Effect of Agronomic Practices on Yield and Quality of Borage at Harvest and During Storage as Minimally-Processed Produce
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
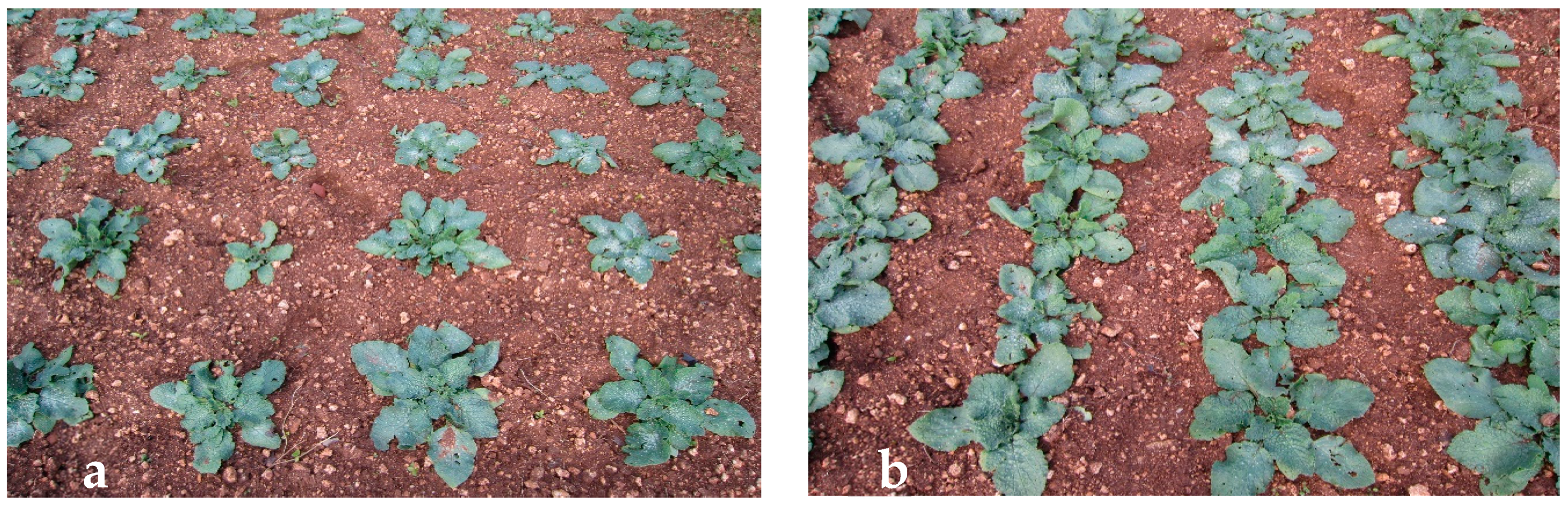
3.1. Effect of Plant Density on Yield, Minimal Processing, and Cold Storage
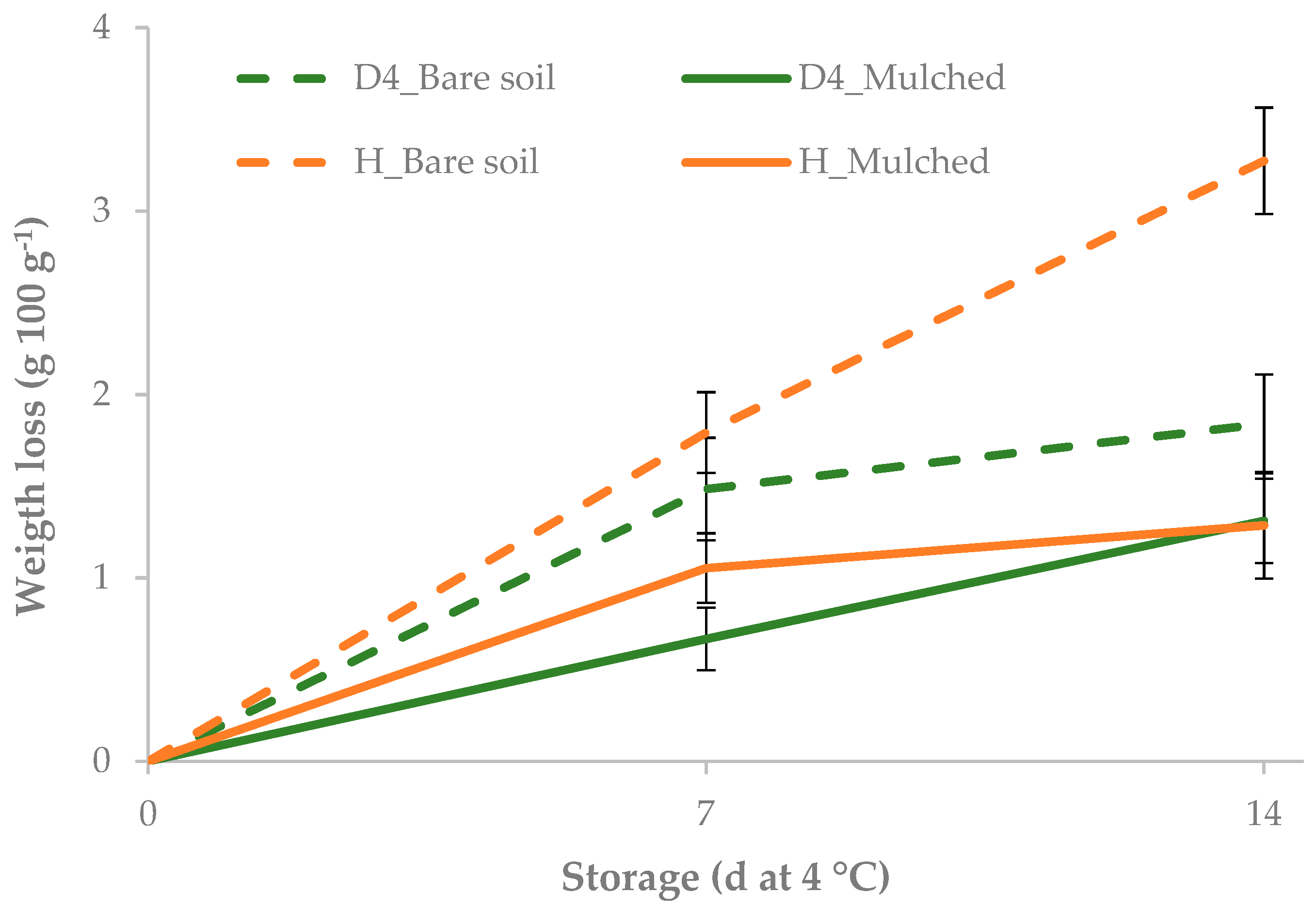
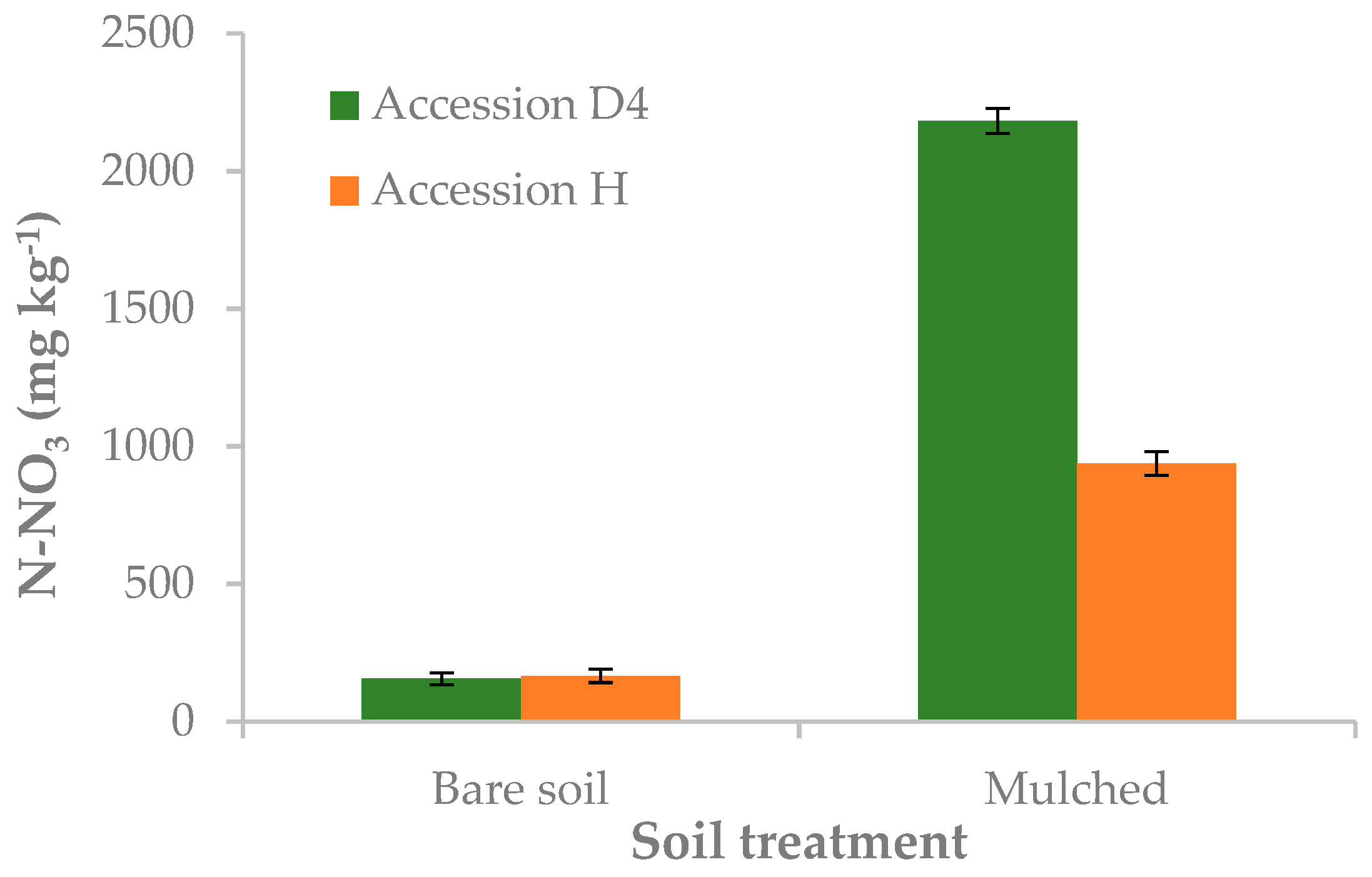
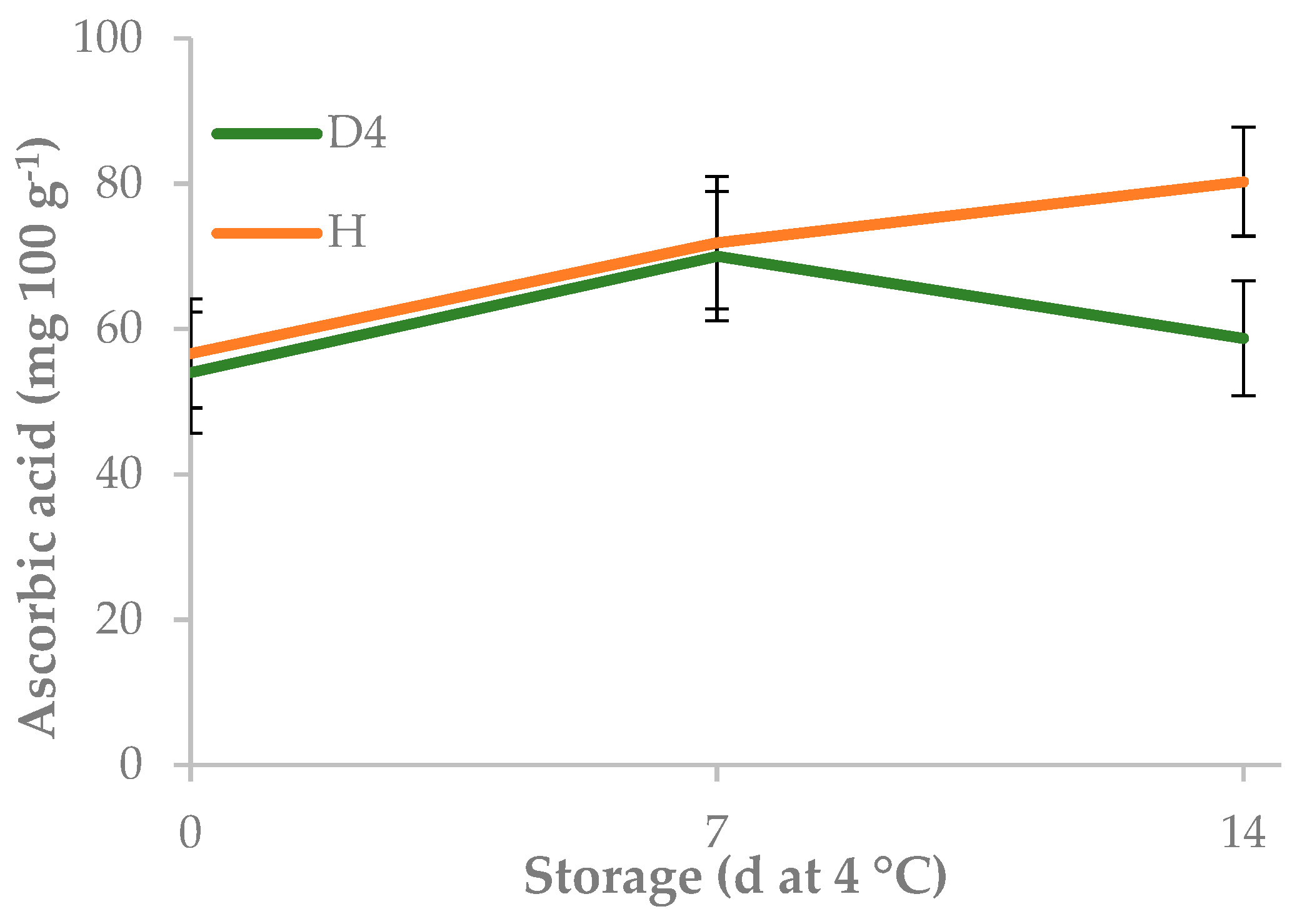
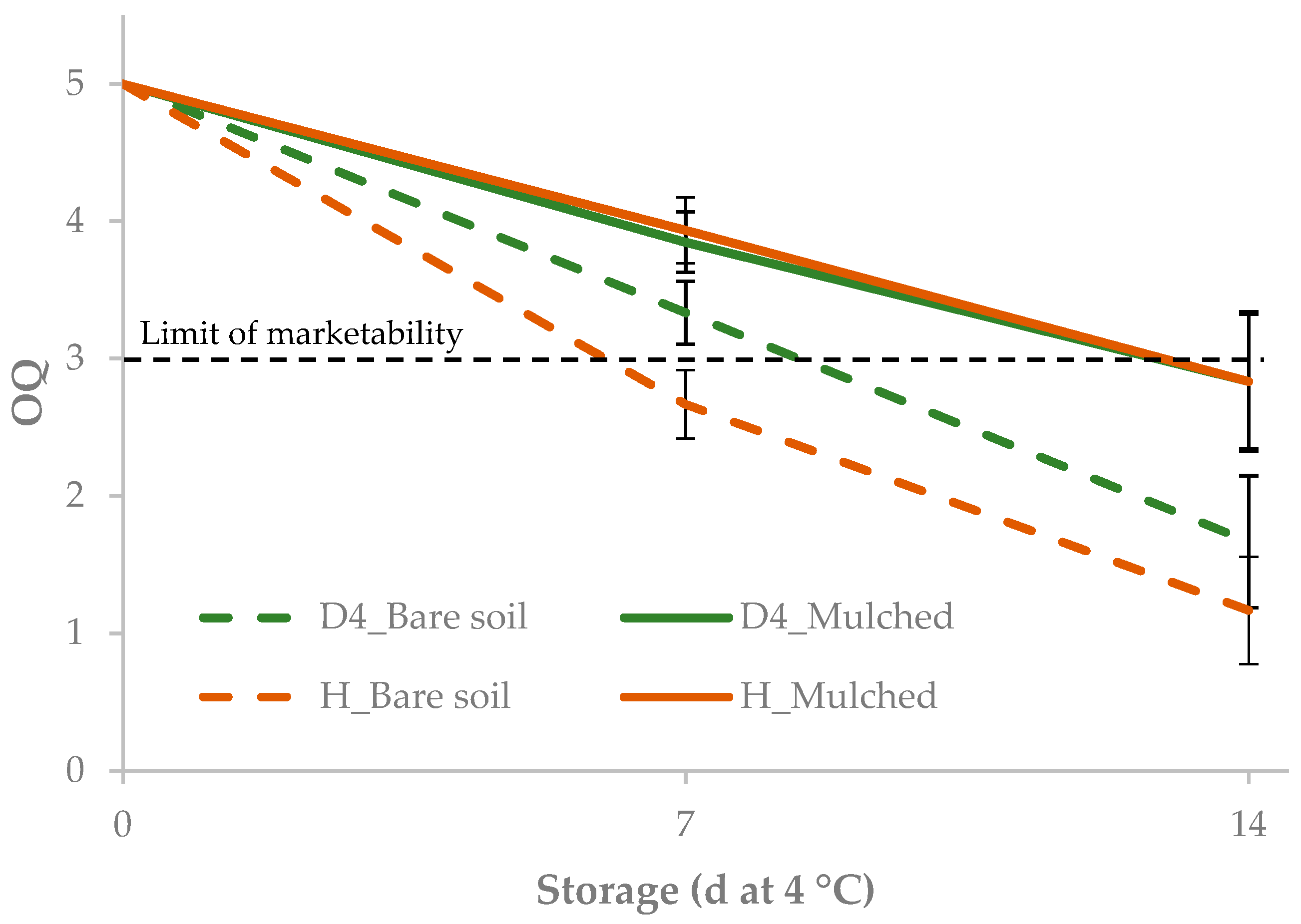
3.2. Effect of Mulching on Yield, Minimal Processing and Cold Storage
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gilani, A.H.; Bashir, S.; Khan, A. Pharmacological basis for the use of Borago officinalis in gastrointestinal, respiratory and cardiovascular disorders. J. Ethnopharmacol. 2007, 114, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Branca, F. Trials related to the cultivation of wild species utilised in Sicily as vegetables. Italus Hortus 2001, 8, 22–26. [Google Scholar]
- Tyler, V.E.; Foster, S. The Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies; Pharmaceutical Products Press: New York, NY, USA, 1993; Volume 37. [Google Scholar]
- Usmanghani, K.; Saeed, A.; Alam, M.T. Indusyunic Medicine; Dept. of Pharmacognosy, Faculty of Pharmacy, University of Karachi: Karachi, Pakistan, 1997; pp. 363–364. [Google Scholar]
- Duke, J.A. Handbook of Phytochemical Constituent Grass, Herbs and Other Economic Plants: Herbal Reference Library; Routledge: Abingdon-on-Thames, UK, 2017. [Google Scholar]
- Bandonien, D.; Murkovic, M. The detection of radical scavenging compounds in crude extract of borage (Borago officinalis L.) by using an on-line HPLC-DPPH method. J. Biochem. Biophys. Methods 2002, 53, 45–49. [Google Scholar] [CrossRef]
- Larson, K.M.; Roby, M.R.; Stermitz, F.R. Unsaturated pyrrolizidines from borage (Borago officinalis), a common garden herb. J. Nat. Prod. 1984, 47, 747–748. [Google Scholar] [CrossRef]
- Mhamdi, B.; Wannes, W.A.; Bourgou, S.; Marzouk, B. Biochemical characterization of borage (Borago officinalis L.) seeds. J. Food Biochem. 2009, 33, 331–341. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Chromatographical analysis of polyphenolic compounds from the herbs of Borago officinalis (L.). Herba Pol. 1996, 42, 252–256. [Google Scholar]
- Zemmouri, H.; Ammar, S.; Boumendjel, A.; Messarah, M.; El Feki, A.; Bouaziz, M. Chemical composition and antioxidant activity of Borago officinalis L. leaf extract growing in Algeria. Arab. J. Chem. 2019, 12, 1954–1963. [Google Scholar] [CrossRef]
- Tewari, D.; Bawari, S.; Patni, P.; Sah, A.N. Borage (Borago officinalis L.). In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 165–170. ISBN 9780128124918. [Google Scholar]
- van Gool, C.J.A.W.; Thijs, C.; Henquet, C.J.M.; van Houwelingen, A.C.; Dagnelie, P.C.; Schrander, J.; Menheere, P.P.C.A.; van den Brandt, P.A. γ-Linolenic acid supplementation for prophylaxis of atopic dermatitis—A randomized controlled trial in infants at high familial risk. Am. J. Clin. Nutr. 2003, 77, 943–951. [Google Scholar] [CrossRef]
- Gerard, J. The Herbal or General History of Plants: The Complete 1633 Edition as Revised and Enlarged by Thomas Johnson; Courier Dover Publications: Mineola, NY, USA, 2015; ISBN 160660080X. [Google Scholar]
- Prakash, V. Leafy Spices.; CRC Press, Inc.: Boca Raton, FL, USA, 1990; ISBN 0849367239. [Google Scholar]
- Abolhassani, M. Antibacterial effect of borage (Echium amoenum) on Staphylococcus aureus. Braz. J. Infect. Dis. 2004, 8, 382–385. [Google Scholar] [CrossRef][Green Version]
- Ahmad, I.; Mehmood, Z.; Mohammad, F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998, 62, 183–193. [Google Scholar] [CrossRef]
