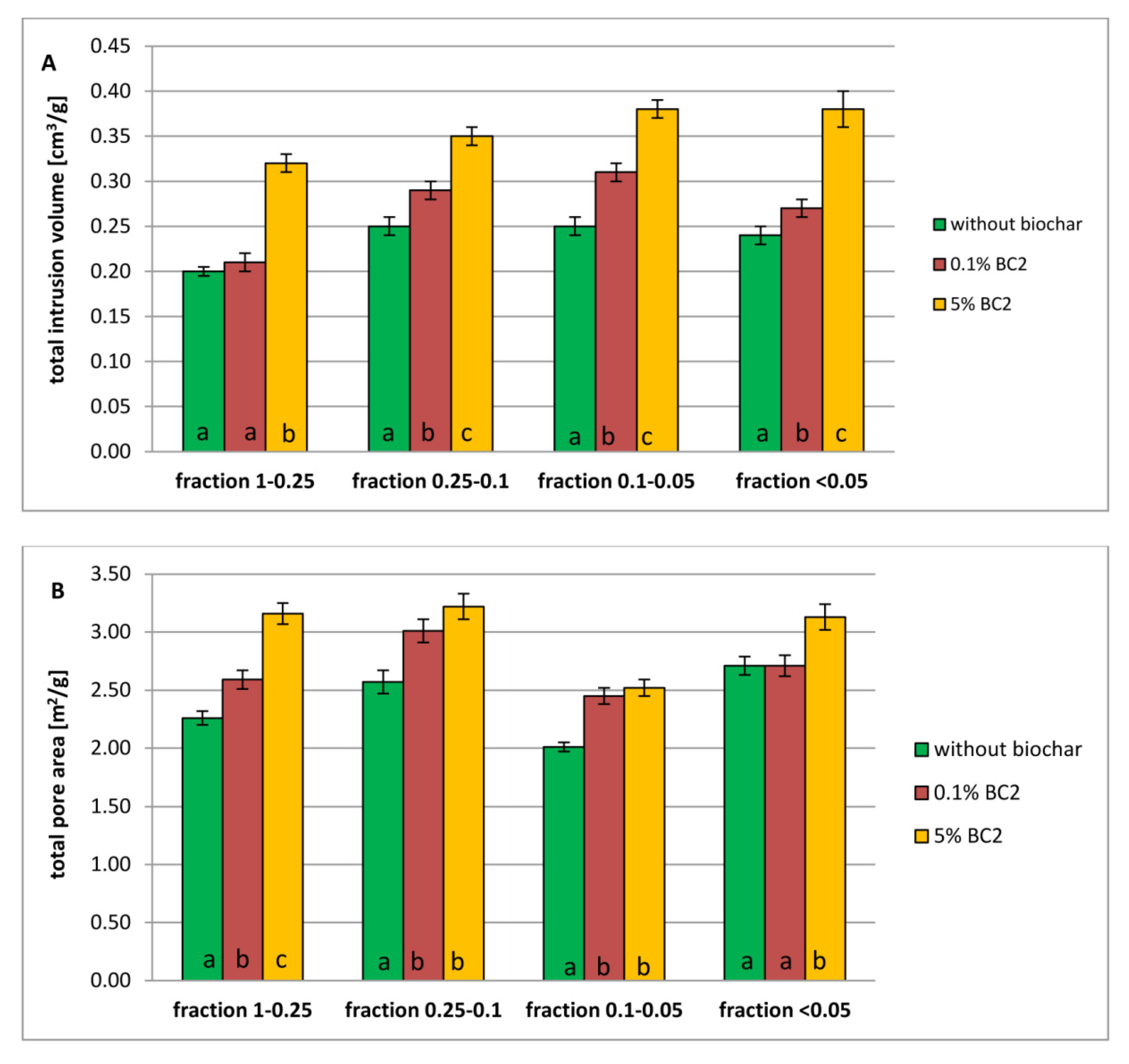
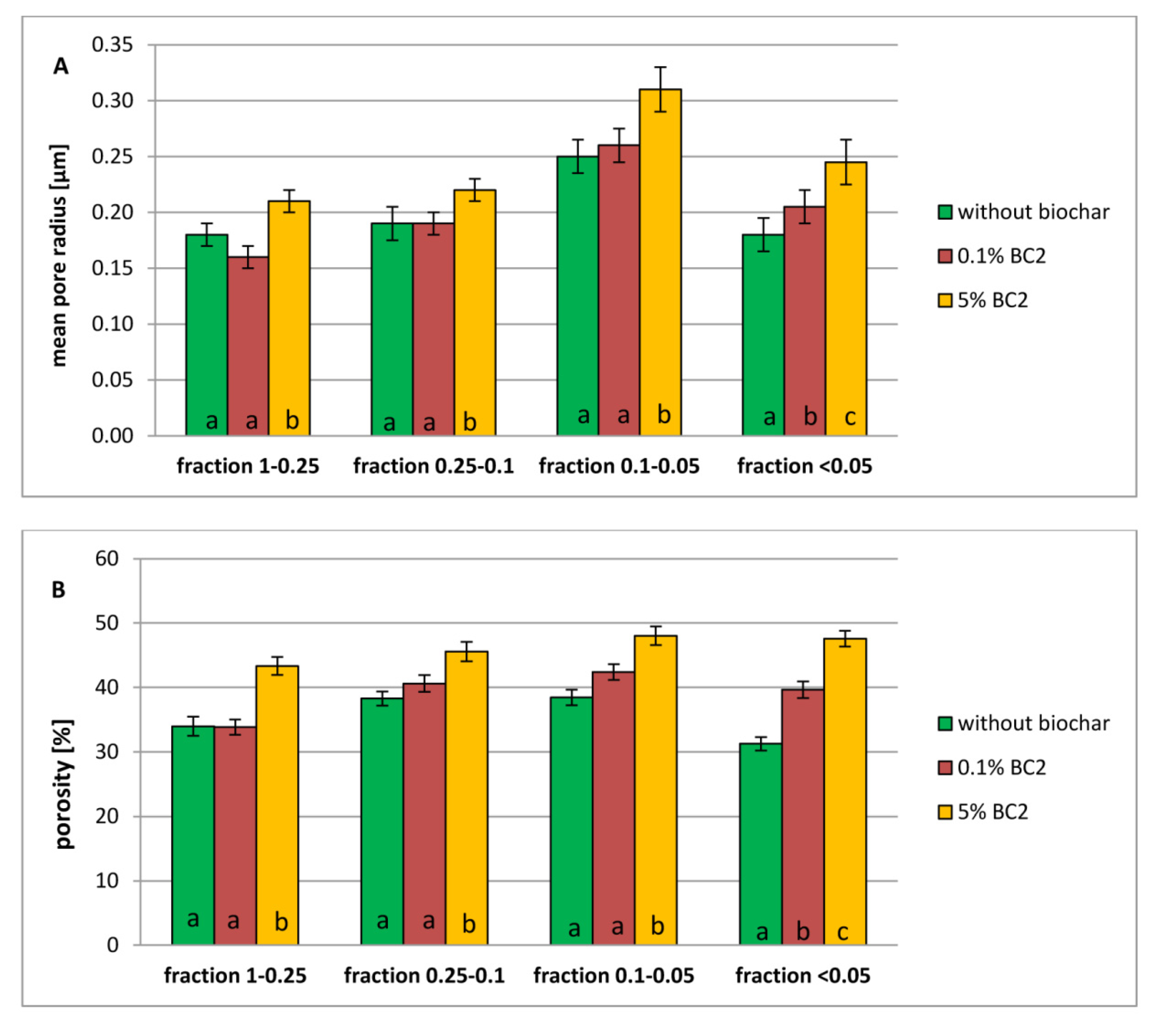
3.2. Porosity of Dystric Cambisol Cylinders with or without Biochar
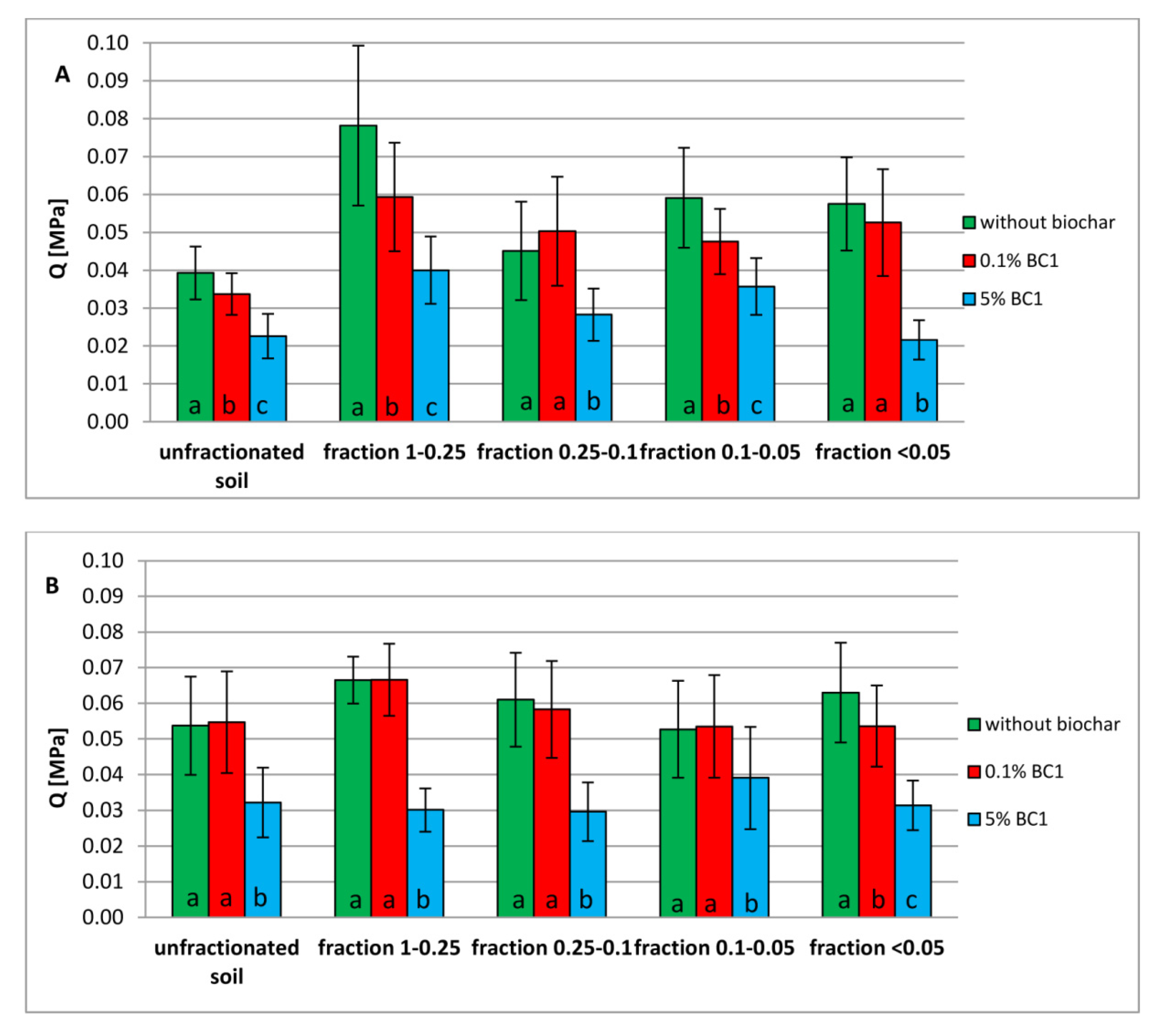
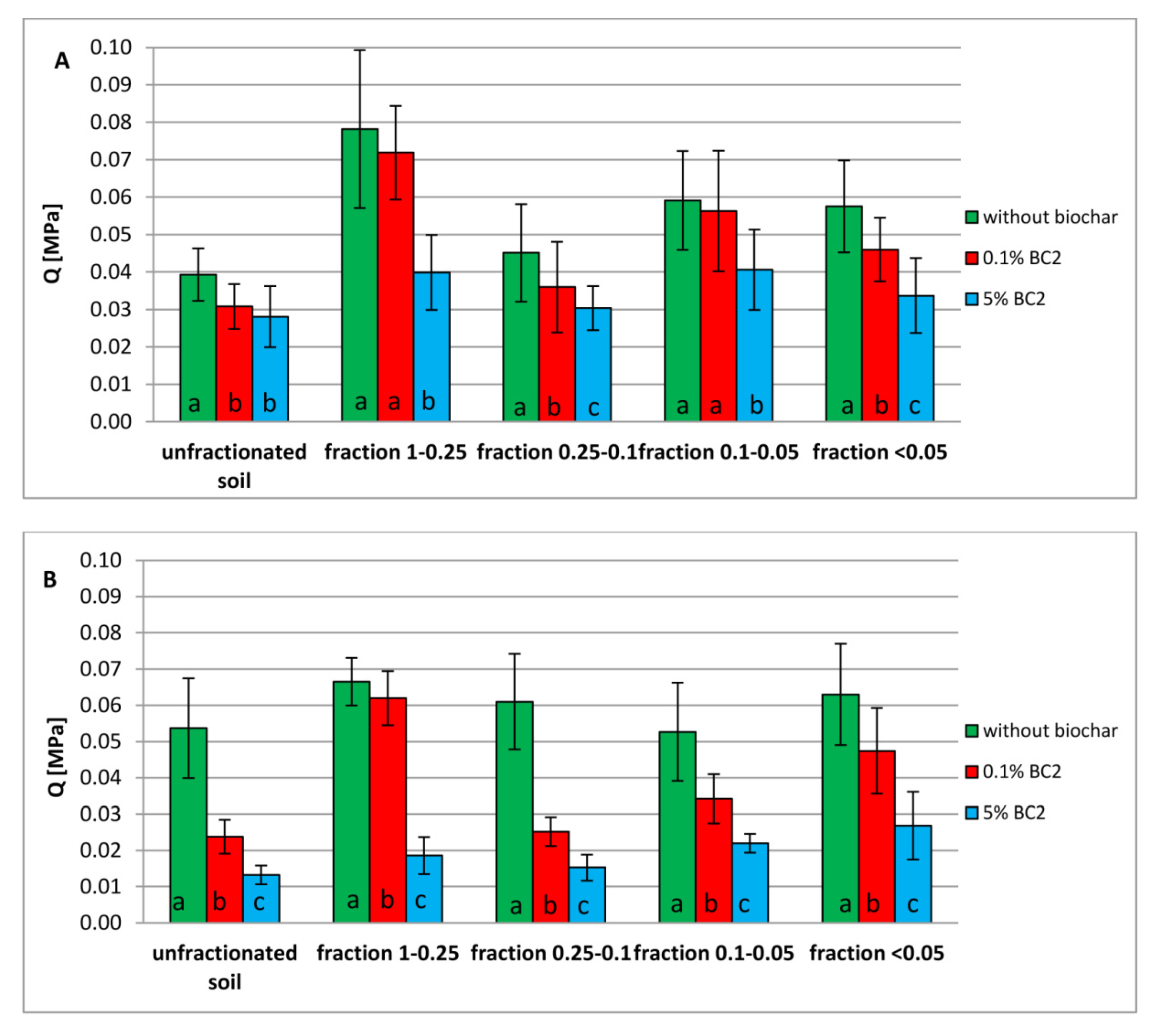
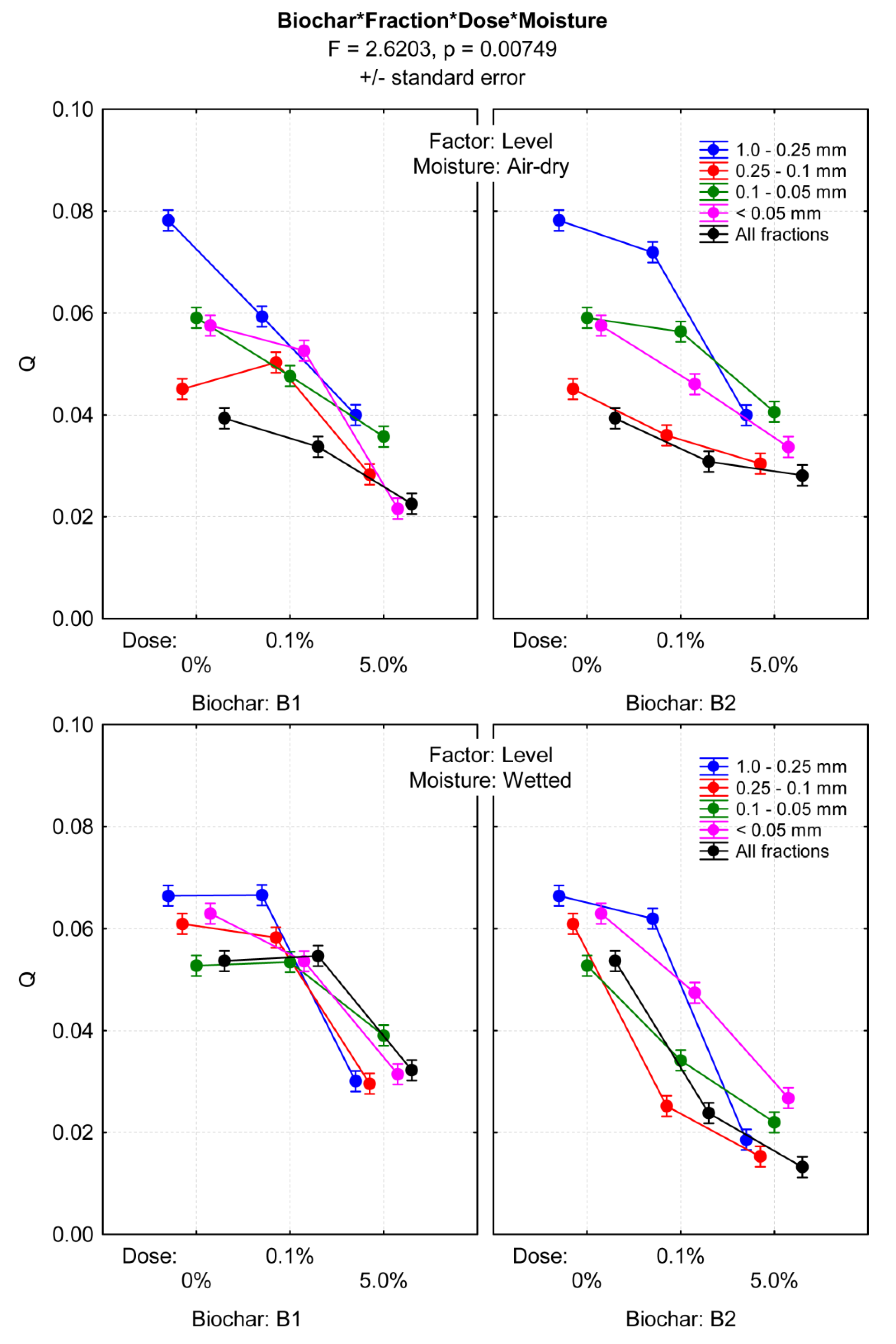
3.3. Tensile Strength of Dystric Cambisol Cylinders with or without Biochar
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarker, T.C.; Incerti, G.; Spaccini, R.; Piccolo, A.; Mazzoleni, S.; Bonanomi, G. Linking organic matter chemistry with soil aggregate stability: Insight from 13C NMR spectroscopy. Soil Biol. Biochem. 2018, 117, 175–184. [Google Scholar] [CrossRef]
- Christensen, B.T. Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur. J. Soil Sci. 2001, 52, 345–353. [Google Scholar] [CrossRef]
- Keil, R.G.; Mayer, L.M. Mineral matrices and organic matter. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 337–359. [Google Scholar]
- Oades, J.M. The role of biology in the formation, stabilization and degradation of soil structure. Geoderma 1993, 56, 377–400. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Bergmann, J.; Verbruggen, E.; Veresoglou, S.D.; Lehmann, A. Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol. 2015, 205, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Doerr, S.H.; Shakesby, R.A.; Walsh, R. Soil water repellency: Its causes, characteristics and hydro-geomorphological significance. Earth Sci. Rev. 2000, 51, 33–65. [Google Scholar] [CrossRef]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jiménez, E.; Pardo, T.; Clemente, R.; Dawson, J.J.C. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Science and Technology, Earthscan: London, UK, 2009; pp. 1–12. [Google Scholar]
