Exogenous Application of Sucrose Promotes Postharvest Ripening of Kiwifruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Experiments with Detached Fruits
2.3. Fruit Firmness
2.4. Ethylene Production
2.5. Quantification of Sucrose
2.6. RNA Extraction and qRT-PCR
2.7. Identification of Kiwifruit Sugar Transporter Genes and Phylogenetic Analyses
2.8. Cis-Elements Analysis
2.9. Statistical Analyses
3. Results
3.1. Exogenous Sucrose Modulates Kiwifruit Ripening
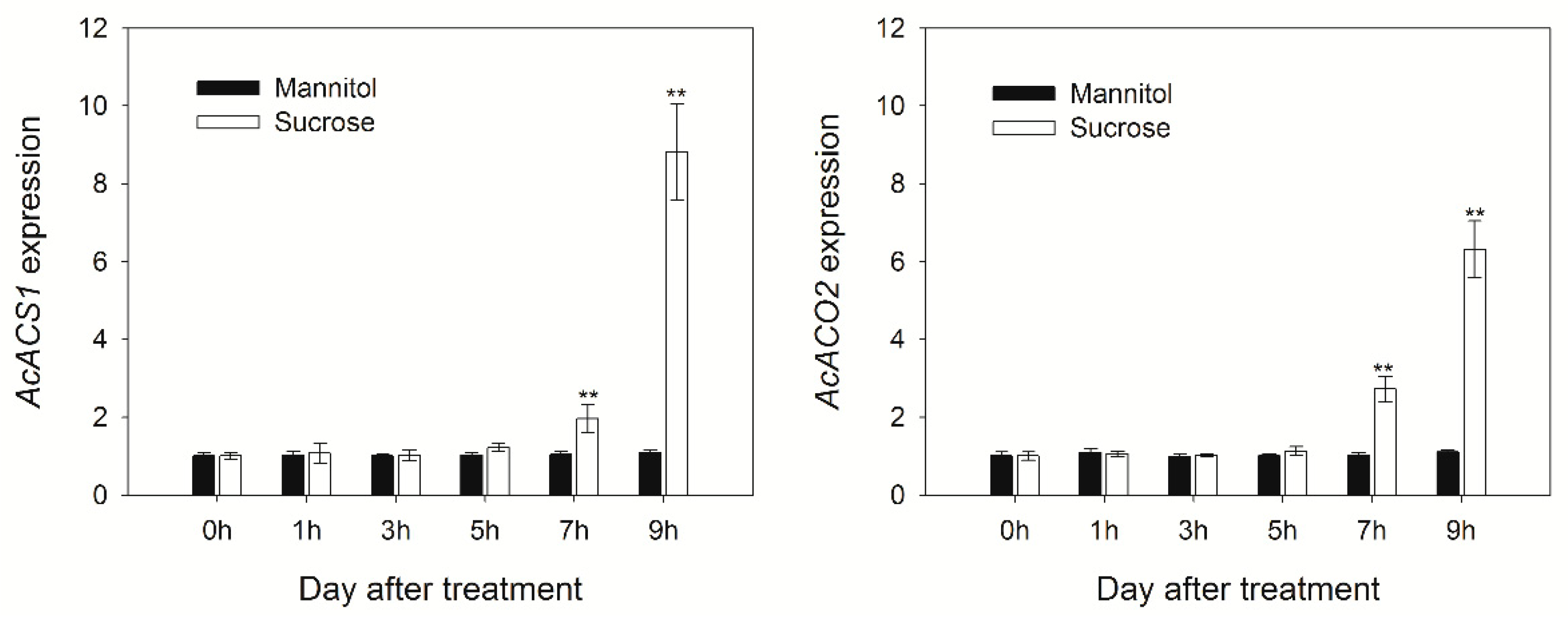
3.2. Changes in the Expression of Genes Involved in Ethylene Biosynthesis and Ripening
3.3. Isolation and Phylogenetic Relationship of the Kiwifruit Sucrose Transporter Genes
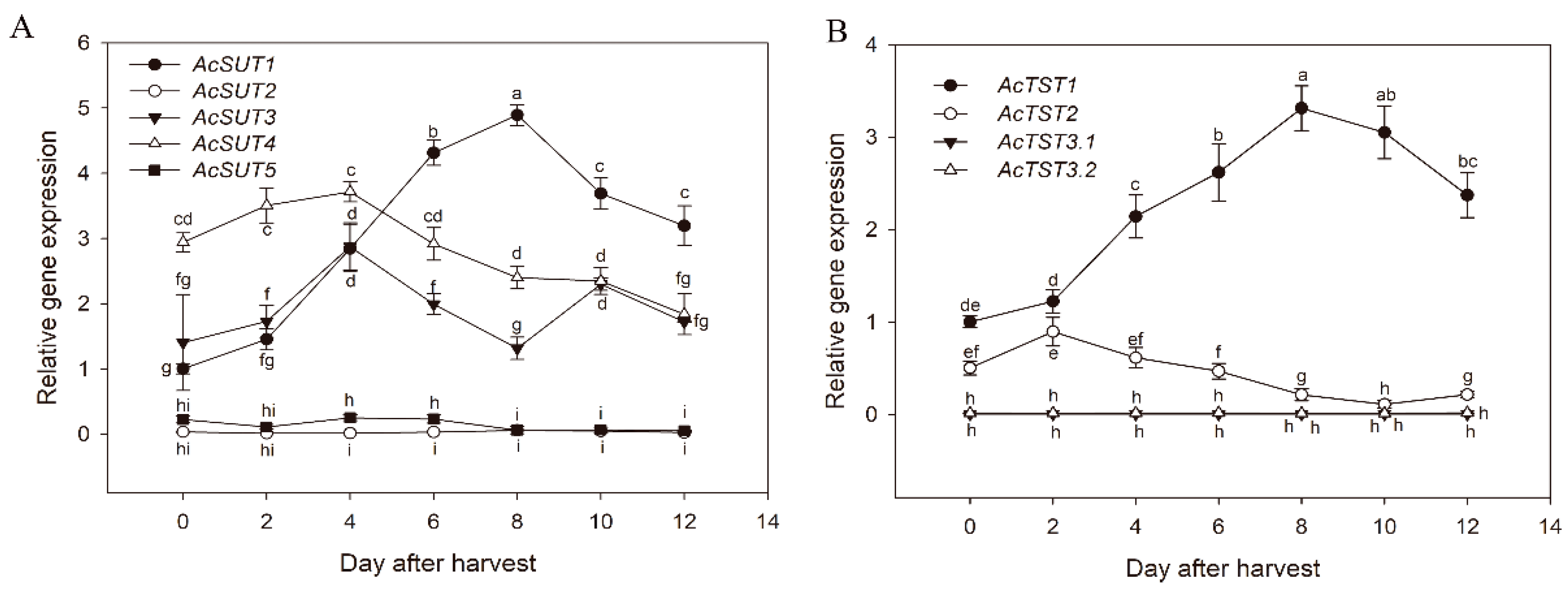
3.4. Expression of Genes during Postharvest Fruit Ripening
3.5. Cis-Elements in Sugar Transporter Gene Promoters in Kiwifruit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1-MCP | 1-Methylcyclopropene |
| MES | 2-(N-Morpholino) ethanesulfonic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| SUT | sucrose transporter |
| TST | tonoplast monosaccharide transporter |
| SWEET | Sugar Will Eventually be Exported Transporters |
| qRT-PCR | Quantitative real time polymerase chain reaction |
| DAH | days after harvest |
References
- Ampa, K.; Saito, T.; Okawa, K.; Ohara, H.; Kondo, S. Effects of ethephon and abscisic acid application on ripening-related genes in ‘kohi’ kiwifruit (Actinidia chinensis) on the vine. Hortic. Plant J. 2017, 3, 29–33. [Google Scholar] [CrossRef]
- Yin, X.; Allan, A.C.; Chen, K.; Ferguson, I.B. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol. 2010, 153, 1280–1292. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Allan, A.C.; Wu, R.; Zhang, B.; Lallu, N.; Ferguson, I.B. Ethylene-induced modulation of genes associated with the ethylene signalling pathway in ripening kiwifruit. J. Exp. Bot. 2008, 59, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, K.; McAtee, P.A.; Nardozza, S.; Pidakala, P.; Wang, R.L.; David, K.; Burdon, J.; Schaffer, R.J. Copy number variants in kiwifruit ETHYLENE RESPONSE FACTOR/APETALA2 (ERF/AP2)-like genes show divergence in fruit ripening associated cold and ethylene responses in C-REPEAT/DRE BINDING FACTOR-like genes. PLoS ONE 2019, 14, e0216120. [Google Scholar] [CrossRef] [PubMed]