- Miceli, A.; Aleo, A.; Corona, O.; Sardina, M.T.; Mammina, C.; Settanni, L. Antibacterial activity of Borago officinalis and Brassica juncea aqueous extracts evaluated invitro and in situ using different food model systems. Food Control 2014, 40, 157–164. [Google Scholar] [CrossRef]
- Miceli, A.; Francesca, N.; Moschetti, G.; Settanni, L. The influence of addition of Borago officinalis with antibacterial activity on the sensory quality of fresh pasta. Int. J. Gastron. Food Sci. 2015, 2, 93–97. [Google Scholar] [CrossRef][Green Version]
- Miceli, C.; Moncada, A.; Vetrano, F.; D’Anna, F.; Miceli, A. Suitability of Borago officinalis for Minimal Processing as Fresh-Cut Produce. Horticulturae 2019, 5, 66. [Google Scholar] [CrossRef]
- Moniruzzaman, M. Effects of plant spacing and mulching on yield and profitability of lettuce (Lactuca sativa L.). J. Agric. Rural Dev. 2006, 4, 107–111. [Google Scholar] [CrossRef]
- Caruso, G.; Stoleru, V.; De Pascale, S.; Cozzolino, E.; Pannico, A.; Giordano, M.; Teliban, G.; Cuciniello, A.; Rouphael, Y. Production, leaf quality and antioxidants of perennial wall rocket as affected by crop cycle and mulching type. Agronomy 2019, 9, 194. [Google Scholar] [CrossRef]
- Miceli, C.; Miceli, A.; Mineo, V.; D’Anna, F. Caratterizzazione di Ecotipi Siciliani di Borragine. In Proceedings of the X Convegno AISSA La valorizzazione del territorio agrario e il controllo del degrado del suolo, Palermo, Italy, 28–29 Novembre 2012; 2012; Volume 7, p. 29. [Google Scholar]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; Boom, T.; Weber, E. Phanologische Entwicklungsstadien von Gemusepflanzen I. Zwiebel-, Wurzel-, Knollen-und Blattgemuse. Nachr. Dtsch. Pflanzenschutzd. 1995, 47, 193–205. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Moreira, M.; Roura, S.I.; del Valle, C.E. Quality of Swiss chard produced by conventional and organic methods. LWT-Food Sci. Technol. 2003, 36, 135–141. [Google Scholar] [CrossRef]
- Miceli, A.; Miceli, C. Effect of nitrogen fertilization on the quality of swiss chard at harvest and during storage as minimally processed produce. J. Food Qual. 2014, 37, 125–134. [Google Scholar] [CrossRef]
- Rodrigo, M.C.; Ramos, C. Nitrate Sap Analysis as A Tool to Assess Nitrogen Nutrition in Artichoke. In Proceedings of the VI International Symposium on Artichoke, Cardoon and Their Wild Relatives 730, Lorca, Spain, 28–31 March 2006; pp. 251–256. [Google Scholar]
- D’Anna, F.; Iapichino, G.; Miceli, A. Effect of clove weight on yield and bulb quality of garlic grown for storage. Acta Hortic. 2000, 533, 589–592. [Google Scholar] [CrossRef]
- Scuderi, D.; Giuffrida, F.; Leonardi, C. Effects of harvest time and plant density on yield and quality of Chinese cabbage for fresh-cut production. Acta Hortic. 2012, 1005, 503–509. [Google Scholar] [CrossRef]
- Mujahid, A.M.; Gupta, A.J. Effect of plant spacing, organic manures and inorganic fertilizers and their combinations on growth, yield and quality of lettuce (Lactuca sativa). Indian J. Agric. Sci. 2010, 80, 177–181. [Google Scholar]
- Bracy, R.P.; Parish, R.L.; Mulkey, W.A. High-density planting in a precision cultural system for vegetable production. Horttechnology 1991, 1, 54–58. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Del-Toro-Sánchez, L.; Alvarez-Parrilla, E.; González-Aguilar, G.A. High relative humidity in-package of fresh-cut fruits and vegetables: Advantage or disadvantage considering microbiological problems and antimicrobial delivering systems? J. Food Sci. 2008, 73, R41–R47. [Google Scholar] [CrossRef] [PubMed]
- Paull, R. Effect of temperature and relative humidity on fresh commodity quality. Postharvest Biol. Technol. 1999, 15, 263–277. [Google Scholar] [CrossRef]
- Robinson, J.E.; Browne, K.M.; Burton, W.G. Storage characteristics of some vegetables and soft fruits. Ann. Appl. Biol. 1975, 81, 399–408. [Google Scholar] [CrossRef]
