Evaluating a Controlled-Release Fertilizer for Plant Establishment in Floating Elements for Bioretention Ponds
Abstract
1. Introduction
2. Materials and Methods
2.1. Setup of the Experiment
2.2. Water Monitoring
2.3. Plant Growth and Analysis
2.4. Statistical Analysis
3. Results
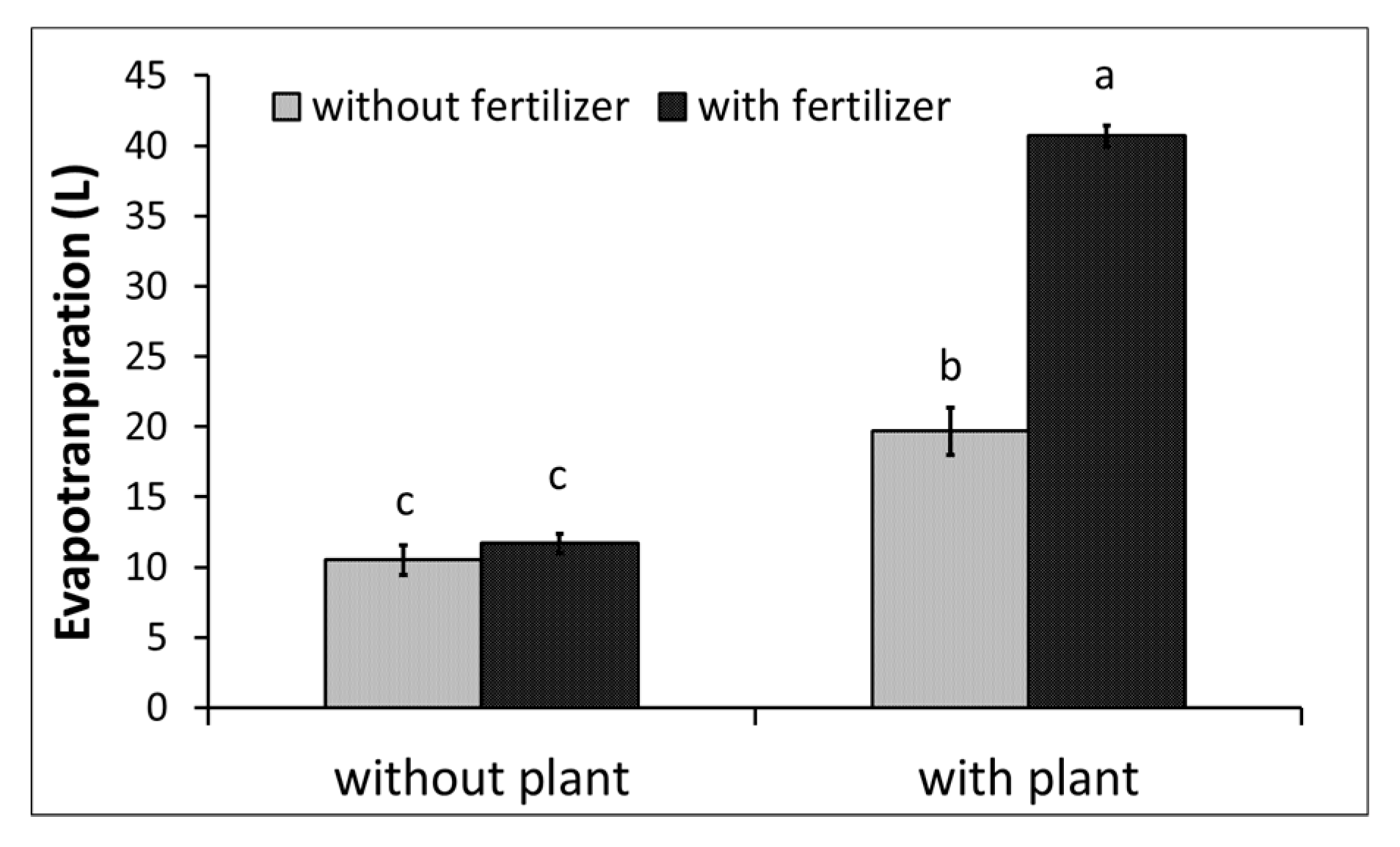
3.1. Water Monitoring
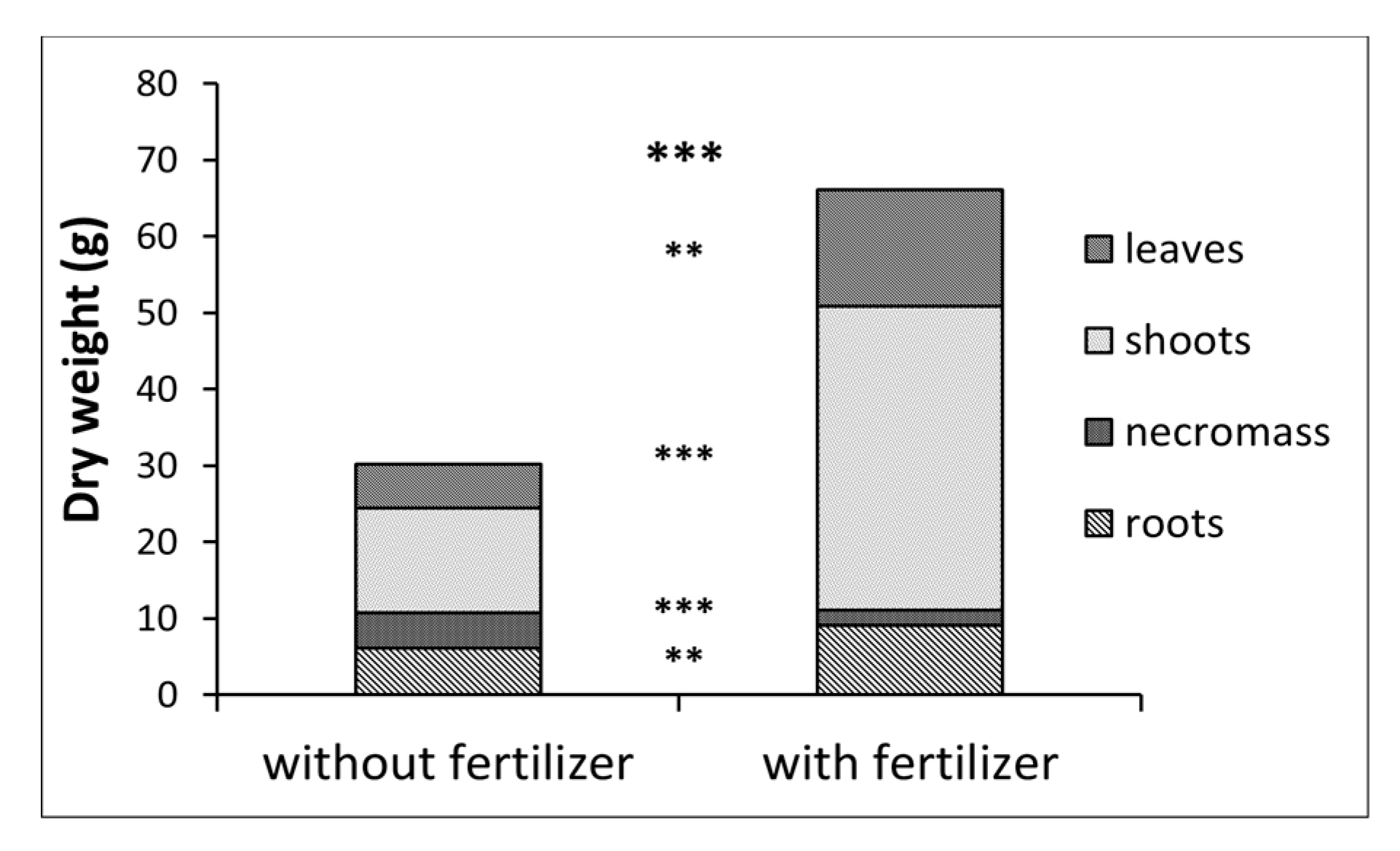
3.2. Plant Growth
3.3. Plant Nutrition and Quality of Water
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mallin, M.A.; Ensign, S.H.; Wheeler, T.L.; Mayes, D.B. Pollutant removal efficacy of three wet detention ponds. J. Environ. Qual. 2002, 31, 654–660. [Google Scholar] [CrossRef] [PubMed]
- German, J.; Svensson, G. Stormwater pond sediments and water—Characterization and assessment. Urban Water J. 2005, 2, 39–50. [Google Scholar] [CrossRef]
- Drake, J.; Guo, Y. Maintenance of wet stormwater ponds in Ontario. Can. Water Resour. J. 2008, 33, 351–368. [Google Scholar] [CrossRef]
- Hancock, G.S.; Holley, J.W.; Chambers, R.M. A Field-Based Evaluation of Wet Retention Ponds: How Effective Are Ponds at Water Quantity Control? J. Am. Water Resour. Assoc. 2010, 46, 1145–1158. [Google Scholar] [CrossRef]
- Tixier, G.; Lafont, M.; Grapentine, L.; Rochfort, Q.; Marsalek, J. Ecological risk assessment of urban stormwater ponds: Literature review and proposal of a new conceptual approach providing ecological quality goals and the associated bioassessment tools. Ecol. Ind. 2011, 11, 1497–1506. [Google Scholar] [CrossRef]
- McAndrew, B.; Ahn, C.; Spooner, J. Nitrogen and sediment capture of a floating treatment wetland on an urban stormwater retention pond—The case of the rain project. Sustainability 2016, 8, 972. [Google Scholar] [CrossRef]
- EC 2013. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Green Infrastructure (GI)—Enhancing Europe’s Natural Capital; (COM (2013) 249 final of 6 May 2013); European Commission: Brussels, Belgium, 2013.
- Strosser, P.; Delacámara, G.; Hanus, A.; Williams, H.; Jaritt, N. A guide to Support the Selection, Design and Implementation of Natural Water Retention Measures in Europe. Capturing the Multiple Benefits of Nature-Based Solutions; Final version, April 2015, Directorate—General for Environment; European Commission: Brussels, Belgium, 2016.