- Asiche, W.O.; Mitalo, O.W.; Kasahara, Y.; Tosa, Y.; Mworia, E.G.; Owino, W.O.; Ushijima, K.; Nakano, R.; Yano, K.; Kubo, Y. Comparative transcriptome analysis reveals distinct ethylene-independent regulation of ripening in response to low temperature in kiwifruit. BMC Plant Biol. 2018, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Minas, I.S.; Tanou, G.; Karagiannis, E.; Belghazi, M.; Molassiotis, A. Coupling of Physiological and Proteomic Analysis to Understand the Ethylene- and Chilling-Induced Kiwifruit Ripening Syndrome. Front. Plant Sci. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.D.; Hu, X.; Kuang, S.; Ge, H.; Yin, X.R.; Chen, K.S. Isolation, classification and transcription profiles of the Ethylene Response Factors (ERFs) in ripening kiwifruit. Sci. Hortic. 2016, 199, 209–215. [Google Scholar] [CrossRef]
- Zhang, A.D.; Wang, W.Q.; Tong, Y.; Li, M.J.; Grierson, D.; Ferguson, I.; Chen, K.S.; Yin, X.R. Transcriptome Analysis Identifies a Zinc Finger Protein Regulating Starch Degradation in Kiwifruit. Plant Physiol. 2018, 178, 850–863. [Google Scholar] [CrossRef]
- Rai, M.K.; Shekhawat, N.S. Recent advances in genetic engineering for improvement of fruit crops. Plant Cell Tissue Organ Cult. 2014, 116, 1–15. [Google Scholar] [CrossRef]
- Zheng, Q.; Tang, Z.; Xu, Q.; Deng, X. Isolation, phylogenetic relationship and expression profiling of sugar transporter genes in sweet orange (Citrus sinensis). Plant Cell Tissue Organ Cult. 2014, 119, 609–624. [Google Scholar] [CrossRef]
- Cakir, B.; Agasse, A.; Gaillard, C.; Saumonneau, A.; Delrot, S.; Atanassova, R. A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 2003, 15, 2165–2180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.; Wang, S.; Xue, H.; Su, Y.; Yang, J.; Li, X. Identification of candidate genes involved in the sugar metabolism and accumulation during pear fruit post-harvest ripening of ‘Red Clapp’s Favorite’ (Pyrus communis L.) by transcriptome analysis. Hereditas 2018, 155, 11. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Jiu, S.; Zhang, C.; Wang, C.; Tariq, P.; Liu, Z.; Wang, B.; Cui, L.; Fang, J. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol. J. 2016, 14, 2045–2065. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhao, Y.; Li, X.; Ding, N.; Li, C.; Ji, W.; et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef]
- Li, D.; Mou, W.; Wang, Y.; Li, L.; Mao, L.; Ying, T.; Luo, Z. Exogenous sucrose treatment accelerates postharvest tomato fruit ripening through the influence on its metabolism and enhancing ethylene biosynthesis and signaling. Acta Physiol. Plant. 2016, 38, 225. [Google Scholar] [CrossRef]
- Orlich, G. Analysis of the driving forces of phloem transport in Ricinus seedlings: Sucrose export and volume flow are determined by the source. Planta 1998, 206, 266–271. [Google Scholar] [CrossRef]