- Miceli, A.; Romano, C.; Moncada, A.; D’Anna, F.; Vetrano, F. Effect of cold storage on the quality of minimally processed cauliflower. Carpathian J. Food Sci. Technol. 2015, 7, 70–74. [Google Scholar]
- Alfonzo, A.; Gaglio, R.; Miceli, A.; Francesca, N.; Di Gerlando, R.; Moschetti, G.; Settanni, L. Shelf life evaluation of fresh-cut red chicory subjected to different minimal processes. Food Microbiol. 2018, 73, 298–304. [Google Scholar] [CrossRef]
- Miceli, A.; Gaglio, R.; Francesca, N.; Ciminata, A.; Moschetti, G.; Settanni, L. Evolution of shelf life parameters of ready-to-eat escarole (Cichorium endivia var. latifolium) subjected to different cutting operations. Sci. Hortic. 2019, 247, 175–183. [Google Scholar] [CrossRef]
- Gaglio, R.; Miceli, A.; Sardina, M.T.; Francesca, N.; Moschetti, G.; Settanni, L. Evaluation of microbiological and physico-chemical parameters of retail ready-to-eat mono-varietal salads. J. Food Process. Preserv. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Watada, A.E.; Qi, L. Quality of fresh-cut produce. Postharvest Biol. Technol. 1999, 15, 201–205. [Google Scholar] [CrossRef]
- Loh, Y.H.; Jakszyn, P.; Luben, R.N.; Mulligan, A.A.; Mitrou, P.N.; Khaw, K.-T. N-nitroso compounds and cancer incidence: The European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk Study. Am. J. Clin. Nutr. 2011, 93, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Blom-Zandstra, M. Nitrate accumulation in vegetables and its relationship to quality. Ann. Appl. Biol. 1989, 115, 553–561. [Google Scholar] [CrossRef]
- Kosma, C.; Triantafyllidis, V.; Papasavvas, A.; Salahas, G.; Patakas, A. Yield and nutritional quality of greenhouse lettuce as affected by shading and cultivation season. Emir. J. Food Agric. 2013, 25, 974–979. [Google Scholar] [CrossRef]
- Ferrante, A.; Incrocci, L.; Serra, G. Quality changes during storage of fresh-cut or intact Swiss chard leafy vegetables. J. Food Agric. Environ. 2008, 6, 60–62. [Google Scholar]
- Roura, S.I.; Davidovich, L.A.; Del Valle, C.E. Postharvest changes in fresh Swiss chard (Beta vulgaris, type cycla) under different storage conditions. J. Food Qual. 2000, 23, 137–147. [Google Scholar] [CrossRef]
- Roura, S.I.; Davidovich, L.A.; Del Valle, C.E. Quality loss in minimally processed swiss chard related to amount of damaged area. LWT-Food Sci. Technol. 2000, 33, 53–59. [Google Scholar] [CrossRef]
- Yamauchi, N.; Watada, A.E. Regulated chlorophyll degradation in spinach leaves during storage. J. Am. Soc. Hortic. Sci. 1991, 116, 58–62. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Sabatino, L.; Iapichino, G.; D’Anna, F.; Vetrano, F. Effect of Molybdenum Rate on Yield and Quality of Lettuce, Escarole, and Curly Endive Grown in a Floating System. Agronomy 2018, 8, 171. [Google Scholar] [CrossRef]
- Howard, L.A.; Wong, A.D.; Perry, A.K.; Klein, B.P. β-Carotene and ascorbic acid retention in fresh and processed vegetables. J. Food Sci. 1999, 64, 929–936. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef]
- Miceli, A.; Romano, C.; Moncada, A.; Piazza, G.; Torta, L.; D’Anna, F.; Vetrano, F. Yield and quality of mini-watermelon as affected bygrafting and mycorrhizal inoculum. J. Agric. Sci. Technol. 2016, 18, 505–516. [Google Scholar]
- Caracciolo, G.; D’Anna, E.; Moncada, A.; D’Anna, F. Evaluation of the quality and antioxidant capacity of woodland strawberry biotypes in Sicily. J. Food Agric. Environ. 2013, 11, 522–525. [Google Scholar]
- Miceli, A.; Vetrano, F.; Sabatino, L.; D’Anna, F.; Moncada, A. Influence of preharvest gibberellic acid treatments on postharvest quality of minimally processed leaf lettuce and rocket. Horticulturae 2019, 5, 63. [Google Scholar] [CrossRef]