- Yang, D.; Yunguo, L.; Shaobo, L.; Xixian, H.; Zhongwu, L.; Xiaofei, T.; Guangming, Z.; Lu, Z. Potential Benefits of Biochar in Agricultural Soils: A Review. Pedosphere 2017, 27, 645–661. [Google Scholar]
- Shrestha, G.; Traina, S.J.; Swanston, C.W. Black carbon’s properties and role in the environment: A comprehensive review. Sustainability 2010, 2, 294–320. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Jatav, H.S.; Jayant, H.; Kumar, S.; Kumar, V.; Chattopadhyay, A.; Dhawal, S.K.; Singh, Y.V. Role of Biochar: In agriculture sector its implication and perspective. Int. J. Chem. Stud. 2017, 5, 14–18. [Google Scholar]
- Sukaronto; Utomo, W.H.; Kusuma, Z.; Nugroho, W.H. Soil Fertility Status, Nutrient Uptake and Maize (Zea mays L.) Yield Following Biochar and Cattle Manure Application on Sandy Soils of Lombok, Indonesia. J. Trop. Agric. 2011, 49, 47–52. [Google Scholar]
- Brandstaka, T.; Helenius, J.; Hovi, J.; Kivela, J.; Koppelmaki, K.; Simojoki, A.; Soinne, H.; Tammeorg, P. Biochar Filter: Use of Biochar in Agriculture as Soil Conditioner. Report for BSAS Commitment; 2010; pp. 1–22, unpublished. [Google Scholar]
- Nigussie, A.; Kissi, E.; Misganaw, M.; Ambaw, G. Effect of Biochar Application on Soil Properties and Nutrient Uptake of Lettuces (Lactuca sativa) Grown in Chromium Polluted Soils. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 369–376. [Google Scholar]