- Collentine, D.; Futter, M.N. Realising the potential of natural water retention measures in catchment flood management: Trade-offs and matching interests. J. Flood Risk Manag. 2018, 11, 76–84. [Google Scholar] [CrossRef]
- Brears, R.C. Blue-Green Infrastructure in Managing Urban Water Resources. In Blue and Green Cities; Palgrave Macmillan Ed: London, UK, 2018. [Google Scholar]
- EPA. Stormwater Wet Pond and Wetland Management Guidebook; EPA 833-B-09-001; United States Environmental Protection Agency: Washington, DC, USA, 2009.
- Wu, J.S.; Holman, R.E.; Dorney, J.R. Systematic evaluation of pollutant removal by urban wet detention ponds. J. Environ. Eng. 1996, 122, 983–988. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Muller, R.A.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef]
- DeBusk, K.M.; Hunt, W.F.; Line, D.E. Bioretention outflow: Does it mimic nonurban watershed shallow interflow? J. Hydrol. Eng. 2010, 16, 274–279. [Google Scholar] [CrossRef]
- Gallagher, M.T.; Snodgrass, J.W.; Ownby, D.R.; Brand, A.B.; Casey, R.E.; Lev, S. Watershed-scale analysis of pollutant distributions in stormwater management ponds. Urban Ecosyst. 2011, 14, 469–484. [Google Scholar] [CrossRef]
- Wium-Andersen, T.; Nielsen, A.H.; Hvitved-Jakobsen, T.; Vollertsen, J. Heavy metals, PAHs and toxicity in stormwater wet detention ponds. Water Sci. Technol. 2011, 64, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.E.; Pitt, R. Targeting treatment technologies to address specific stormwater pollutants and numeric discharge limits. Water Res. 2012, 46, 6715–6730. [Google Scholar] [CrossRef] [PubMed]
- Shelef, O.; Gross, A.; Rachmilevitch, S. Role of plants in a constructed wetland: Current and new perspectives. Water 2013, 5, 405–419. [Google Scholar] [CrossRef]
- Winston, R.J.; Hunt, W.F.; Kennedy, S.G.; Merriman, L.S.; Chandler, J.; Brown, D. Evaluation of floating treatment wetlands as retrofits to existing stormwater retention ponds. Ecol. Eng. 2013, 54, 254–265. [Google Scholar] [CrossRef]
- Al-Rubaei, A.M.; Engström, M.; Viklander, M.; Blecken, G.T. Effectiveness of a 19-year old combined pond-wetland system in removing particulate and dissolved pollutants. Wetlands 2017, 37, 485–496. [Google Scholar] [CrossRef]
- Schwartz, D.; Sample, D.J.; Grizzard, T.J. Evaluating the performance of a retrofitted stormwater wet pond for treatment of urban runoff. Environ. Monit. Assess. 2017, 189, 256. [Google Scholar] [CrossRef]
- Wang, C.Y.; Sample, D.J. Assessment of the nutrient removal effectiveness of floating treatment wetlands applied to urban retention ponds. J. Environ. Manag. 2014, 137, 23–35. [Google Scholar] [CrossRef]
- Headley, T.R.; Tanner, C.C. Constructed wetlands with floating emergent macrophytes: An innovative stormwater treatment technology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2261–2310. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Xie, H.; Yang, Z. Constructed wetlands: A review on the role of radial oxygen loss in the rhizosphere by macrophytes. Water 2018, 10, 678. [Google Scholar] [CrossRef]
- Kivaisi, A.K. The potential for constructed wetlands for wastewater treatment and reuse in developing countries: A review. Ecol. Eng. 2001, 16, 545–560. [Google Scholar] [CrossRef]
- Headley, T.; Tanner, C.C. Application of Floating Wetlands for Enhanced for Stormwater Treatment: A Review; Auckland Regional Council: Auckland, New Zealand, 2008.