- La Scalia, G.; Aiello, G.; Miceli, A.; Nasca, A.; Alfonzo, A.; Settanni, L. Effect of Vibration on the Quality of Strawberry Fruits Caused by Simulated Transport. J. Food Process Eng. 2016, 39, 140–156. [Google Scholar] [CrossRef]
- Lament, W.J. Plastic mulches for the production of vegetable crops. Horttechnology 1993, 3, 35–39. [Google Scholar] [CrossRef]
- Núñez-Zofío, M.; Larregla, S.; Garbisu, C. Application of organic amendments followed by soil plastic mulching reduces the incidence of Phytophthora capsici in pepper crops under temperate climate. Crop Prot. 2011, 30, 1563–1572. [Google Scholar] [CrossRef]
- Lalitha, M.; Kasthuri Thilagam, V.; Balakrishnan, N.; Mansour, M. Effect of plastic mulch on soil properties and crop growth-a review. Agric. Rev. 2010, 31, 145–149. [Google Scholar]
- Ashrafuzzaman, M.; Halim, M.A.; Ismail, M.R.; Shahidullah, S.M.; Hossain, M.A. Effect of plastic mulch on growth and yield of chilli (Capsicum annuum L.). Brazilian Arch. Biol. Technol. 2011, 54, 321–330. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Vetrano, F.; Moncada, A.; Miceli, A.; De Pasquale, C.; D’Anna, F.; Giurgiulescu, L. Effects of polyethylene and biodegradable starch-based mulching films on eggplant production in a Mediterranean area. Carpathian J. Food Sci. Technol. 2018, 10, 81–89. [Google Scholar]
- Vetrano, F.; Fascella, S.; Iapichino, G.; Incalcaterra, G.; Girgenti, P.; Sutera, P.; Buscemi, G. Response of melon genotypes to polyethylene and biodegradable starch-based mulching films used for fruit production in the Western Coast of Sicily. Acta Hortic. 2009, 807, 109–114. [Google Scholar] [CrossRef]
- Iapichino, G.; Vetrano, F.; Moncada, A.; Fascella, S.; Incalcaterra, G. Effects of plastic mulch and floating cover on lettuce production in Sicily. Acta Hortic. 2012, 936, 491–494. [Google Scholar] [CrossRef]
- Abdullah, K.; Ismail, T.G.; Yusuf, U.; Belgin, C. Effects of mulch and irrigation water amounts on lettuce’s yield, evapotranspiration, transpiration and soil evaporation in Isparta location, Turkey. J. Biol. Sci. 2004, 4, 751–755. [Google Scholar]
- Siwek, P.; Kalisz, A.; Wojciechowska, R. Effect of mulching with film of different colours made from original and recycled polyethylene on the yield of butterhead lettuce and celery. Folia Hortic. 2007, 19, 25–35. [Google Scholar]
- Pernice, R.; Scuderi, D.; Napolitano, A.; Fogliano, V.; Leonardi, C. Polyphenol composition and qualitative characteristics of fresh-cut lettuce in relation to cultivar, mulching, and storage. J. Hortic. Sci. Biotechnol. 2007, 82, 420–427. [Google Scholar] [CrossRef]
- Sanders, D.C. Using plastic mulches and drip irrigation for vegetable production. N. C. State Univ. Coop. Ext. Serv. Hort. Info. Lf. 2001, 33, 4. [Google Scholar]
- Li, F.-M.; Song, Q.-H.; Jjemba, P.K.; Shi, Y.-C. Dynamics of soil microbial biomass C and soil fertility in cropland mulched with plastic film in a semiarid agro-ecosystem. Soil Biol. Biochem. 2004, 36, 1893–1902. [Google Scholar] [CrossRef]
- Ekinci, M.; Dursun, A. Mulching for vegetable growing. Derim 2006, 23, 20–27. [Google Scholar]
- Miceli, A.; Settanni, L. Influence of agronomic practices and pre-harvest conditions on the attachment and development of Listeria monocytogenes in vegetables. Ann. Microbiol. 2019, 69, 185–199. [Google Scholar] [CrossRef]
- Goñi, M.G.; Agüero, M.V.; Moreira, M.D.E.L.R.; Ponce, A.; Roura, S.I. Ring Characterization of Quality Indices In Butterhead Lettuce Cultivated Under Mulch And Bare Soil. J. Food Qual. 2010, 33, 439–460. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Elia, A. Pre-harvest nitrogen and Azoxystrobin application enhances postharvest shelf-life in Butterhead lettuce. Postharvest Biol. Technol. 2013, 85, 67–76. [Google Scholar] [CrossRef]
- Cantliffe, D.J. Nitrate accumulation in vegetable crops as affected by photoperiod and light duration. Am. Soc. Hortic. Sci. J. 1972, 97, 414–418. [Google Scholar]
