Variations in Growth, Physiology, and Antioxidative Defense Responses of Two Tomato (Solanum lycopersicum L.) Cultivars after Co-Infection of Fusarium oxysporum and Meloidogyne incognita
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Fungal and Nematode Culture
2.2. Screening of Tomato Cultivars
2.3. Experimental Design and Treatment Plan
2.4. Scoring of Chlorotic or Necrotic Symptoms and Disease Intensity
2.5. Disease Assessment of Root Galls
2.6. Assessment of Antioxidant Activities in Roots and Leaves of Tomato
2.7. Determination of Oxidative Stress Indicators
2.8. Plant Biomass Accumulation
2.9. Total Chlorophyll Contents
2.10. Proline Contents
2.11. Electrolyte Leakage
2.12. Leaf Gas Exchange and Fluorescence Analysis
2.13. Transmission Electron Microscopy
2.14. Statistical Analyses
3. Results
3.1. Disease Assessment
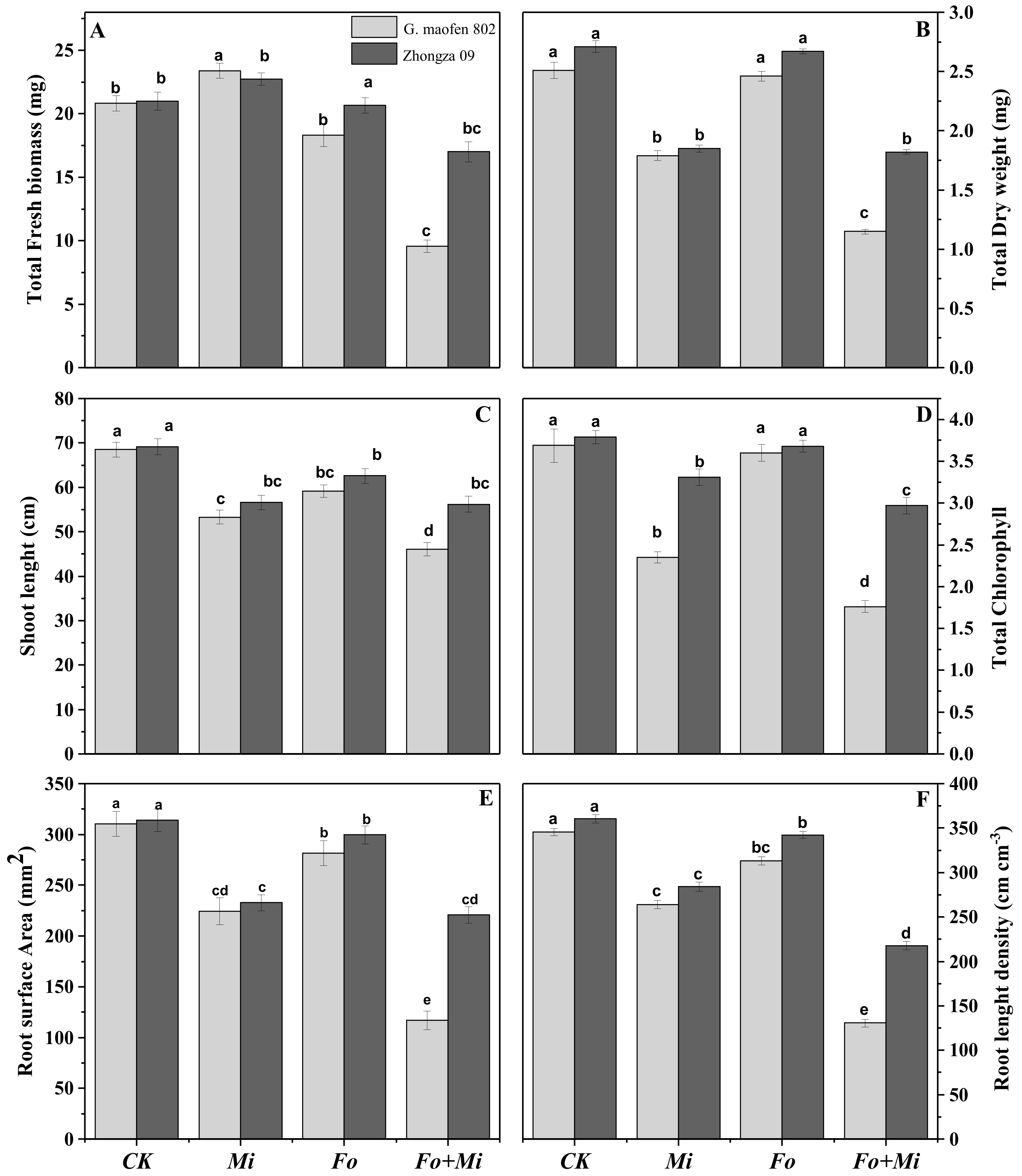
3.2. Interactive Effects of Fo and Mi on Biomass Accumulation
3.3. Interactive Effect of Fo and Mi on Chlorophyll Contents
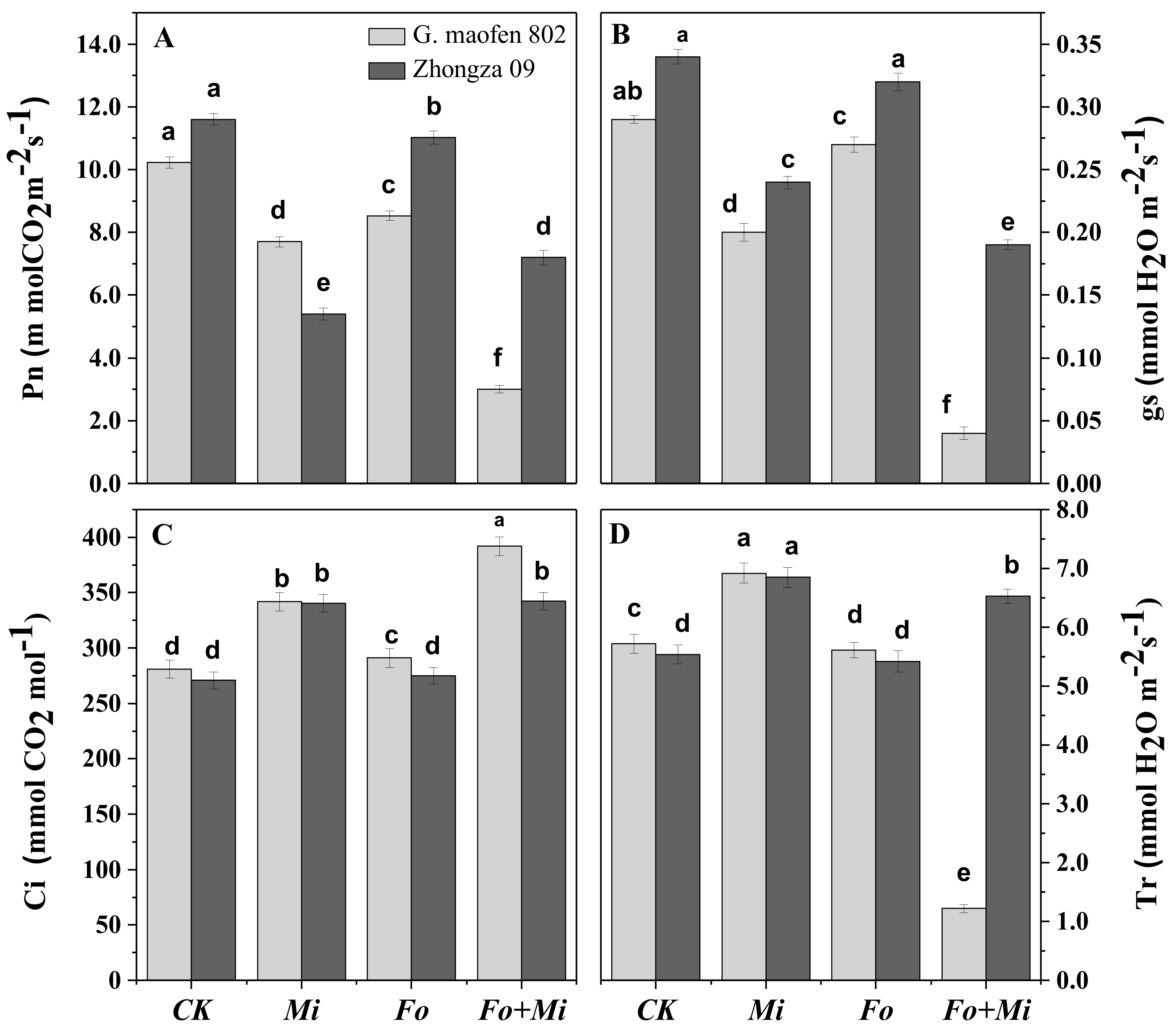
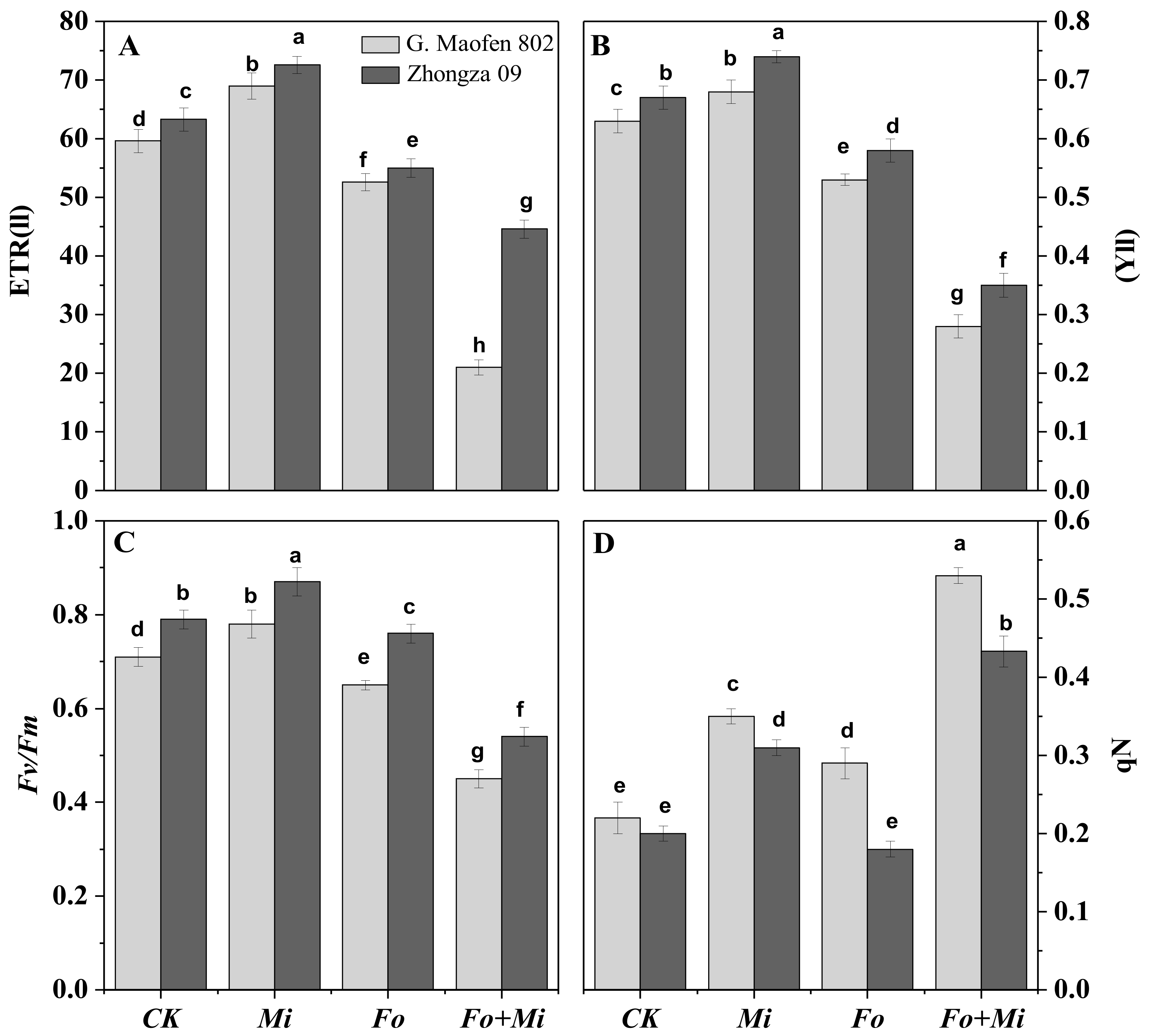
3.4. Interactive Effect of Fo and Mi on Photosynthetic Traits
3.5. Interactive Effect of Fo and Mi on Oxidative Defense Mechanism in Leaves and Roots
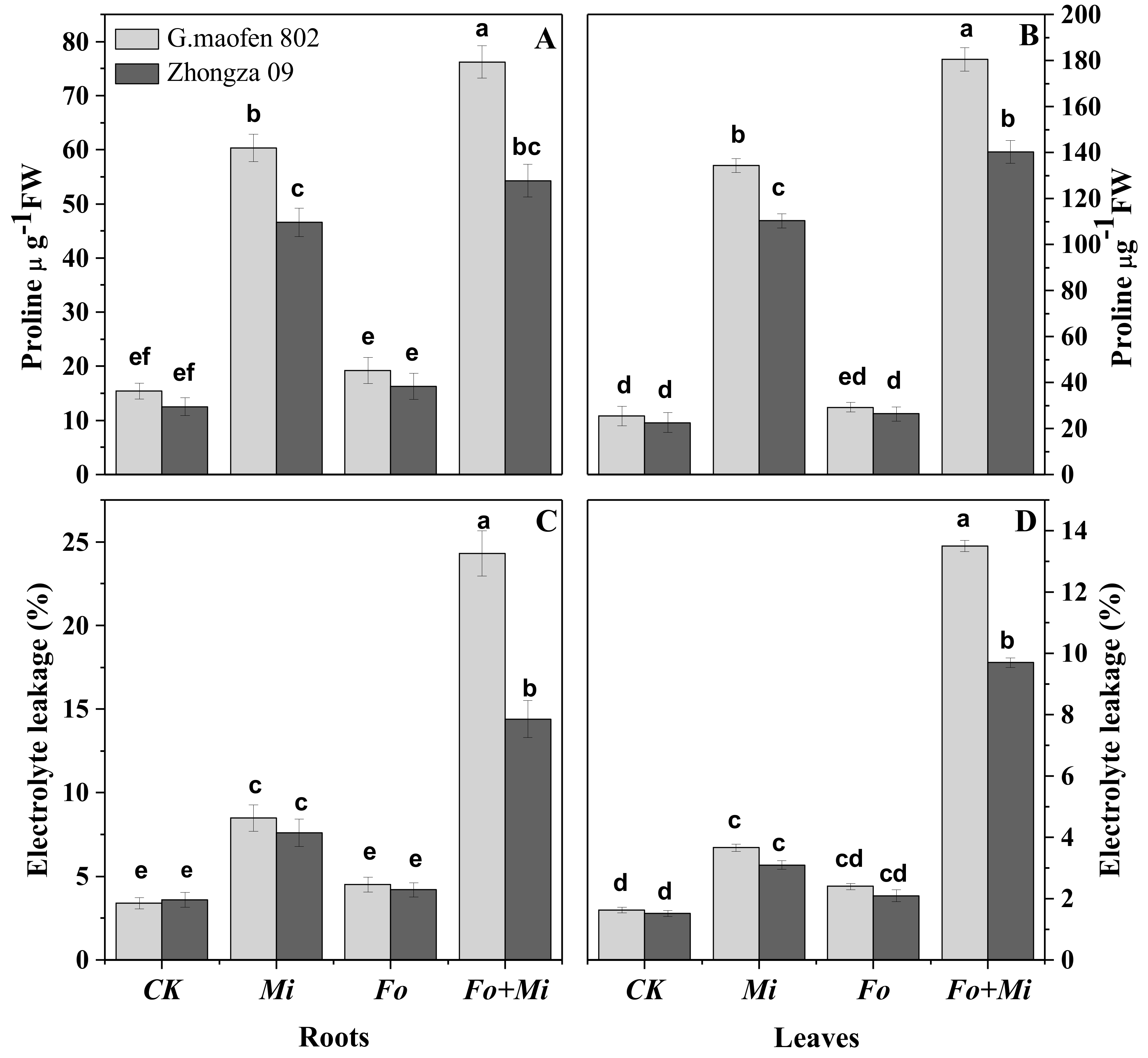
3.6. Proline Contents and Electrolyte Leakage
3.7. Root Galls and Mi Population in Roots and Soil
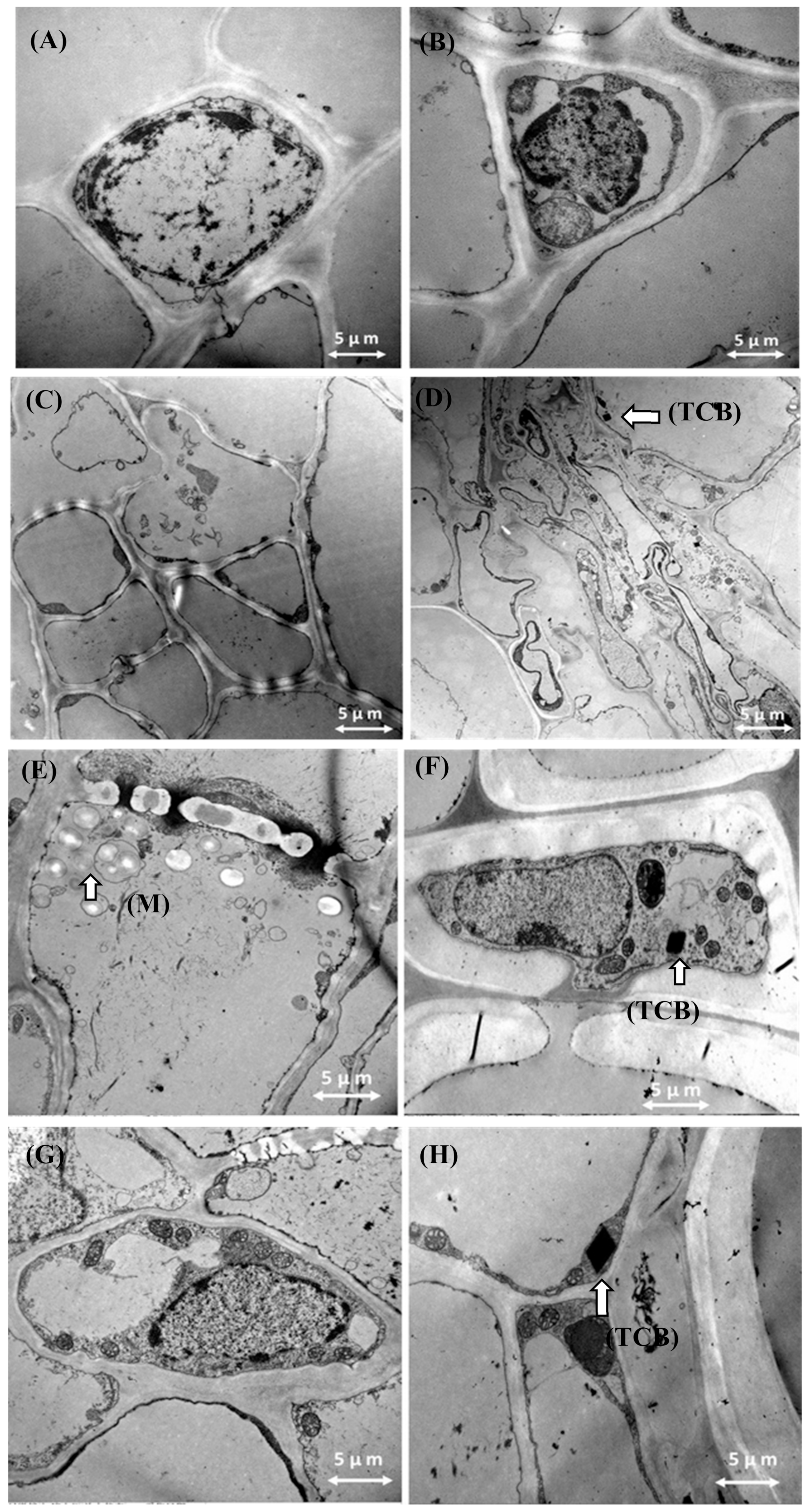
3.8. Transmission Electron Microscopy Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, X.; Lewis, E.E.; Liu, Q.; Li, H.; Bai, C.; Wang, Y. Effects of long-term continuous cropping on soil nematode community and soil condition associated with replant problem in strawberry habitat. Sci. Rep. 2016, 6, 30466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Li, X.; Yang, Q.; Chi, X.; Pan, L.; Chen, N.; Yang, Z.; Wang, T.; Wang, M.; Yu, S. Soil eukaryotic microorganism succession as affected by continuous cropping of peanut-pathogenic and beneficial fungi were selected. PLoS ONE 2012, 7, e40659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The good, the bad, and the ugly of rhizosphere microbiome. In Probiotics and Plant Health; Springer: Berlin, Germany, 2017; pp. 253–290. [Google Scholar]
- Gordon, T.R. Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Shafique, H.A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of soil-borne diseases of organic vegetables. J. Plant Prot. Res. 2016, 56, 221–230. [Google Scholar] [CrossRef]
- Sharma, I.P.; Sharma, A. Physiological and biochemical changes in tomato cultivar PT-3 with dual inoculation of mycorrhiza and PGPR against root-knot nematode. Symbiosis 2017, 71, 175–183. [Google Scholar] [CrossRef]