- Islam, M.K. Nutrient Removal from Urban Stormwater Using Floating Treatment Wetland System. Master’s Thesis, University of Central Florida, Orlando, FL, USA, 2011. [Google Scholar]
- De Stefani, G.; Tocchetto, D.; Salvato, M.; Borin, M. Performance of a floating treatment wetland for in-stream water amelioration in NE Italy. Hydrobiologia 2011, 674, 157–167. [Google Scholar] [CrossRef]
- Chang, N.B.; Xuan, Z.; Marimon, Z.; Islam, K.; Wanielista, M.P. Exploring hydrobiogeochemical processes of floating treatment wetlands in a subtropical stormwater wet detention pond. Ecol. Eng. 2013, 54, 66–76. [Google Scholar] [CrossRef]
- Zanin, G.; Bortolini, L.; Borin, M. Assessing stormwater nutrient and heavy metal plant uptake in an experimental bioretention pond. Land 2018, 7, 150. [Google Scholar] [CrossRef]
- Backhaus, A.; Fryd, O. The aesthetic performance of urban landscape-based stormwater management systems: A review of twenty projects in Northern Europe. J. Landsc. Archit. 2013, 8, 52–63. [Google Scholar] [CrossRef]
- Zancan, S.; Cesco, S.; Ghisi, R. Effect of UV-B radiation on iron content and distribution in maize plants. Environ. Exp. Bot. 2006, 55, 266–272. [Google Scholar] [CrossRef]
- Haynes, R.J. Active ion uptake and maintenance of cation-anion balance: A critical examination of their role in regulating rhizosphere pH. Plant Soil 1990, 126, 247–264. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Rhizosphere chemistry in relation to plant nutrition. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2012; pp. 347–368. [Google Scholar]
- Savvas, D.; Gianquinto, G.; Tuzel, Y.; Gruda, N. Soilless culture. In Good Agricultural Practices for Greenhouse Vegetable Crops: Principles for Mediterranean Climate Areas; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2013; pp. 303–354. [Google Scholar]
- Husby, C.E.; Niemiera, A.X.; Harris, J.R.; Wright, R.D. Influence of diurnal temperature on nutrient release patterns of three polymer-coated fertilizers. HortScience 2003, 38, 387–389. [Google Scholar] [CrossRef]
- Merhaut, D.J.; Blythe, E.K.; Newman, J.P.; Albano, J.P. Nutrient release from controlled-release fertilizers in acid substrate in a greenhouse environment: I. Leachate electrical conductivity, pH, and nitrogen, phosphorus, and potassium concentrations. HortScience 2006, 41, 780–787. [Google Scholar] [CrossRef]
- Newman, J.P.; Albano, J.P.; Merhaut, D.J.; Blythe, E.K. Nutrient release from controlled-release fertilizers in a neutral-pH substrate in an outdoor environment: I. Leachate electrical conductivity, pH, and nitrogen, phosphorus, and potassium concentrations. HortScience 2006, 41, 1674–1682. [Google Scholar] [CrossRef]
- Signore, A.; Serio, F.; Santamaria, P. A targeted management of the nutrient solution in a soilless tomato crop according to plant needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Shaviv, A. Advances in controlled-release fertilizers. Adv. Agron. 2000, 71, 1–49. [Google Scholar]
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilisers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, X. Controlled-Release Fertilizers as a Means to Reduce Nitrogen Leaching and Runoff in Container-Grown Plant Production. In Nitrogen in Agriculture-Updates; IntechOpen: London, UK, 2018. [Google Scholar]
- Ehret, D.L.; Edwards, D.; Helmer, T.; Lin, W.; Jones, G.; Dorais, M.; Papadopoulos, A.P. Effects of oxygen-enriched nutrient solution on greenhouse cucumber and pepper production. Sci. Hortic. 2010, 125, 602–607. [Google Scholar] [CrossRef]
- Lara, L.J.; Egea-Gilabert, C.; Niñirola, D.; Conesa, E.; Fernández, J.A. Effect of aeration of the nutrient solution on the growth and quality of purslane (Portulaca oleracea). J. Hortic. Sci. Biotechnol. 2011, 86, 603–610. [Google Scholar] [CrossRef]
- Morard, P.; Silvestre, J. Plant injury due to oxygen deficiency in the root environment of soilless culture: A review. Plant Soil 1996, 184, 243–254. [Google Scholar] [CrossRef]
- Lynch, J.; Marschner, P.; Rengel, Z. Effect of internal and external factors on root growth and development. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2012; pp. 331–346. [Google Scholar]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of zeolites for sustainable agriculture: A review on water and nutrient retention. Water Air Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Jakkula, V.S.; Wani, S.P. Zeolites: Potential soil amendments for improving nutrient and water use efficiency and agriculture productivity. Sci. Rev. Chem. Commun. 2018, 8, 1–15. [Google Scholar]
- Mahesh, M.; Thomas, J.; Arun Kumar, K.; Bhople, B.S.; Saresh, N.V.; Vaid, S.K.; Sahu, S.K. Zeolite farming: A sustainable agricultural prospective. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2912–2924. [Google Scholar] [CrossRef]
- Shaviv, A.; Raban, S.; Zaidel, E. Modeling controlled nutrient release from polymer coated fertilizers: Diffusion release from single granules. Environ. Sci. Technol. 2003, 37, 2251–2256. [Google Scholar] [CrossRef]
- Lammel, J. Cost of the different options available to the farmers: Current situation and prospects. In IFA International Workshop on En Hanced-Efficiency Fertilizers; International Fertilizer Industry Association: Paris, France, 2005. [Google Scholar]
- Jeke, N.; Zvomuya, F. Nutrient supply rates and phytoextraction during Wetland Phytoremediation of an End-of-Life Municipal Lagoon. Soil Sci. Soc. Am. J. 2018, 82, 1004–1012. [Google Scholar] [CrossRef]
- Flimlin, G. Establishing an Ornamental Aquatic Plant Culture Facility. Fact Sheet FS535. Rutgers NJAES Cooperative Extension. 2004. Available online: https://agrilifecdn.tamu.edu/fisheries/files/2013/09/Establishing-an-Ornamental-Aquatic-Plant-Culture-Facility.pdf (accessed on 8 July 2019).