- Burns, I.G.; Zhang, K.; Turner, M.K.; Lynn, J.; McClement, S.; Hand, P.; Pink, D. Genotype and environment effects on nitrate accumulation in a diversity set of lettuce accessions at commercial maturity: The influence of nitrate uptake and assimilation, osmotic interactions and shoot weight and development. J. Sci. Food Agric. 2011, 91, 2217–2233. [Google Scholar] [CrossRef]
- Filipović, V.; Romić, D.; Romić, M.; Borošić, J.; Filipović, L.; Mallmann, F.J.K.; Robinson, D.A. Plastic mulch and nitrogen fertigation in growing vegetables modify soil temperature, water and nitrate dynamics: Experimental results and a modeling study. Agric. Water Manag. 2016, 176, 100–110. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Siwek, P.; Libik, A. Effect of mulching with various films on the yield quality of butterhead lettuce and celery stalks with special reference to nitrate metabolism. Folia Hortic 2007, 19, 37–44. [Google Scholar]
- Maggio, A.; De Pascale, S.; Paradiso, R.; Barbieri, G. Quality and nutritional value of vegetables from organic and conventional farming. Sci. Hortic. 2013, 164, 532–539. [Google Scholar] [CrossRef]
- Benoit, F.; Ceustermans, N. Ecological Vegetable Growing with Plastics. In Proceedings of the 12. Congreso Internacional de Plasticos en Agricultura, Granada, Spain, 3–8 May 1992. [Google Scholar]
- Qiu, W.; Wang, Z.; Huang, C.; Chen, B.; Yang, R. Nitrate accumulation in leafy vegetables and its relationship with water. J. Soil Sci. Plant Nutr. 2014, 14, 761–768. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EC) No. 1258/2011 of 2 December 2011 amending Regulation (EC) No. 1881/2006 as regards maximum levels for nitrates in foodstuffs. Off. J. Eur. Union 2011, 320, 15–17. [Google Scholar]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Miceli, A.; Vetrano, F.; Romano, C. Effect of hot air treatment on minimally processed cauliflower. Acta Hortic. 2013, 1005, 309–314. [Google Scholar] [CrossRef]
- Panchal, S.C.; Bhatnagar, R.; Momin, R.A.; Chauhan, N.P. Capsaicin and ascorbic acid content of chilli as influenced by cultural practices. Capsicum Eggplant Newsl. 2001, 20, 19–22. [Google Scholar]
- Najda, A.; Dyduch, J. The effect of length of vegetation and soil mulching on yielding of two cultivars of celery. Zesz. Nauk. AR Poznań Ser. Rol. 2005, 515, 363–366. [Google Scholar]
- Govedarica-Lučić, A.; Milić, V. Influence variety and mulching land on mass head and contents vitamin C by lettuce. Technol. Acta 2011, 4, 47–50. [Google Scholar]
- Dvořák, P.; Tomašek, J.; Hajšlova, J.; Schulzova, V. Influence of Surface Mulching on the Quality of Potato Tubers. In Proceedings of the 3rd Scientific Conference 2011-Proceedings. New findings in organic farming research and their possible use for Central and Eastern Europe, Bioinstitut, Prague, Czech Republic, 14–15 November 2011; pp. 49–52. [Google Scholar]
- Eheart, M.S.; Odland, D. Storage of fresh broccoli and green beans. Effect of ascorbic acid, sugars, and total acids. J. Am. Diet. Assoc. 1972, 60, 402–406. [Google Scholar]
- Wu, Y.; Perry, A.K.; Klein, B.P. Vitamin C and β-carotene in fresh and frozen green beans and broccoli in a simulated system. J. Food Qual. 1992, 15, 87–96. [Google Scholar] [CrossRef]
- Esteve, M.J.; Farre, R.; Frigola, A.; Clemente, G. Changes in ascorbic acid content of green asparagus during the harvesting period and storage. J. Agric. Food Chem. 1995, 43, 2058–2061. [Google Scholar] [CrossRef]
- Leskovar, D.I.; Stein, L.A.; Dainello, F.J. Planting systems influence growth dynamics and quality of fresh market spinach. HortScience 2000, 35, 1238–1240. [Google Scholar] [CrossRef]