- Al-Hammouri, A.A.; Lindemann, W.; Thomas, S.; Sanogo, S. Effect of inoculum levels of Rhizoctonia solani and Meloidogyne incognita on chile pepper in soil simultaneously infested with both pathogens. Res. Crop. 2018, 19. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Ozbay, N.; Newman, S.E. Fusarium crown and root rot of tomato and control methods. Plant Pathol. J. 2004, 3, 9–18. [Google Scholar]
- Simbaqueba, J. Analysis of Fusarium Oxysporum Effectors Shared between Strains that Infect Cape Gooseberry and Tomato. Ph.D. Thesis, The Australian National University, Canberra, Australia, 2017. [Google Scholar]
- Singh, V.K.; Singh, H.B.; Upadhyay, R.S. Role of fusaric acid in the development of ‘Fusarium wilt’symptoms in tomato: Physiological, biochemical and proteomic perspectives. Plant Physiol. Biochem. 2017, 118, 320–332. [Google Scholar] [CrossRef]
- Castagnone-Sereno, P.; Danchin, E.G.; Perfus-Barbeoch, L.; Abad, P. Diversity and evolution of root-knot nematodes, genus Meloidogyne: New insights from the genomic era. Annu. Rev. Phytopathol. 2013, 51, 203–220. [Google Scholar] [CrossRef]
- Kyndt, T.; Vieira, P.; Gheysen, G.; de Almeida-Engler, J. Nematode feeding sites: Unique organs in plant roots. Planta 2013, 238, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Favery, B.; Quentin, M.; Jaubert-Possamai, S.; Abad, P. Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. J. Insect Physiol. 2016, 84, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Grundler, F.M.; Hofmann, J. Water and nutrient transport in nematode feeding sites. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Berlin, Germany, 2011; pp. 423–439. [Google Scholar]
- Kassie, Y.G. Status of root knot nematode (Meloidogyne species) and fusarium wilt (Fusarium oxysporum) disease complex on tomato (Solanum lycopersicum L.) in the Central Rift Valley Ethopia. Agri. Sci. 2019, 10, 1090–1103. [Google Scholar]
- Powell, N. Interaction of plant parasitic nematodes with other disease-causing agents. In Plant Parasitic Nematodes; Zuckerman, B.M., Mai, W.F., Rohde, R.A., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 119–136. [Google Scholar]
- Horrigue-Raouani, N. Variabilité de la relation hôte parasite dans le cas des Meloidogyne spp.(Nematoda: Meloidogynidae). Ph.D. Thesis, Université Tunis-El Manar, Faculté des Sciences de Tunis, Tunis, Tunisia, 2003. [Google Scholar]
- Kazan, K.; Lyons, R. Intervention of phytohormone pathways by pathogen effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef] [Green Version]
- Hossain, Z.; Komatsu, S. Contribution of proteomic studies towards understanding plant heavy metal stress response. Front. Plant Sci. 2013, 3, 310. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zuo, C.; Deng, G.; Kuang, R.; Yang, Q.; Hu, C.; Sheng, O.; Zhang, S.; Ma, L.; Wei, Y. Contamination of bananas with beauvericin and fusaric acid produced by Fusarium oxysporum f. sp. cubense. PLoS ONE 2013, 8, e70226. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.S. Vegetable breeding for nutritional quality and health benefits. In Cultivar: Chemical Properties, Antioxidant Activities and Health Benefits; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 1–81. [Google Scholar]