- Flimlin, G.; Pomeroy, R. Growing Ornamental Aquatic Plants as a Business in the Northeastern United States. Northeastern Regional Aquaculture Center. 2008. Available online: https://agrilifecdn.tamu.edu/fisheries/files/2013/09/NRAC-Publication-No.-301-2008-%E2%80%93-Growing-Ornamental-Aquatic-Plants-as-a-Business-in-the-Northeastern-United-States.pdf (accessed on 8 July 2019).
- Sink, T.; Gwinn, J.; Gerke, H. Ornamental Ponds & Water Gardens in Texas; Texas A&M AgriLife Extension Service: College Station, TX, USA, 2014.
- Ye, Y.; Liang, X.; Chen, Y.; Liu, J.; Gu, J.; Guo, R.; Li, L. Alternate wetting and drying irrigation and controlled-release nitrogen fertilizer in late-season rice. Effects on dry matter accumulation, yield, water and nitrogen use. Field Crops Res. 2013, 144, 212–224. [Google Scholar] [CrossRef]
- Chalk, P.M.; Craswell, E.T.; Polidoro, J.C.; Chen, D. Fate and efficiency of 15 N-labelled slow-and controlled-release fertilizers. Nutr. Cycl. Agroecosyst. 2015, 102, 167–178. [Google Scholar] [CrossRef]
- Sirvi, R. Effect of Polymer Coated Urea on Growth and Yield of Rice (Oryza sativa L.). Ph.D. Thesis, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, India, 2015. [Google Scholar]


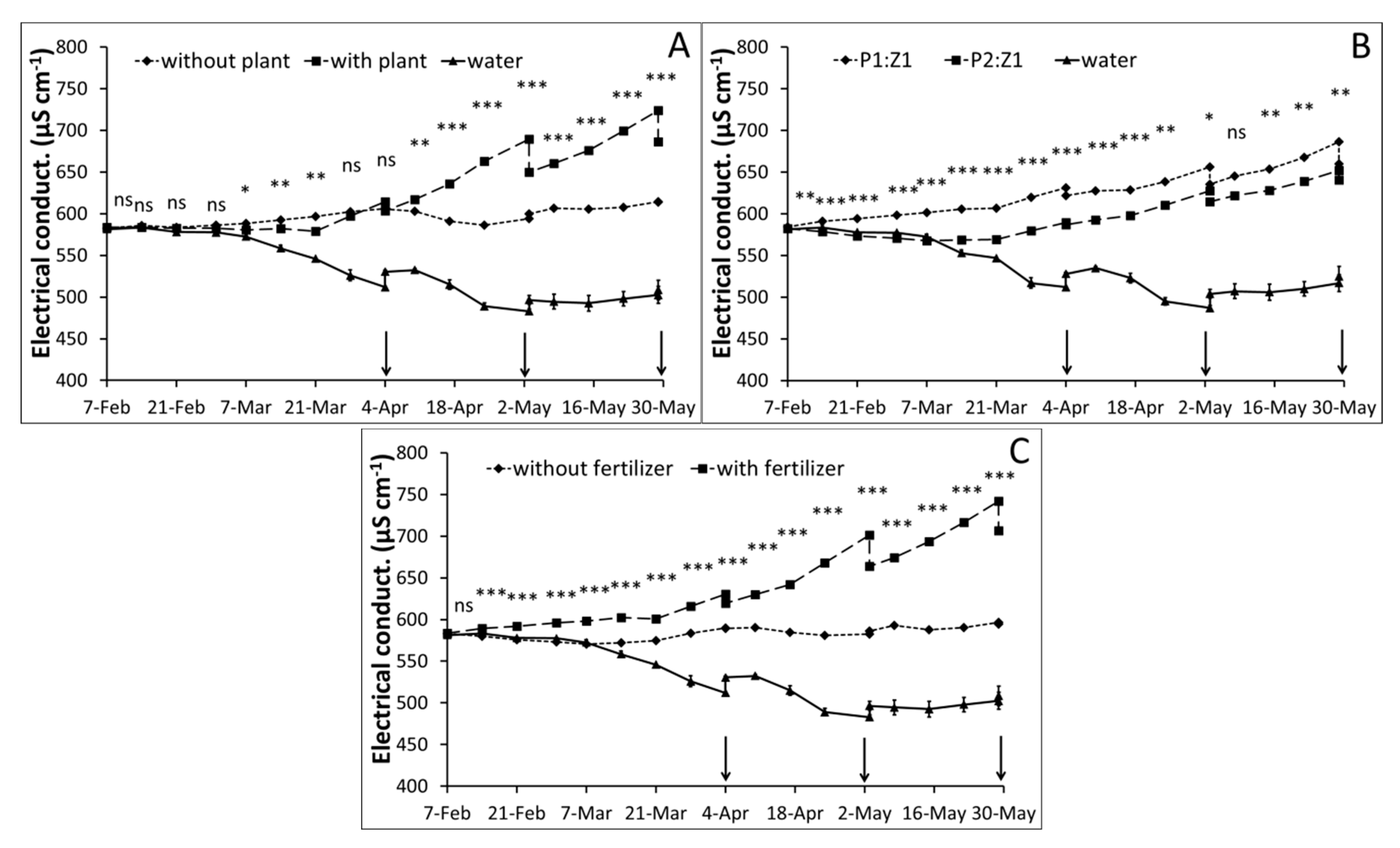
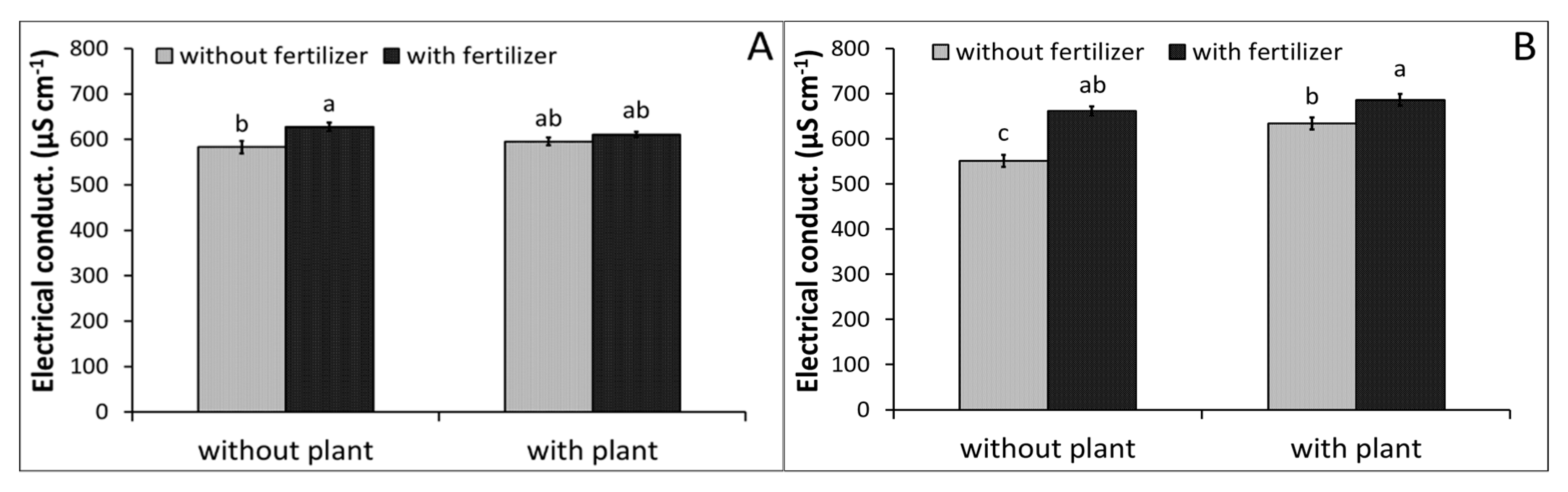
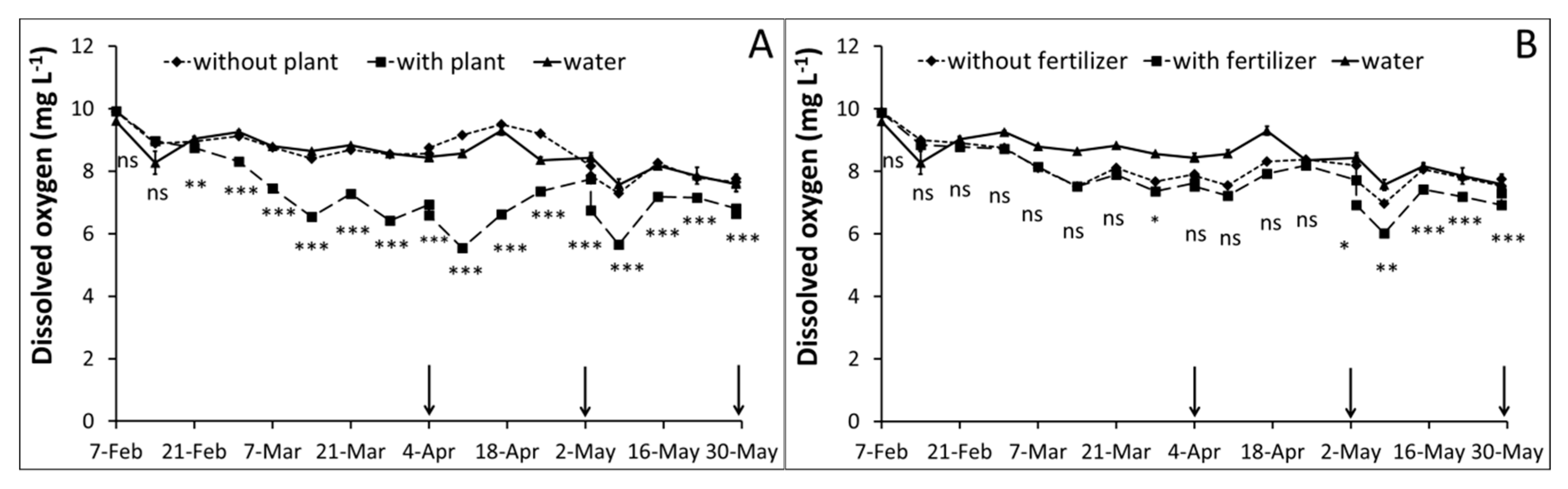
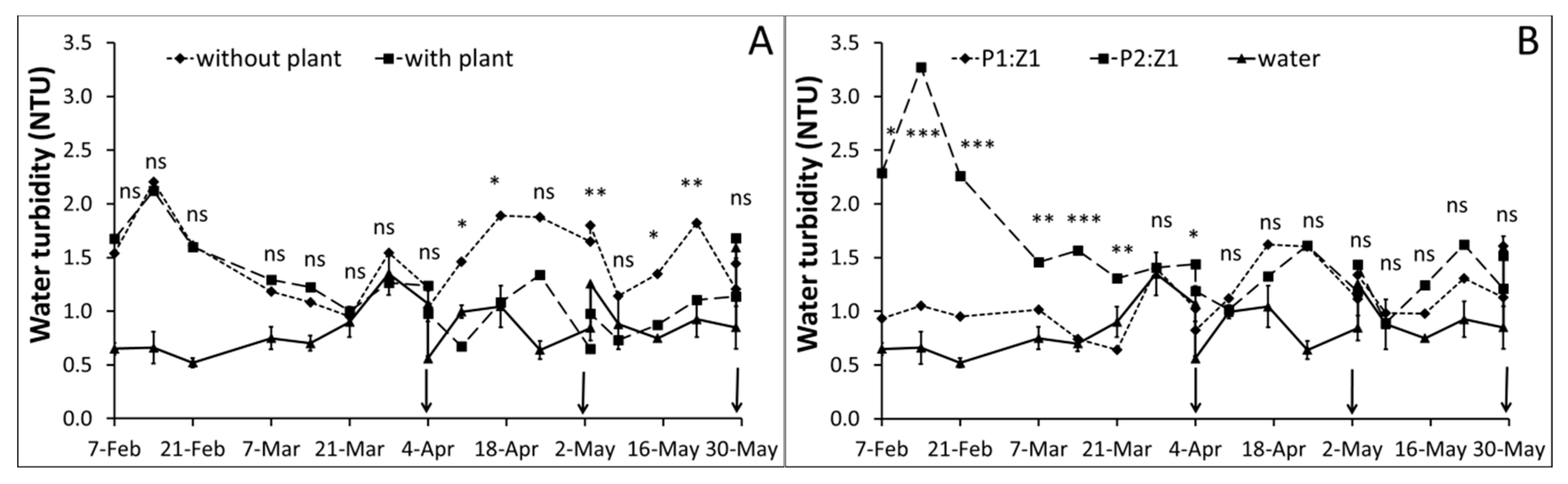

| Parameter | Peat (P) | Zeolite (Z) | P2:Z1 | P1:Z1 | Method |
|---|---|---|---|---|---|
| Bulk Density (g cm−3) * | 126 | 1052 | 425 | 583 | EN 13040 |
| pH | 4.3 | 7.7 | 4.8 | 5.1 | EN 13037 |
| EC (µS cm−1) * | 50 | 91 | 53 | 63 | EN 13038 |