- Ponce, A.G.; Agüero, M.V.; Roura, S.I.; Del Valle, C.E.; Moreira, M.R. Dynamics of indigenous microbial populations of butter head lettuce grown in mulch and on bare soil. J. Food Sci. 2008, 73, M257–M263. [Google Scholar] [CrossRef]
- Agüero, M.V.; Ponce, A.G.; Moreira, M.R.; Roura, S.I. Plastic mulch improves microbial quality and shelf life of cold stored butter lettuce (Lactuca sativa var Lores). Fresh Prod. 2008, 2, 6–13. [Google Scholar]



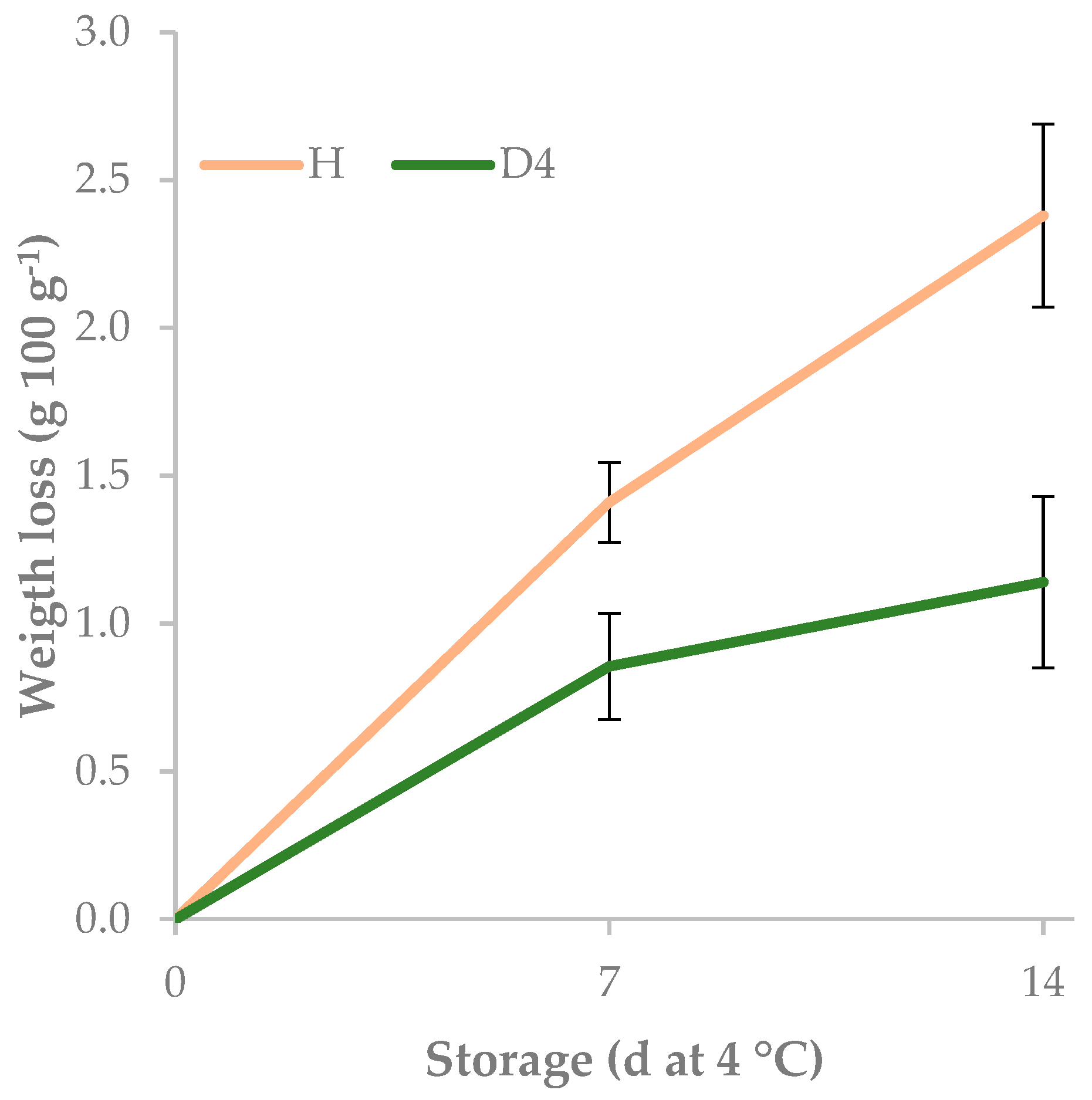
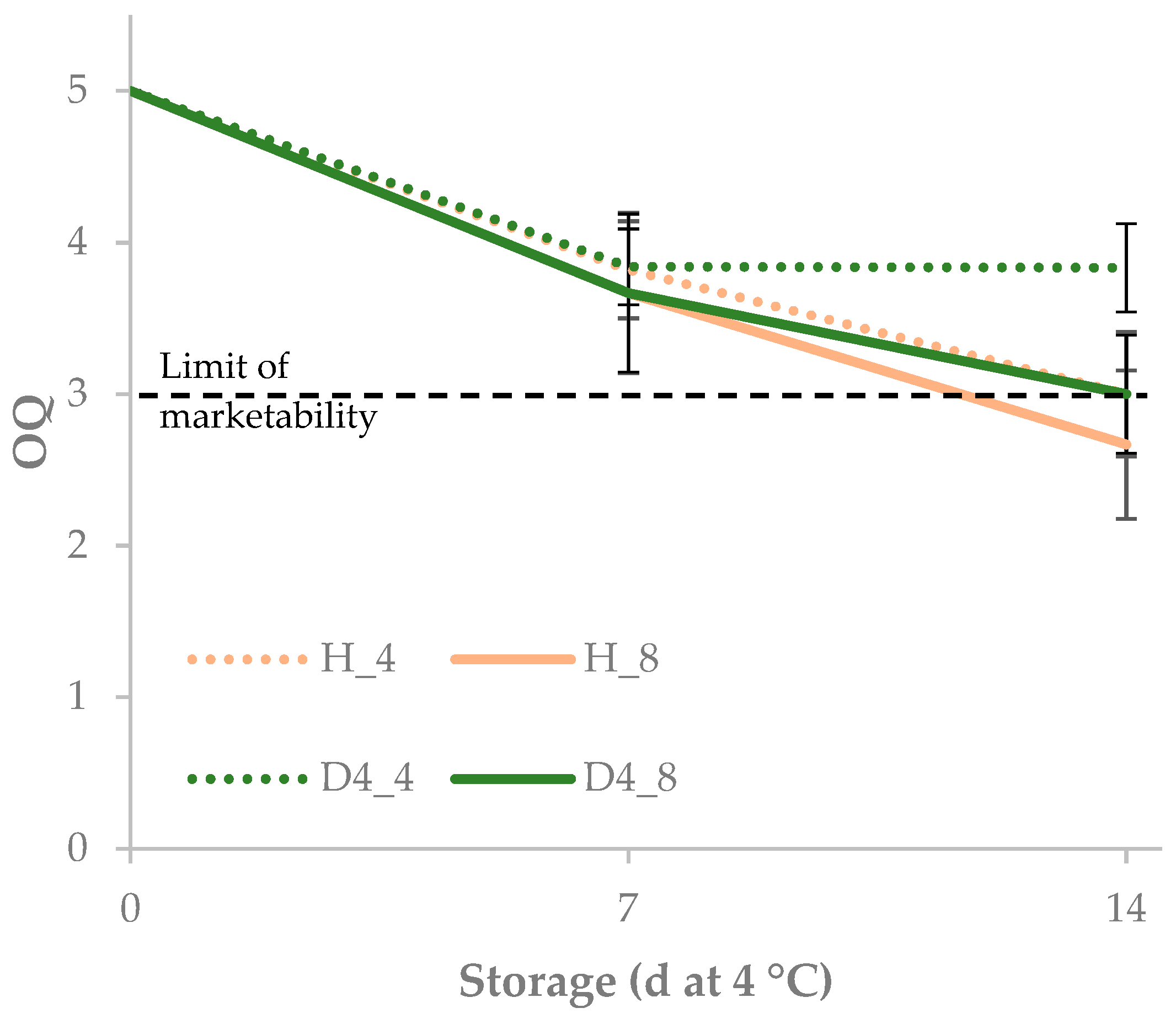
| Multi-Crop Passport Descriptors | Borage Accessions | |
|---|---|---|
| Accession name | D4 | H |
| Accession number | BoPA005 | BoPA001 |
| Holding institute | Vegetable laboratory—Department of Agricultural, Food, and Forest Sciences (SAAF—University of Palermo, Italy) | |
| Genus | Borago | |
| Species | officinalis | |
| Common crop name | Borage | |
| Country of origin | ITA | ITA |
| Location of collecting site | Monte Pellegrino-Palermo (PA) | Piana dei colli-Palermo (PA) |
| Latitude of collecting site | 381031 N | 380925 N |
| Longitude of collecting site | 0132136 E | 0131957 E |
| Elevation of collecting site | 438 m | 35 m |
| Collecting date | 201102-- | 201103-- |
| Biological status of accession | Wild | Wild |
| Collecting/acquisition source | Woodland | Fallow land |
| Type of germplasm storage | Seed collection | Seed collection |
| Source of Variance | Plant | Total Yield (g m−2) | Minimally Processed Leaves | |||||
|---|---|---|---|---|---|---|---|---|
| Fresh Weight (g plant−1) | Dry Weight (g plant−1) | Dry Matter (%) | Yield | Dry Matter | ||||
| (%) | (g m−2) | (%) | ||||||
| Accession | ||||||||
| D4 | z 370.9 | 40.0 | 10.9 | 2152.5 | 38.5 | 806.3 a | 8.3 | |
| H | 368.4 | 43.4 | 11.9 | 2078.3 | 32.3 | 673.3 b | 8.1 | |
| Plant density | ||||||||
| 4 (plant m−2) | 421.0 a | 46.1 a | 11.0 | 1683.8 b | 37.0 | 620.1 b | 8.3 | |
| 8 (plant m−2) | 318.4 b | 37.3 b | 11.8 | 2547.0 a | 33.7 | 859.5 a | 8.1 | |
| Accession × Density | ||||||||
| D4 | 4 | 407.4 | 42.5 | 10.5 | 1629.5 | 42.5 a | 693.3 | 8.3 |
| 8 | 334.4 | 37.5 | 11.3 | 2675.6 | 34.4 b | 919.2 | 8.3 | |
| H | 4 | 434.5 | 49.7 | 11.4 | 1738.1 | 31.5 b | 546.8 | 8.3 |
| 8 | 302.3 | 37.1 | 12.3 | 2418.4 | 33.1 b | 799.8 | 7.8 | |
| Significance x | ||||||||
| Accession | ns | ns | ns | ns | ** | * | ns | |
| Density | *** | ** | ns | *** | * | ** | ns | |
| Accession × Density | ns | ns | ns | ns | * | ns | ns | |
| Source of Variance | Weight Loss | TSS | N-NO3 | Chlorophyll | ||
|---|---|---|---|---|---|---|
| (g 100 g−1) | (°Brix) | (mg kg−1) | (mg 100 g−1) | |||
| Accession | ||||||
| D4 | z 0.67 a | 6.0 | 227.8 | 50.2 a | ||
| H | 1.26 b | 5.5 | 249.5 | 35.7 b | ||
| Plant density | ||||||
| 4 (plant m−2) | 0.91 | 5.7 | 227.8 | 44.6 | ||
| 8 (plant m−2) | 1.02 | 5.8 | 249.5 | 41.3 | ||
| Storage (d at 4 °C) | ||||||
| 0 | 0.00 c | 5.9 | 228.3 b | 39.5 | ||
| 7 | 1.13 b | 5.9 | 226.7 b | 43.8 | ||
| 14 | 1.76 a | 5.5 | 260.9 a | 45.5 | ||
| Accession × Density × Storage | ||||||
| D4 | 4 | 0 | 0.00 | 5.5 | 233.3 | 46.4 |
| 7 | 0.94 | 6.0 | 193.3 | 50.7 | ||
| 14 | 1.27 | 5.4 | 223.3 | 58.8 | ||
| 8 | 0 | 0.00 | 6.5 | 210.0 | 42.9 | |
| 7 | 0.77 | 6.3 | 230.0 | 46.1 | ||
| 14 | 1.01 | 6.1 | 276.7 | 56.2 | ||
| H | 4 | 0 | 0.00 | 5.9 | 233.3 | 40.4 |
| 7 | 1.34 | 5.9 | 206.7 | 36.5 | ||
| 14 | 1.92 | 5.5 | 276.7 | 34.6 | ||
| 8 | 0 | 0.00 | 5.5 | 236.7 | 28.3 | |
| 7 | 1.48 | 5.3 | 276.7 | 42.0 | ||
| 14 | 2.84 | 5.1 | 266.7 | 32.4 | ||
| Significance x | ||||||
| Accession | *** | ns | ns | * | ||
| Plant density | ns | ns | ns | ns | ||
| Storage | *** | ns | * | ns | ||
| Accession × density | * | ** | ns | ns | ||
| Accession × Storage | *** | ns | ns | ns | ||
| Density × Storage | ns | ns | ns | ns | ||
| Accession × Density × Storage | ns | ns | ns | ns | ||