- Palomares-Rius, J.E.; Castillo, P.; Trapero-Casas, J.L.; Jiménez-Díaz, R.M. Infection by Meloidogyne javanica does not breakdown resistance to the defoliating pathotype of Verticillium dahliae in selected clones of wild olive. Sci. Hortic. 2016, 199, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elsalam, K.A.; Asran-Amal, A.; Schnieder, F.; Migheli, Q.; Verreet, J.A. Molecular detection of Fusarium oxysporum f. sp. vasinfectum in cotton roots by PCR and real-time PCR assay/Molekularer Nachweis von Fusarium oxysporum f. sp. vasinfectum in baumwolle mittels PCR und Real-Time-PCR. J. Plant Dis. Prot. 2006, 113, 14–19. [Google Scholar]
- Koch, R. The aetiology of tuberculosis. Berl. Klin. Wochenschr. 1882, 19, 221–230. [Google Scholar]
- Barker, K.; Hussey, R. Histopathology of nodular tissues of legumes infected with certain nematodes. Phytopathology 1976, 66, 851–855. [Google Scholar] [CrossRef]
- Latif Khan, A.; Ahmed Halo, B.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J.; Al-Harrasi, A.; Lee, I.J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron. J. Biotechnol. 2016, 19, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Purwati, R.D.; Hidayah, N. Inoculation methods and conidial densities of Fusarium oxysporum f. sp. cubense in Abaca. HAYATI J. Biosci. 2008, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bybd, D., Jr.; Kirkpatrick, T.; Barker, K. An improved technique for clearing and staining plant tissues for detection of nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar] [PubMed]
- Viglierchio, D.; Schmitt, R.V. On the methodology of nematode extraction from field samples: Baermann funnel modifications. J. Nematol. 1983, 15, 438–444. [Google Scholar] [PubMed]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Yong, Z.; Hao-Ru, T.; Ya, L. Variation in antioxidant enzyme activities of two strawberry cultivars with short-term low temperature stress. World J. Agric. Sci. 2008, 4, 458–462. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Esterbauer, H.; Schwarzl, E.; Hayn, M. A rapid assay for catechol oxidase and laccase using 2-nitro-5-thiobenzoic acid. Anal. Biochem. 1977, 77, 486–494. [Google Scholar] [CrossRef]
- Li, F.; Ling, X.; Cao, X.; Wang, Z.; Shi, W.; Zhang, S. High specific monoclonal antibody production and development of an ELISA method for monitoring T-2 toxin in rice. J. Agric. Food Chem. 2014, 62, 1492–1497. [Google Scholar] [CrossRef]