- Ding, Y.; Liu, Y.G.; Liu, S.B.; Li, Z.W.; Tan, X.F.; Huang, X.X.; Zeng, G.M.; Zhou, L.; Zheng, B.H. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Cybulak, M.; Sokołowska, Z.; Boguta, P. Impact of biochar on physicochemical properties of Haplic Luvisol soil under different land use: A plot experiment. Agronomy 2019, 9, 531. [Google Scholar] [CrossRef]
- Cybulak, M.; Sokołowska, Z.; Boguta, P.; Tomczyk, A. Influence of pH and grain size on physicochemical properties of biochar and released humic substances. Fuel 2019, 240, 334–338. [Google Scholar] [CrossRef]
- Huang, X.X.; Liu, Y.G.; Liu, S.B.; Tan, X.F.; Ding, Y.; Zeng, G.M.; Zhou, Y.Y.; Zhang, M.M.; Wang, S.F.; Zheng, B.H. Effective removal of Cr(VI) using β-cyclodextrin-chitosan modified biochars with adsorption/reduction bifunctional roles. RSC Adv. 2016, 6, 223–228. [Google Scholar]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, C.W.T.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nosal-Wiercińska, A.; Ostolska, I.; Sternik, D.; Nowicki, P.; Pietrzak, R.; Bazan-Woźniak, A.; Goncharuk, O. Nanostructure of poly(acrylic acid) adsorption layer on the surface of activated carbon obtained from residue after supercritical extraction of hops. Nanoscale Res. Lett. 2017, 12, 2. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P.; Nosal-Wiercińska, A.; Pietrzak, R.; Szewczuk-Karpisz, K.; Ostolska, I.; Sternik, D. Adsorption of poly(acrylic acid) on the surface of microporous activated carbon obtained from cherry stones. Colloids Surf. A 2017, 514, 137–145. [Google Scholar] [CrossRef]