| Source of Variance | L* | a* | b* | Chroma | Hue Angle | Overall Quality | ||
|---|---|---|---|---|---|---|---|---|
| Accession | ||||||||
| D4 | z 38.6 | −13.1 a | 17.4 b | 21.8 b | 127.2 | 4.1 | ||
| H | 38.8 | −13.9 b | 19.4 a | 23.9 a | 126.0 | 3.9 | ||
| Plant density | ||||||||
| 4 (plant m−2) | 38.4 | −13.1 a | 17.7 b | 22.0 b | 126.9 | 4.1 | ||
| 8 (plant m−2) | 39.0 | −13.9 b | 19.1 a | 23.6 a | 126.3 | 3.9 | ||
| Storage (d at 4 °C) | ||||||||
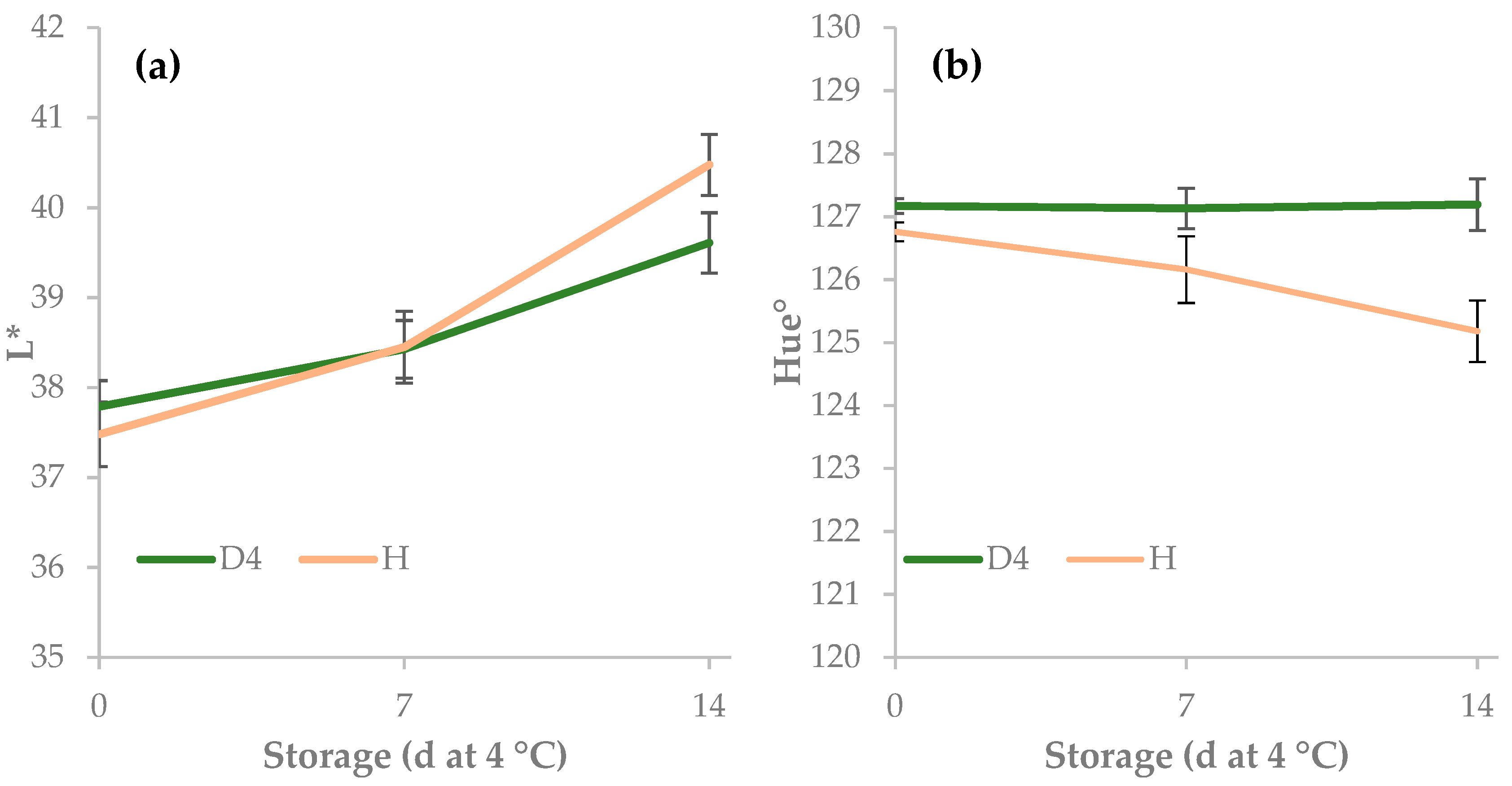
| 0 | 37.6 | −13.7 | 18.3 | 22.9 | 127.0 | 5.0 | ||
| 7 | 38.4 | −13.3 | 18.0 | 22.4 | 126.6 | 3.8 | ||
| 14 | 40.0 | −13.5 | 18.8 | 23.2 | 126.2 | 3.1 | ||
| Accession × Density × Storage | ||||||||
| D4 | 4 | 0 | 37.0 | −13.1 | 17.0 | 21.5 | 127.8 | 5.0 |
| 7 | 38.0 | −12.4 | 16.1 | 20.4 | 127.7 | 3.8 | ||
| 14 | 38.8 | −12.7 | 16.4 | 20.8 | 128.2 | 3.8 | ||
| 8 | 0 | 38.5 | −13.3 | 18.0 | 22.4 | 126.5 | 5.0 | |
| 7 | 38.9 | −13.4 | 18.2 | 22.6 | 126.6 | 3.7 | ||
| 14 | 40.4 | −13.4 | 18.5 | 22.9 | 126.2 | 3.0 | ||
| H | 4 | 0 | 37.6 | −13.8 | 18.4 | 23.0 | 127.2 | 5.0 |
| 7 | 38.6 | −13.3 | 18.5 | 22.8 | 125.9 | 3.8 | ||
| 14 | 40.7 | −13.3 | 19.7 | 23.8 | 124.6 | 3.0 | ||
| 8 | 0 | 37.3 | −14.5 | 20.0 | 24.7 | 126.3 | 5.0 | |
| 7 | 38.3 | −14.1 | 19.2 | 23.8 | 126.5 | 3.7 | ||
| 14 | 40.3 | −14.5 | 20.6 | 25.3 | 125.7 | 2.7 | ||
| Significance x | ||||||||
| Accession | ns | *** | *** | *** | *** | ns | ||
| Plant density | ns | *** | *** | *** | * | * | ||
| Storage | *** | ns | ns | ns | * | *** | ||
| Accession × Density | ** | ns | ns | ns | *** | ns | ||
| Accession × Storage | * | ns | ns | ns | * | * | ||
| Density × Storage | ns | ns | ns | ns | ns | ns | ||
| Accession × Density × Storage | ns | ns | ns | ns | ns | ns | ||
| Source of Variance | Plant | Total Yield (g m−2) | Minimally Processed Leaves | |||||
|---|---|---|---|---|---|---|---|---|
| fresh Weight (g plant−1) | Dry Weight (g plant−1) | Dry Matter (%) | Yield | Dry Matter | ||||
| (%) | (g m−2) | (%) | ||||||
| Accession | ||||||||
| D4 | z 426.4 | 43.7 | 10.5 | 3434.3 | 27.1 | 974.0 | 7.8 b | |
| H | 443.5 | 42.9 | 9.9 | 3519.3 | 28.9 | 1107.5 | 8.2 a | |
| Soil treatment | ||||||||
| bare soil | 379.1 b | 44.7 a | 11.8 a | 3027.4 b | 22.7 b | 798.7 b | 9.6 a | |
| mulched | 490.8 a | 42.0 b | 8.6 b | 3926.1 a | 33.3 a | 1282.8 a | 6.5 b | |
| Accession × Soil treatment | ||||||||
| D4 | bare soil | 369.5 | 45.8 | 12.4 | 3001.9 | 20.9 | 707.4 | 9.3 |
| mulched | 483.3 | 41.6 | 8.6 | 3866.7 | 33.4 | 1240.6 | 6.3 | |
| H | bare soil | 388.7 | 43.5 | 11.2 | 3053.0 | 24.5 | 889.9 | 9.8 |
| mulched | 498.2 | 42.3 | 8.5 | 3985.6 | 33.3 | 1325.1 | 6.6 | |
| Significance x | ||||||||
| Accession | ns | ns | ns | ns | ns | ns | * | |
| Soil treatment | ** | ** | *** | *** | *** | *** | *** | |
| Accession × Soil treatment | ns | ns | ns | ns | ns | ns | ns | |
| Source of Variance | Weight Loss | TA y | TSS | N-NO3 | Ascorbic Acid | ||
|---|---|---|---|---|---|---|---|
| (g 100 g−1) | (mg 100 g−1) | (°Brix) | (mg kg−1) | (mg 100 g−1) | |||
| Accession | |||||||
| D4 | z 0.88 | 169.6 | 4.6 a | 1168.4 | 60.9 | ||
| H | 1.23 | 181.4 | 5.0 b | 551.6 | 69.6 | ||
| Soil treatment | |||||||
| bare soil | 1.40 | 182.0 | 5.9 a | 160.0 | 87.9 | ||
| mulched | 0.72 | 169.0 | 3.8 b | 1560.0 | 42.5 | ||
| Storage (d at 4 °C) | |||||||
| 0 | 0.00 | 101.6 c | 4.7 | 913.3 | 55.3 | ||
| 7 | 1.25 | 166.5 b | 4.9 | 860.7 | 70.9 | ||
| 14 | 1.93 | 258.4 a | 4.9 | 806.0 | 69.5 | ||
| Accession × Soil treatment × Storage | |||||||
| D4 | bare soil | 0 | 0.00 e | 88.8 | 5.9 | 176.7 | 84.0 |
| 7 | 1.48 bc | 203.2 | 5.8 | 133.3 | 98.4 | ||
| 14 | 1.84 bc | 225.4 | 5.3 | 154.0 | 82.4 | ||
| mulched | 0 | 0.00 e | 100.8 | 3.4 | 2290.0 | 24.0 | |
| 7 | 0.67 d | 140.9 | 3.4 | 2200.0 | 41.6 | ||