- Abbasi, H.; Akhtar, A.; Sharf, R. Vesicular arbuscular mycorrhizal (VAM) fungi: A tool for sustainable agriculture. Am. J. Plant Nutr. Fertil. Technol. 2015, 5, 40–49. [Google Scholar]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metal immobilization in soil irrigated with untreated wastewater. J. Plant Growth Regul. 2019, 1–16. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzyme in isolated chloroplast and chlorophyll expressed in terms of mg per gram. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Balestrini, R.; Cosgrove, D.J.; Bonfante, P. Differential location of α-expansin proteins during the accommodation of root cells to an arbuscular mycorrhizal fungus. Planta 2005, 220, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J.; Toulmin, C.; Williams, S. Sustainable intensification in African agriculture. Int. J. Agric. Sustain. 2011, 9, 5–24. [Google Scholar] [CrossRef]
- Dracatos, P.M.; Haghdoust, R.; Singh, D.; Park, R.F. Exploring and exploiting the boundaries of host specificity using the cereal rust and mildew models. New Phytol. 2018, 218, 453–462. [Google Scholar] [CrossRef]
- Ramalingam, K. Exploring the disease severity by the interaction of fusarium wilt and root knot nematode in tomato. Int. J. Fauna Biol. Stud. 2019, 6, 1–5. [Google Scholar]
- Al-Hazmi, A.; Al-Nadary, S. Interaction between Meloidogyne incognita and Rhizoctonia solani on green beans. Saudi J. Biol. Sci. 2015, 22, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Saeedizadeh, A.; Kheiri, A.; Okhovat, M.; Hoseininejad, A. Study on interaction between root-knot nematode Meloidogyne javanica and wilt fungus Verticillium dahliae on olive seedlings in greenhouse. Commun. Agric. Appl. Biol. Sci. 2003, 68, 139–143. [Google Scholar] [PubMed]
- Mallaiah, B.; Muthamilan, M.; Prabhu, S.; Ananthan, R. Studies on interaction of nematode, Pratylenchus delattrei and fungal pathogen, Fusarium incarnatum associated with crossandra wilt in Tamil Nadu, India. Curr. Biot. 2014, 8, 157–164. [Google Scholar]
- Hua, G.K.H.; Timper, P.; Ji, P. Meloidogyne incognita intensifies the severity of fusarium wilt on watermelon caused by Fusarium oxysporum f. sp. niveum. Can. J. Plant Pathol. 2019, 41, 261–269. [Google Scholar] [CrossRef]
- Mai, W.; Abawi, G. Interactions among root-knot nematodes and Fusarium wilt fungi on host plants. Annu. Rev. Phytopathol. 1987, 25, 317–338. [Google Scholar] [CrossRef]
- Manzanilla-López, R.H.; Esteves, I.; Devonshire, J. Biology and management of Pochonia chlamydosporia and plant-parasitic nematodes. In Perspectives in Sustainable Nematode Management through Pochonia Chlamydosporia Applications for Root and Rhizosphere Health; Springer: Berlin, Germany, 2017; pp. 47–76. [Google Scholar]
- Pshibytko, N.; Zenevich, L.; Kabashnikova, L. Changes in the photosynthetic apparatus during Fusarium wilt of tomato. Russian J. Plant Physiol. 2006, 53, 25–31. [Google Scholar] [CrossRef]