- Kazmierczak-Razna, J.; Nowicki, P.; Wiśniewska, M.; Nosal-Wiercińska, A.; Pietrzak, R. Thermal and physicochemical properties of phosphorus-containing activated carbons obtained from biomass. J. Taiwan Inst. Chem. Eng. 2017, 80, 1006–1013. [Google Scholar] [CrossRef]
- Horel, A.; Barna, G.; Mako, A. Soil physical properties affected by biochar addition at different plant phaenological phases. Part I. Int. Agrophys. 2019, 33, 255–262. [Google Scholar] [CrossRef]
- Hartley, W.; Riby, P.; Waterson, J. Effects of three different biochars on aggregate stability, organic carbon mobility and micronutrient bioavailability. J. Environ. Manag. 2016, 181, 770–778. [Google Scholar] [CrossRef]
- Kelly, C.N.; Benjamin, J.; Calderon, F.C.; Mikha, M.M.; Rutherford, D.W.; Rostad, C.E. The incorporation of biochar carbon into stable soil aggregates: The role of clay mineralogy and other soil characteristics. Pedosphere 2017, 27, 694–704. [Google Scholar] [CrossRef]
- Sun, F.; Lu, S. Biochars improve aggregate stability, water retention and pore-space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Ouyang, L.; Wang, F.; Tang, J.; Yu, L.; Zhang, R. Effects of biochar amendment on soil aggregates and hydraulic properties. J. Soil Sci. Plant Nutr. 2013, 13, 991–1002. [Google Scholar] [CrossRef]