| 14 | 1.31 c | 258.3 | 3.8 | 2056.7 | 35.0 | ||
| H | bare soil | 0 | 0.00 e | 100.8 | 5.6 | 197.3 | 74.3 |
| 7 | 1.79 b | 192.1 | 6.5 | 197.3 | 93.9 | ||
| 14 | 3.27 a | 281.8 | 6.1 | 101.3 | 94.7 | ||
| mulched | 0 | 0.00 e | 116.1 | 4.0 | 989.3 | 38.9 | |
| 7 | 1.05 c | 129.8 | 3.8 | 912.0 | 49.9 | ||
| 14 | 1.29 c | 267.9 | 4.2 | 912.0 | 65.9 | ||
| Significance x | |||||||
| Accession | *** | ns | ** | *** | ns | ||
| Soil treatment | *** | ns | *** | *** | *** | ||
| Storage | *** | *** | ns | ns | *** | ||
| Accession × Soil treatment | *** | ns | ns | *** | ns | ||
| Accession × Storage | *** | ns | ns | ns | * | ||
| Soil treatment × Storage | *** | ns | ns | ns | ns | ||
| Accession × Soil treatment × Storage | *** | ns | ns | ns | ns | ||
| Source of Variance | L* | a* | b* | Chroma | Hue Angle | Overall Quality | ||
|---|---|---|---|---|---|---|---|---|
| Accession | ||||||||
| D4 | z 39.4 | −14.7 | 20.8 | 25.6 | 126.2 | 3.6 | ||
| H | 41.0 | −15.1 | 22.2 | 26.9 | 125.1 | 3.4 | ||
| Soil treatment | ||||||||
| bare soil | 41.4 | −15.3 | 23.6 | 28.2 | 124.0 | 3.1 | ||
| mulched | 39.0 | −14.5 | 19.4 | 24.3 | 127.4 | 3.9 | ||
| Storage (d at 4 °C) | ||||||||
| 0 | 36.2 | −13.5 | 17.3 | 22.0 | 128.2 | 5.0 | ||
| 7 | 39.9 | −14.9 | 20.6 | 25.5 | 126.5 | 3.4 | ||
| 14 | 44.6 | −16.3 | 26.5 | 31.2 | 122.3 | 2.1 | ||
| Accession × Soil treatment × Storage | ||||||||
| D4 | bare soil | 0 | 37.3 | −14.6 | 19.5 cd | 24.4 c | 127.0 b | 5.0 a |
| 7 | 38.6 | −14.6 | 20.2 c | 25.0 c | 126.1 bc | 3.3 c | ||
| 14 | 45.0 | −16.1 | 28.5 ab | 32.9 ab | 120.6 d | 1.7 ef | ||
| mulched | 0 | 35.3 | −12.8 | 15.3 d | 20.0 d | 130.2 a | 5.0 a | |
| 7 | 38.7 | −14.5 | 18.1 cd | 23.2 cd | 128.8 ab | 3.8 b | ||
| 14 | 41.6 | −15.6 | 23.0 bc | 27.9 bc | 124.5 c | 2.8 e | ||
| H | bare soil | 0 | 36.6 | −13.6 | 18.1 cd | 22.6 cd | 127.1 b | 5.0 a |
| 7 | 42.9 | −15.6 | 25.4 b | 29.9 b | 122.2 cd | 2.7 d | ||
| 14 | 48.1 | −17.3 | 29.7 a | 34.4 a | 120.8 d | 1.2 f | ||
| mulched | 0 | 35.6 | −13.1 | 16.5 d | 21.0 d | 128.5 ab | 5.0 a | |
| 7 | 39.4 | −14.9 | 18.7 dc | 23.9 cd | 128.8 ab | 3.9 b | ||
| 14 | 43.6 | −16.2 | 24.9 b | 29.8 b | 123.5 c | 2.8 e | ||
| Significance x | ||||||||
| Accession | *** | * | ** | ** | ** | * | ||
| Soil treatment | *** | *** | *** | *** | *** | *** | ||
| Storage | *** | *** | *** | *** | *** | *** | ||
| Accession × Soil treatment | ns | ns | ns | ns | ns | * | ||
| Accession × Storage | ** | ** | * | * | ns | ns | ||
| Soil treatment × Storage | * | ns | ns | ns | * | ** | ||
| Accession × Soil treatment × Storage | ns | ns | ** | ** | ** | ns | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, C.; Moncada, A.; Vetrano, F.; Iapichino, G.; D’Anna, F.; Miceli, A. Effect of Agronomic Practices on Yield and Quality of Borage at Harvest and During Storage as Minimally-Processed Produce. Agronomy 2020, 10, 242. https://doi.org/10.3390/agronomy10020242
Miceli C, Moncada A, Vetrano F, Iapichino G, D’Anna F, Miceli A. Effect of Agronomic Practices on Yield and Quality of Borage at Harvest and During Storage as Minimally-Processed Produce. Agronomy. 2020; 10(2):242. https://doi.org/10.3390/agronomy10020242
Chicago/Turabian StyleMiceli, Claudia, Alessandra Moncada, Filippo Vetrano, Giovanni Iapichino, Fabio D’Anna, and Alessandro Miceli. 2020. "Effect of Agronomic Practices on Yield and Quality of Borage at Harvest and During Storage as Minimally-Processed Produce" Agronomy 10, no. 2: 242. https://doi.org/10.3390/agronomy10020242
APA StyleMiceli, C., Moncada, A., Vetrano, F., Iapichino, G., D’Anna, F., & Miceli, A. (2020). Effect of Agronomic Practices on Yield and Quality of Borage at Harvest and During Storage as Minimally-Processed Produce. Agronomy, 10(2), 242. https://doi.org/10.3390/agronomy10020242