| Total–N (%) | 1.01 | 0.01 | 0.18 (689) | 0.08 (420) | Kjeldahl |
| ICP-OES: | |||||
| P (‰) | 0.266 | 0.914 | 0.604 (231) | 0.705 (370) | Zancan et al. [32] |
| K (%) | 0.044 | 3.328 | 1.328 (5080) | 2.328 (12215) | Zancan et al. [32] |
| Ca (%) | 0.982 | 1.920 | 1.699 (6499) | 1.874 (9833) | Zancan et al. [32] |
| Mg (%) | 1.027 | 6.225 | 3.540 (13541) | 4.628 (24283) | Zancan et al. [32] |
| Ion Chromatography: | |||||
| Nitrate-N (mg L−1) | 3.656 | 2.593 | 3.254 (14.6) | 2.967 (13.4) | EN 13652 |
| Ammonium-N (mg L−1) | 3.240 | 0.382 | 0.894 (4.0) | 0.623 (2.8) | EN 13652 |
| Phosphate-P (mg L−1) | 1.606 | 0.188 | 0.734 (3.3) | 0.419 (1.9) | EN 13652 |
| K+ (mg L−1) | 2.892 | 13.699 | 7.685 (34.6) | 7.898 (35.5) | EN 13652 |
| Ca2+ (mg L−1) | 16.989 | 8.376 | 15.482 (69.7) | 13.887 (62.5) | EN 13652 |
| Mg2+ (mg L−1) | 3.877 | 1.242 | 3.040 (13.7) | 2.289 (10.3) | EN 13652 |
| Parameters | Value ± S.D. |
|---|---|
| pH | 6.58 ± 0.6 |
| Electrical conductivity (µS cm−1) | 586 ± 28 |
| Nitrate-NO3− (mg L−1) | 18.15 ± 1.11 |
| PO43− (mg L−1) | traces |
| SO42− (mg L−1) | 22.57 ± 1.98 |
| Cl− (mg L−1) | 9.07 ± 0.62 |
| HCO3− (mg L−1) | 293 ± 14 |
| NH4+ (mg L−1) | 0.14 ± 0.04 |
| K+ (mg L−1) | 0.78 ± 0.09 |
| Ca2+ (mg L−1) | 36.40 ± 7.63 |
| Mg2+ (mg L−1) | 23.99 ± 1.29 |
| Na+ (mg L−1) | 4.33 ± 0.21 |
| N | P | K | Ca | Mg | |||
| Tap water | 184 | traces | 34.2 | 1595 | 1051 | ||
| Plant | Fertilizer | Substrate | |||||
| Without | Without | P2:Z1 | 192 c | traces | 35.7 c | 1663 c | 1096 c |
| P1:Z1 | 191 c | traces | 35.6 c | 1655 c | 1090 c | ||
| With | P2:Z1 | 203 c | traces | 37.6 c | 1752 c | 1154 c | |