- Berthier, A.; Desclos, M.; Amiard, V.; Morvan-Bertrand, A.; Demmig-Adams, B.; Adams, W.W., III; Turgeon, R.; Prud’homme, M.-P.; Noiraud-Romy, N. Activation of sucrose transport in defoliated lolium Perenne l.: An example of apoplastic phloem loading plasticity. Plant Cell Physiol. 2009, 50, 1329–1344. [Google Scholar] [CrossRef]
- Rennie, E.A.; Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA 2009, 106, 14162–14167. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Xie, Z.; Zou, X.L.; Casaretto, J.; Ho, T.H.D.; Shen, Q.X.J. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 2004, 134, 1500–1513. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Peng, Y.B.; Pelleschi-Travier, S.; Fan, Y.; Lu, Y.F.; Lu, Y.M.; Gao, X.P.; Shen, Y.Y.; Delrot, S.; Zhang, D.P. Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol. 2004, 135, 574–586. [Google Scholar] [CrossRef]
- Koch, K.E.; Avigne, W.T. Postphloem, nonvascular transfer in citrus—Kinetics, metabolism, and sugar gradients. Plant Physiol. 1990, 93, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Wang, X.; Xia, G.; Pan, Q.; Fan, R.; Wu, F.; Yu, X.; Zhang, D. A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol. 2006, 142, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L.; Patrick, J.W. The cellular pathway of postphloem sugar-transport in developing tomato fruit. Planta 1995, 196, 434–444. [Google Scholar] [CrossRef]
- Chen, C.; Yuan, Y.; Zhang, C.; Li, H.; Ma, F.; Li, M. Sucrose phloem unloading follows an apoplastic pathway with high sucrose synthase in Actinidia fruit. Plant Sci. 2017, 255, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, X.; Hou, B.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Sivitz, A.B.; Reinders, A.; Ward, J.M. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 2008, 147, 92–100. [Google Scholar] [CrossRef]
- Jung, B.; Ludewig, F.; Schulz, A.; Meißner, G.; Wöstefeld, N.; Flügge, U.I.; Pommerrenig, B.; Wirsching, P.; Sauer, N.; Koch, W. Identification of the transporter responsible for sucrose accumulation in sugar beet taproots. Nat. Plants 2015, 1, 14001. [Google Scholar] [CrossRef]
- Wormit, A.; Trentmann, O.; Feifer, I.; Lohr, C.; Tjaden, J.; Meyer, S.; Schmidt, U.; Martinoia, E.; Neuhaus, H.E. Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell 2006, 18, 3476–3490. [Google Scholar] [CrossRef]
- Schneider, S.; Hulpke, S.; Schulz, A.; Yaron, I.; Höll, J.; Imlau, A.; Schmitt, B.; Batz, S.; Wolf, S.; Hedrich, R. Vacuoles release sucrose via tonoplast-localised SUC4-type transporters. Plant Biol. 2012, 14, 325–336. [Google Scholar] [CrossRef]