- Šimanský, V. Effects of biochar and biochar with nitrogen on soil organic matter and soil structure in Haplic Luvisol. Acta Fytotech. Zootech. 2016, 19, 129–138. [Google Scholar] [CrossRef]
- Khademalrasoul, A.; Naveed, M.; Heckrath, G.; Kumari, I.; dr Jonge, L.W.; Elsgaard, L.; Vogel, H.-J.; Iversen, B.V. Biochar effects on soil aggregate properties under no-till maize. Soil Sci. 2014, 179, 273–283. [Google Scholar] [CrossRef]
- Ajayi, A.E.; Horn, R. Biochar-induced changes in soil resilience: Effects of soil texture and biochar dosage. Pedosphere 2017, 27, 236–247. [Google Scholar] [CrossRef]
- Zhou, H.; Feng, H.; Zhang, Q.; Wang, Q.; Chen, C.; Mooney, S.J.; Peng, X.; Du, Z. Biochar enhances soil hydraulic function but not soil aggregation in a sandy loam. Eur. J. Soil Sci. 2018, 70, 291–300. [Google Scholar] [CrossRef]
- Polish Standard PN-Z-19010–1 Soil Quality. Determination of Specific Surface Area of Soils by Water Sorption (BET); ALFA-WERO: Warsaw, Poland, 1997. (In Polish)
- Tyurin, I.V. Tyurin’s method. In Analytical Methods of Nutrients in Soil; Committee of the Analytical Methods of Nutrients in Soil, Ed.; Japanese Society of Soil Science and Plant Nutrition, Yakendo Co.: Tokyo, Japan, 1980; pp. 120–124. [Google Scholar]
- Alten, F.; Wandrowski, B.; Knippenberg, E. Beitrag zur Humusbestimmung. Ergebn. Agrikulturchemie 1935, IV B, 61–69. [Google Scholar]
- ASTM D4284–12. Standard Test Method for Determining Pore Volume Distribution of Catalysts and Catalyst Carriers by Mercury Intrusion Porosimetr; ASTM International: West Conshohocken, PA, USA, 1994. [Google Scholar]