| P1:Z1 | 191 c | traces | 35.4 c | 1650 c | 1087 c | ||
| With | Without | P2:Z1 | 239 b | traces | 44.3 b | 2064 b | 1360 a |
| P1:Z1 | 222 bc | traces | 41.2 bc | 1920 bc | 1265 bc | ||
| With | P2:Z1 | 319 a | traces | 59.2 a | 2758 a | 1817 a | |
| P1:Z1 | 319 a | traces | 59.3 a | 2759 a | 1817 a | ||
| Significance | |||||||
| Plant (P) | *** | n.d. | *** | *** | *** | ||
| Fertilizer (F) | *** | n.d. | *** | *** | *** | ||
| Substrate (S) | n.s. | n.d. | n.s. | n.s. | n.s. | ||
| P x F | ***. | n.d. | *** | *** | *** | ||
| P x S | n.s. | n.d. | n.s. | n.s. | n.s. | ||
| F x S | n.s. | n.d. | n.s. | n.s. | n.s. | ||
| P x F x S | n.s. | n.d. | n.s. | n.s. | n.s. |
| N | P | K | Ca | Mg | |||
| Tap water | 163 | traces | 34.7 | 733 | 913 | ||
| Plant | Fertilizer | Substrate | |||||
| Without | Without | P2:Z1 | 79 b | 1 c | 307 b | 1225 b | 724 c |
| P1:Z1 | 85 b | traces c | 428 b | 951 b | 796 bc | ||
| With | P2:Z1 | 243 a | 46.5 a | 403 b | 1679 a | 854 ab | |
| P1:Z1 | 233 a | 35.3 b | 577 a | 1703 a | 857 ab | ||
| With | Without | P2:Z1 | 6 c | traces c | 3 d | 1307 ab | 865 ab |
| P1:Z1 | 7 c | traces c | 114 c | 1145 b | 794 bc | ||
| With | P2:Z1 | 4 c | traces c | 4 d | 1206 b | 872 ab | |
| P1:Z1 | 6 c | traces c | 3 d | 1286 ab | 926 a | ||
| Significance | |||||||
| Plant (P) | *** | *** | *** | * | n.s. | ||
| Fertilizer (F) | *** | *** | n.s. | *** | * | ||
| Substrate (S) | n.s. | n.s. | *** | n.s. | n.s. | ||
| PxF | *** | *** | *** | *** | n.s. | ||
| PxS | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| FxS | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| PxFxS | n.s. | n.s. | n.s. | n.s. | n.s. |