- Schulz, A.; Beyhl, D.; Marten, I.; Wormit, A.; Neuhaus, E.; Poschet, G.; Büttner, M.; Schneider, S.; Sauer, N.; Hedrich, R. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 2011, 68, 129–136. [Google Scholar] [CrossRef]
- Reinders, A.; Sivitz, A.; Starker, C.; Gantt, J.; Ward, J. Functional analysis of LjSUT4, a vacuolar sucrose transporter from Lotus japonicus. Plant Mol. Biol. 2008, 68, 289–299. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van, P.Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 2000, 12, 599–609. [Google Scholar] [CrossRef]
- Baumann, K.; Paolis, A.D.; Costantino, P.; Gualberti, G. The DNA binding site of the Dof protein NtBBF1 is essential for tissue-specific and auxin-regulated expression of the rolB oncogene in plants. Plant Cell 1999, 11, 323–333. [Google Scholar] [CrossRef]
- Grierson, C.; Du, J.S.; Zabala, M.D.; Beggs, K.; Smith, C.; Holdsworth, M.; Bevan, M. Separate cis sequences and trans factors direct metabolic and developmental regulation of a potato-tuber storage protein gene. Plant J. 1994, 5, 815–826. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Karrer, E.E.; Thomas, B.R.; Chen, L.; Rodriguez, R.L. Three cis-elements required for rice alpha-amylase Amy3D expression during sugar starvation. Plant Mol. Biol. 1998, 36, 331–341. [Google Scholar] [CrossRef]
- Hu, X.; Kuang, S.; Zhang, A.D.; Zhang, W.S.; Chen, M.J.; Yin, X.R.; Chen, K.S. Characterization of Starch Degradation Related Genes in Postharvest Kiwifruit. Int. J. Mol. Sci. 2016, 17, 2112. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wu, J.; Chen, S.; Chen, H.; Chai, L.; Yi, H. Comparative transcriptome analyses between a spontaneous late-ripening sweet orange mutant and its wild type suggest the functions of ABA, sucrose and ja during citrus fruit ripening. PLoS ONE 2014, 9, e116056. [Google Scholar] [CrossRef]
- Barker, L.; Kuhn, C.; Weise, A.; Schulz, A.; Gebhardt, C.; Hirner, B.; Hellmann, H.; Schulze, W.; Ward, J.M.; Frommer, W.B. SUT2, a putative sucrose sensor in sieve elements. Plant Cell 2000, 12, 1153–1164. [Google Scholar] [CrossRef]
- Weise, A.; Barker, L.; Kuhn, C.; Lalonde, S.; Buschmann, H.; Frommer, W.B.; Ward, J.M. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 2000, 12, 1345–1355. [Google Scholar] [CrossRef]
- Manning, K.; Davies, C.; Bowen, H.C.; White, P.J. Functional characterization of two ripening-related sucrose transporters from grape berries. Ann. Bot. 2001, 87, 125–129. [Google Scholar] [CrossRef]