- Verbančič, J.; Lunn, J.E.; Stitt, M.; Persson, S. Carbon supply and the regulation of cell wall synthesis. Mol. Plant 2018, 11, 75–94. [Google Scholar] [CrossRef]
- Voiniciuc, C.; Pauly, M.; Usadel, B. Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol. 2018, 176, 2590–2600. [Google Scholar] [CrossRef] [Green Version]
- Bleve-Zacheo, T.; Melillo, M.T.; Castagnone-Sereno, P. The contribution of biotechnology to root-knot nematode control in tomato plants. Pest Technol. 2007, 1, 1–16. [Google Scholar]
- Pask, A.; Pietragalla, J.; Mullan, D.; Reynolds, M. Physiological Breeding II: A Field Guide to Wheat Phenotyping; CIMMYT: El Batán, Texcoco, Mexico, 2012. [Google Scholar]
- Stoeva, N.; Bineva, T. Oxidative changes and photosynthesis in oat plants grown in As-contaminated soil. Bulg. J. Plant Physiol. 2003, 29, 87–95. [Google Scholar]
- de Oliveira Silva, F.M.; Lichtenstein, G.; Alseekh, S.; Rosado-Souza, L.; Conte, M.; Suguiyama, V.F.; Lira, B.S.; Fanourakis, D.; Usadel, B.; Bhering, L.L. The genetic architecture of photosynthesis and plant growth-related traits in tomato. Plant Cell Environ. 2018, 41, 327–341. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barna, B.; Ádám, A.; Király, Z. Juvenility and resistance of a superoxide tolerant plant to diseases and other stresses. Naturwissenschaften 1993, 80, 420–422. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Zhao, X.; Zhen, W.; Qi, Y.; Liu, X.; Yin, B. Coordinated effects of root autotoxic substances and Fusarium oxysporum Schl. f. sp. fragariae on the growth and replant disease of strawberry. Front. Agric. China 2009, 3, 34. [Google Scholar] [CrossRef]
- Lu, P.; Davis, R.F.; Kemerait, R.C.; van Iersel, M.W.; Scherm, H. Physiological effects of Meloidogyne incognita infection on cotton genotypes with differing levels of resistance in the greenhouse. J. Nematol. 2014, 46, 352–359. [Google Scholar] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Sharf, R.; Hisamuddin, A.; Akhtar, A. Management of root-knot disease in Phaseolus vulgaris using potassium fertilizer and biocontrol agents. J. Plant Pathol. Microbiol. 2014, 5, 1. [Google Scholar]
- Barker, K.; Olthof, T.H. Relationships between nematode population densities and crop responses. Annu. Rev. Phytopathol. 1976, 14, 327–353. [Google Scholar] [CrossRef]
- Meena, K.S.; Ramyabharathi, S.; Raguchander, T.; Jonathan, E. Interaction of Meloidogyne incognita and Fusarium oxysporum in carnation and physiological changes induced in plants due to the interaction. SAARC J. Agric. 2016, 14, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Jones, H. Partitioning stomatal and non-stomatal limitations to photosynthesis. Plant Cell Environ. 1985, 8, 95–104. [Google Scholar] [CrossRef]