- Lipiec, J.; Hajnos, M.; Świeboda, R. Estimating effects of compaction on pore size distribution of soil aggregates by mercury porosimeter. Geoderma 2012, 179, 20–27. [Google Scholar] [CrossRef]
- Brussaard, L.; van Faassen, H. Effects of compaction on soil biota and soil biological processes. In Soil Compaction in Crop Production; Soane, B.D., van Ouerkerk, C., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 215–235. [Google Scholar]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Haryanto, Y.; Widyaningrum, A.; Sudibyo, G.H.; Maryoto, A. Mechanical properties of lightweight aggregate concrete reinforced with soda can waste fibre. MATEC Web Conf. 2017, 38, 01021. [Google Scholar] [CrossRef][Green Version]
- Sing, K.S.W. Characterization of porous solids: An introductory survey. Stud. Surf. Sci. Catal. 1991, 62, 1–9. [Google Scholar]
- Lei, O.; Zhang, R. Effects of biochars derived from different feedstocks and pyrolysis temperatures on soil physical and hydraulic properties. J. Soil Sediments 2013, 13, 1561–1572. [Google Scholar] [CrossRef]
- Hass, A.; Gonzalez, J.M.; Lima, I.; Godwin, H.W.; Halvorson, J.J.; Boyer, D.G. Chicken manure biochar as liming and nutrient source for acid Appalachian soil. J. Environ. Qual. 2012, 41, 1096–1106. [Google Scholar] [CrossRef]
- Meleka, N.N.; Bashandy, A.A.; Arab, M.A. Economical reactive powder concrete cast using available materials in North Sinai, Egypt. Arch. Civ. Eng. 2013, LIX 2, 175–195. [Google Scholar] [CrossRef][Green Version]