- Li, C.Y.; Shi, J.X.; Weiss, D.; Goldschmidt, E.E. Sugars regulate sucrose transporter gene expression in citrus. Biochem. Biophys. Res. Commun. 2003, 306, 402–407. [Google Scholar] [CrossRef]
- Zanon, L.; Falchi, R.; Hackel, A.; Kuehn, C.; Vizzotto, G. Expression of peach sucrose transporters in heterologous systems points out their different physiological role. Plant Sci. 2015, 238, 262–272. [Google Scholar] [CrossRef]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef]
- Cheng, J.; Wen, S.; Xiao, S.; Lu, B.; Ma, M.; Bie, Z. Overexpression of the tonoplast sugar transporter CmTST2 in melon fruit increases sugar accumulation. J. Exp. Bot. 2018, 69, 511–523. [Google Scholar] [CrossRef]
- Chen, L.; Lin, I.W.; Qu, X.; Sosso, D.; McFarlane, H.E.; Londono, A.; Samuels, A.L.; Frommer, W.B. A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef]
- Eom, J.S.; Chen, L.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef]
- Lin, I.W.; Sosso, D.; Chen, L.; Gase, K.; Kim, S.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.; Qu, X.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef]






| Cis-Element | Sequence | Response | Copies/Promoter | ||
|---|---|---|---|---|---|
| AcSUT1 | AcSUT4 | AcTST1 | |||
| DPBFCOREDCDC3 | ACACNNG | Abscisic acid | 1 | 4 | 5 |
| MYCCONSENSUSAT | CANNTG | Abscisic acid | 7 | 9 | 6 |
| WRKY71OS | TGACY | Gibberellin | 11 | 17 | 25 |
| NTBBF1ARROLB | ACTTTA | Auxin | 4 | 2 | 1 |
| CGACGOSAMY3 | CGACG | Sugar repress | 1 | 0 | 2 |
| AMYBOX1 | TAACAAA | Sugar repress | 0 | 1 | 0 |
| WBOXHVISO1 | TGACT | Sugar induce | 4 | 3 | 4 |
| SUCROSE BOX 3 | AAATCA…AA | Sucrose induce | 5 | 5 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, L.; Yuan, X.; Chen, C.; Wan, C.; Fu, Y.; Chen, J.; Gan, Z. Exogenous Application of Sucrose Promotes Postharvest Ripening of Kiwifruit. Agronomy 2020, 10, 245. https://doi.org/10.3390/agronomy10020245
Fei L, Yuan X, Chen C, Wan C, Fu Y, Chen J, Gan Z. Exogenous Application of Sucrose Promotes Postharvest Ripening of Kiwifruit. Agronomy. 2020; 10(2):245. https://doi.org/10.3390/agronomy10020245
Chicago/Turabian StyleFei, Liuying, Xin Yuan, Chuying Chen, Chunpeng Wan, Yongqi Fu, Jinyin Chen, and Zengyu Gan. 2020. "Exogenous Application of Sucrose Promotes Postharvest Ripening of Kiwifruit" Agronomy 10, no. 2: 245. https://doi.org/10.3390/agronomy10020245
APA StyleFei, L., Yuan, X., Chen, C., Wan, C., Fu, Y., Chen, J., & Gan, Z. (2020). Exogenous Application of Sucrose Promotes Postharvest Ripening of Kiwifruit. Agronomy, 10(2), 245. https://doi.org/10.3390/agronomy10020245