- Gomes, V.M.; Souza, R.M.; Midorikawa, G.; Miller, R.; Almeida, A.M. Guava Decline: Evidence of Nationwide Incidence in Brazil [deterioro del guayabo: Evidencia de la incidencia en brasil]. Nematropica 2012, 42, 153–162. [Google Scholar]
- Allahverdiyeva, Y.; Aro, E.-M. Photosynthetic responses of plants to excess light: Mechanisms and conditions for photoinhibition, excess energy dissipation and repair. In Photosynthesis; Springer: Berlin, Germany, 2012; pp. 275–297. [Google Scholar]
- Yu, J.; Zhang, Y.; Liu, J.; Wang, L.; Liu, P.; Yin, Z.; Guo, S.; Ma, J.; Lu, Z.; Wang, T. Proteomic discovery of H2O2 response in roots and functional characterization of PutGLP gene from alkaligrass. Planta 2018, 248, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kesba, H.H.; El-Beltagi, H.S. Biochemical changes in grape rootstocks resulted from humic acid treatments in relation to nematode infection. Asian Pac. J. Trop. Biomed. 2012, 2, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Zhang, X.; Yang, L.; Tang, M.; Wang, K.; Wang, L.; Bai, L.; Song, C. Hydrogen peroxide plays an important role in PERK4-mediated abscisic acid-regulated root growth in Arabidopsis. Funct. Plant Biol. 2019, 46, 165–174. [Google Scholar] [CrossRef]
- Molinari, S.; Fanelli, E.; Leonetti, P. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol. Plant Pathol. 2014, 15, 255–264. [Google Scholar] [CrossRef]
- Eissa, M.F.; Abd-Elgawad, M.M. Nematophagous bacteria as biocontrol agents of phytonematodes. In Biocontrol Agents of Phytonematodes; CAB International: Wallingford, UK, 2015; pp. 217–243. [Google Scholar]
- Arora, A.; Sairam, R.; Srivastava, G. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Woin, N.; Tata Ngome, P.; Waingeh, N.; Adjoudji, O.; Nossi, E.; Simo, B.; Yingchia, Y.; Nsongang, A.; Adama, F.; Mveme, M. Suitability of Different Processing Techniques and Sales Options for Irish Potato (Solanum Tuberusum) Cultivars in Cameroon; FARA Research Report: Accra, Ghana, 2019; pp. 2550–3359. [Google Scholar]
- Awan, Z.A.; Shoaib, A.; Khan, K.A. Variations in total phenolics and antioxidant enzymes cause phenotypic variability and differential resistant response in tomato genotypes against early blight disease. Sci. Hortic. 2018, 239, 216–223. [Google Scholar] [CrossRef]
- Arshad, W.; Waheed, M.T.; Mysore, K.S.; Mirza, B. Agrobacterium-mediated transformation of tomato with rolB gene results in enhancement of fruit quality and foliar resistance against fungal pathogens. PLoS ONE 2014, 9, e96979. [Google Scholar] [CrossRef] [PubMed]
- Hlatshwayo, S.G. Involvement of abscisic acid and H2O2 in antioxidant enzyme activities mediated by nitric oxide synthase-like activity in maize. Ph.D. Thesis, University of the Western Cape, Cape Town, South Africa, 2018. [Google Scholar]