- Batremieux, R.; Le Borgne, E.; Monnier, G. Evolution de certaines prorietes du sol sous l’influence du chauffage. C. R. Acad. Sci. 1960, 251, 2752–2755. [Google Scholar]
- Giovannini, G.; Lucchesi, S.; Giachetti, M. Beneficial and detrimental effects of heating on soil quality. In Fire in Ecosystem Dynamics; Goldammer, J.C., Jenkins, M.J., Eds.; SPB Academic Publishing: Ann Arbor, MI, USA, 1990; pp. 95–102. [Google Scholar]
- Arcenegui, V.; Mataix-Solera, J.; Guerro, C.; Zornoza, R.; Mataix-Beneyto, J.; Garcia-Orenes, F. Immediate effects of wildfires on water repellency and aggregate stability in Mediterranean calcareous soils. Catena 2008, 74, 219–226. [Google Scholar] [CrossRef]
- Albalasmeh, A.; Ghazzehei, T. Soil aggregate formation: The role of wetting-drying cycles in the genesis of interparticle bonding. Geophys. Res. Abstr. 2013, 15. [Google Scholar]

| Parameter | Soil | BC1 | BC2 | |
|---|---|---|---|---|
| Specific surface area [m2/g] | 15.2 ± 0.7 | 69.9 ± 2.2 | 80.9 ± 2.1 | |
| pHH20 | 6.3 ± 0.1 | 8.2 ± 0.1 | 9.7 ± 0.2 | |
| pHKCl | 6.1 ± 0.1 | 7.6 ± 0.1 | 9.2 ± 0.2 | |
| Density [g/cm3] | 2.6 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | |
| Ash content [w%] | 97.0 ± 1.5 | 43.7 ± 1.8 | 35.4 ± 1.1 | |
| Organic carbon content [%] | 1.2 ± 0.1 | 12.3 ± 0.2 | 22.5 ± 0.2 | |
| Texture [%] | Sand (2–0.05 mm) | 16 ± 1.2 | - | - |
| Silt (0.05–0.002 mm) | 75 ± 1.5 | - | - | |
| Clay (<0.002 mm) | 9 ± 0.8 | - | - | |