| Fertilizer | Substrate | N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|
| Without | P2:Z1 | 279 b | 33 b | 762 b | 366 b | 147 b |
| P1:Z1 | 235 b | 23 b | 697 b | 330 b | 101 c | |
| With | P2:Z1 | 701 a | 123 a | 1695a | 640 a | 274 a |
| P1:Z1 | 615 a | 139 a | 1632 a | 699 a | 241 ab | |
| Significance | ||||||
| Fertilizer (F) | *** | *** | *** | *** | *** | |
| Substrate (S) | n.s. | n.s. | n.s. | n.s. | * | |
| FxS | n.s. | * | n.s. | n.s. | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanin, G.; Maucieri, C.; Dal Ferro, N.; Bortolini, L.; Borin, M. Evaluating a Controlled-Release Fertilizer for Plant Establishment in Floating Elements for Bioretention Ponds. Agronomy 2020, 10, 199. https://doi.org/10.3390/agronomy10020199
Zanin G, Maucieri C, Dal Ferro N, Bortolini L, Borin M. Evaluating a Controlled-Release Fertilizer for Plant Establishment in Floating Elements for Bioretention Ponds. Agronomy. 2020; 10(2):199. https://doi.org/10.3390/agronomy10020199
Chicago/Turabian StyleZanin, Giampaolo, Carmelo Maucieri, Nicola Dal Ferro, Lucia Bortolini, and Maurizio Borin. 2020. "Evaluating a Controlled-Release Fertilizer for Plant Establishment in Floating Elements for Bioretention Ponds" Agronomy 10, no. 2: 199. https://doi.org/10.3390/agronomy10020199
APA StyleZanin, G., Maucieri, C., Dal Ferro, N., Bortolini, L., & Borin, M. (2020). Evaluating a Controlled-Release Fertilizer for Plant Establishment in Floating Elements for Bioretention Ponds. Agronomy, 10(2), 199. https://doi.org/10.3390/agronomy10020199