- Yang, Y.X.; Ahammed, G.J.; Wu, C.; Yang, Z.; Wan, C.; Chen, J. Red light-induced systemic resistance against root-knot nematode is mediated by a coordinated regulation of salicylic acid, jasmonic acid and redox signaling in watermelon. Front. Plant Sci. 2018, 9, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGovern, R. Management of tomato diseases caused by Fusarium oxysporum. Crop Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]

| Treatments | Disease Intensity (%) | |
|---|---|---|
| G. maofen 802 | Zhongza 09 | |
| Control | 0.0 | 0.0 |
| Meloidogyne incognita (Mi) | 0.0 | 0.0 |
| Fusarium oxysporum (Fo) | 0.0 | 0.0 |
| Meloidogyne incognita + Fusarium oxysporum | 100 | 8.5 |
| Fusarium oxysporumAfter7days→Meloidogyne incognita | 85.4 | 3.1 |
| Meloidogyne incognitaAfter7days→Fusarium oxysporum | 95.1 | 7.6 |
| Plant Parts | Cultivars | Treatments | Superoxide Dismutase (Unit mg−1 Protein) | Catalase (Unit mg−1 Protein) | Polyphenol Oxidase (Unit mg−1 Protein) | Ascorbate Peroxidase (Unit mg−1 F.W) | Ascorbate Oxidase (Unit mg-1 Protein) | Proteins (mg g−1 F.W) |
|---|---|---|---|---|---|---|---|---|
| Leaves | G. maofen 802 | CK | 8.06 ± 0.15 e | 8.93 ± 0.15 c | 2.15 ± 0.04 f | 9.56 ± 0.17 f | 0.591 ± 0.04 d | 1.61 ± 0.03 c |
| Mi | 9.54 ± 0.17 b | 6.63 ± 0.12 g | 2.53 ± 0.03 d | 13.3 ± 0.16 c | 0.442 ± 0.05 f | 1.53 ± 0.04 d | ||
| Fo | 7.18 ± 0.16 f | 7.45 ± 0.13 e | 1.86 ± 0.03 g | 12.4 ± 0.14 d | 0.622 ± 0.03 b | 1.5 ± 0.03 e | ||
| Fo + Mi | 2.33 ± 0.12 h | 2.75 ± 0.04 h | 0.15 ± 0.01 h | 5.083 ± 0.13 g | 0.323 ± 0.01 h | 0.28 ± 0.01 h | ||
| Zhongza 09 | CK | 8.24 ± 0.16 d | 9.43 ± 0.14 b | 2.25 ± 0.05 c | 10.32 ± 0.18 e | 0.604 ± 0.03 c | 1.98 ± 0.04 b | |
| Mi | 11.34 ± 0.13 a | 8.23 ± 0.13 d | 3.43 ± 0.03 a | 12.56 ± 0.16 d | 0.535 ± 0.04 e | 1.4 ± 0.03 f | ||
| Fo | 8.83 ± 0.17 c | 10.64 ± 0.11 a | 2.83 ± 0.04 b | 14.72 ± 0.13 a | 0.784 ± 0.03 a | 2.03 ± 0.03 a | ||
| Fo + Mi | 6.73 ± 0.13 g | 6.75 ± 0.17 f | 2.22 ± 0.03 e | 13.85 ± 0.15 b | 0.422 ± 0.04 g | 1.01 ± 0.02 g | ||
| Roots | G. maofen 802 | CK | 4.19 ± 0.16 g | 6.83 ± 0.12 d | 1.25 ± 0.02 e | 5.66 ± 0.15 d | 0.351 ± 0.03 d | 1.21 ± 0.03 d |
| Mi | 5.76 ± 0.15 e | 5.67 ± 0.11 f | 0.94 ± 0.03 f | 4.28 ± 0.14 g | 0.232 ± 0.02 f | 0.85 ± 0.04 f | ||
| Fo | 6.61 ± 0.16 c | 6.35 ± 0.14 e | 1.6 ± 0.02 c | 6.23 ± 0.14 b | 0.427 ± 0.04 b | 0.93 ± 0.05 e | ||
| Fo + Mi | 1.20 ± 0.10 h | 1.87 ± 0.01 g | 0.3 ± 0.04 g | 1.99 ± 0.16 h | 0.125 ± 0.05 h | 0.03 ± 0.01 g | ||
| Zhongza 09 | CK | 4.85 ± 0.14 f | 7.54 ± 0.12 b | 1.77 ± 0.05 b | 5.94 ± 0.14 c | 0.406 ± 0.03 c | 1.43 ± 0.02 b | |
| Mi | 7.54 ± 0.15 b | 7.1 ± 0.13 c | 1.43 ± 0.03 d | 5.53 ± 0.12 e | 0.335 ± 0.02 a | 1.13 ± 0.04 c | ||