| Total Intrusion Volume [cm3/g] | Average Pore Radius [μm] | Total Pore Area [m2/g] | Total Porosity [%] | |
|---|---|---|---|---|
| Soil | 0.38 ± 0.02 | 0.55 ± 0.03 | 1.38 ± 0.07 | 49.18 ± 1.53 |
| BC1 | 2.31 ± 0.14 | 0.20 ± 0.01 | 22.72 ± 1.41 | 74.45 ± 2.54 |
| BC2 | 2.28 ± 0.11 | 0.24 ± 0.01 | 19.01 ± 0.89 | 75.92 ± 3.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokołowska, Z.; Szewczuk-Karpisz, K.; Turski, M.; Tomczyk, A.; Cybulak, M.; Skic, K. Effect of Wood Waste and Sunflower Husk Biochar on Tensile Strength and Porosity of Dystric Cambisol Artificial Aggregates. Agronomy 2020, 10, 244. https://doi.org/10.3390/agronomy10020244
Sokołowska Z, Szewczuk-Karpisz K, Turski M, Tomczyk A, Cybulak M, Skic K. Effect of Wood Waste and Sunflower Husk Biochar on Tensile Strength and Porosity of Dystric Cambisol Artificial Aggregates. Agronomy. 2020; 10(2):244. https://doi.org/10.3390/agronomy10020244
Chicago/Turabian StyleSokołowska, Zofia, Katarzyna Szewczuk-Karpisz, Marcin Turski, Agnieszka Tomczyk, Marta Cybulak, and Kamil Skic. 2020. "Effect of Wood Waste and Sunflower Husk Biochar on Tensile Strength and Porosity of Dystric Cambisol Artificial Aggregates" Agronomy 10, no. 2: 244. https://doi.org/10.3390/agronomy10020244
APA StyleSokołowska, Z., Szewczuk-Karpisz, K., Turski, M., Tomczyk, A., Cybulak, M., & Skic, K. (2020). Effect of Wood Waste and Sunflower Husk Biochar on Tensile Strength and Porosity of Dystric Cambisol Artificial Aggregates. Agronomy, 10(2), 244. https://doi.org/10.3390/agronomy10020244