| F0 | 8.48 ± 0.13 a | 8.25 ± 0.13 a | 1.84 ± 0.04 a | 6.75 ± 0.15 a | 0.55 ± 0.04 e | 1.82 ± 0.03 a | ||
| Fo + Mi | 5.94 ± 0.11 d | 5.66 ± 0.1 f | 0.83 ± 0.02 f | 4.73 ± 0.12 f | 0.246 ± 0.02 g | 0.95 ± 0.01 e |
| Varieties | Treatments | No. of Females (Per g Root) | Root Population Soil Population | |||
|---|---|---|---|---|---|---|
| No. of Eggs (Per g Root) | Galling Index (0–5) | Size of Galls (mm) | No. of Juveniles (Per 250 cm3 Soil) | |||
| G. maofen 802 | CK | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mi | 83 ± 3.5 a | 11445 ± 10 a | 5.0 | 3.8 ± 0.1 a | 644 ± 5 a | |
| Fo | 0.0 | 0.0 | 0.0 | 0.00 | 0.0 | |
| Fo + Mi | 66 ± 2.5 b | 870 ± 8 c | 3.0 | 1.8 ± 0.2 c | 538 ± 13 b | |
| FoAfter7days→Mi | 51 ± 1.5 cd | 755 ± 12 d | 2.0 | 1.6 ± 0.1 c | 480 ± 8 cd | |
| MiAfter7days→Fo | 64 ± 2.6 b | 875 ± 6.5 c | 3.0 | 1.7 ± 0.2 c | 541 ± 5 b | |
| Zhongza 09 | CK | 0.0 | 0.00 | 0.0 | 0.0 | 0.0 |
| Mi | 56 ± 2.0 c | 957 ± 17 b | 3.0 | 2.2 ± 0.1 b | 558 ± 6 b | |
| Fo | 0.0 | 0.0 | 0.0 | 0.00 | 0.0 | |
| Fo + Mi | 45 ± 2.6 d | 675 ± 6 e | 2.0 | 1.1 ± 0.1 d | 492 ± 6 c | |
| FoAfter7days→Mi | 36 ± 1.5 e | 564 ± 18 f | 1.0 | 1.0 ± 0.1 d | 409 ± 5 e | |
| MiAfter7days→Fo | 46 ± 2.5 d | 683 ± 6 e | 2.0 | 1.2 ± 0.18 d | 490 ± 7 c | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqsood, A.; Wu, H.; Kamran, M.; Altaf, H.; Mustafa, A.; Ahmar, S.; Hong, N.T.T.; Tariq, K.; He, Q.; Chen, J.-T. Variations in Growth, Physiology, and Antioxidative Defense Responses of Two Tomato (Solanum lycopersicum L.) Cultivars after Co-Infection of Fusarium oxysporum and Meloidogyne incognita. Agronomy 2020, 10, 159. https://doi.org/10.3390/agronomy10020159
Maqsood A, Wu H, Kamran M, Altaf H, Mustafa A, Ahmar S, Hong NTT, Tariq K, He Q, Chen J-T. Variations in Growth, Physiology, and Antioxidative Defense Responses of Two Tomato (Solanum lycopersicum L.) Cultivars after Co-Infection of Fusarium oxysporum and Meloidogyne incognita. Agronomy. 2020; 10(2):159. https://doi.org/10.3390/agronomy10020159
Chicago/Turabian StyleMaqsood, Ambreen, Haiyan Wu, Muhammad Kamran, Hussain Altaf, Adnan Mustafa, Sunny Ahmar, Nguyen Thi Thang Hong, Kinza Tariq, Qiong He, and Jen-Tsung Chen. 2020. "Variations in Growth, Physiology, and Antioxidative Defense Responses of Two Tomato (Solanum lycopersicum L.) Cultivars after Co-Infection of Fusarium oxysporum and Meloidogyne incognita" Agronomy 10, no. 2: 159. https://doi.org/10.3390/agronomy10020159
APA StyleMaqsood, A., Wu, H., Kamran, M., Altaf, H., Mustafa, A., Ahmar, S., Hong, N. T. T., Tariq, K., He, Q., & Chen, J.-T. (2020). Variations in Growth, Physiology, and Antioxidative Defense Responses of Two Tomato (Solanum lycopersicum L.) Cultivars after Co-Infection of Fusarium oxysporum and Meloidogyne incognita. Agronomy, 10(2), 159. https://doi.org/10.3390/agronomy10020159









