1. Introduction
February daphne,
Daphne mezereum L., belongs to the Thymelaeaceae family of plants. In Poland, the shrub is legally protected. It needs protection as its habitat, deciduous forests, is being replaced by conifers. Its natural stands are often vandalized by breaking off the flowering branches or digging bushes out for transfer into gardens [
1]. Apart from flowers, which appear very early in spring, ornamental features of the plant include ornamental red berries of 1 cm in diameter which ripen at the end of June. In horticulture, the shrub is mainly used in gardens, on flower beds, and in rock gardens [
2].
Propagation of February daphne is difficult. Its stem-cuttings root poorly even under optimal conditions so this method is inefficient and not commonly used [
3]. So far, no effective method of propagation of this species has been discovered. Propagation by seed is currently used in production systems but it does not always bring about the expected results, and often produces non-uniform material, due to genetic segregation. For these reasons, new propagation methods for this valuable species are being sought. One of these could be micropropagation via tissue culture [
4,
5].
The standard culture medium used for propagation of woody plants is WPM (Woody Plant Medium). In comparison to other commonly used media it, contains less nitrogen [
6]. So far, no recommendations for the optimal medium composition and growing conditions for the in vitro propagation of
Daphne mezereum have been published. However, several closely related species have been tested. Among those,
D. jasminea Sm. showed the highest regenerative potential [
5,
7]. Noshad et al. [
5] attempted the in vitro propagation of seven species from genus Daphne:
D. caucasica Pall.,
D. cneorum L.,
D. giraldii Nitsche.,
D. jasminea,
D. laureola L.,
D. retusa Hemsl, and
D. tangutica Maxim., and each was tested on five different culture media: MS [
8], WPM [
9], B5 [
10], SH [
11], and LS [
12]. For
D. tangutica,
D. laureola,
D. caucasica,
D. retusa, and
D. giraldii, the best responses were on the MS while,
D. cneorum and
D. jasminea responded better to the WPM. Those cultures were made from apical meristems as well as axillary buds [
5].
Growth regulators commonly used in in vitro plant propagation are cytokinins. They are applied during the proliferation stage to produce as many axillary buds, and hence microcuttings, as possible [
13]. Cytokinins play an important role in regulating different aspects of plant growth and development: they stimulate cell divisions, delay aging of leaves and flower development, define defensive mechanisms helping the plant to adapt to different stressors [
14]. In woody plants, they stimulate proliferation of shoots and in small concentration, and with the addition of auxin, they lead to spontaneous development of adventitious roots [
15]. Each species, and sometimes even difference variety or cultivar, reacts differently to the type and concentration of a cytokinin. Such different reactions are best observed among closely related plants, such as members of the same family or genus. The best for the proliferation of shoots in
Daphne gnidium L. seems to be BA of 5 µM [
16] and for
D. jasminea, 12.3 µM 2iP [
17].
Meta-Topolin (6-(3-hydroxylbenzylamino) purine) is coming into more common use in the in vitro reproduction of plants. It is a cytokinin originally isolated from leaves of a poplar and so it is considered a natural, aromatic cytokinin. Biochemically, it differs from isoprenoid cytokinins, such as zeatin and 2-iP, and its biological activity profile is different [
18]. It has been shown that topolins increase shoot proliferation, maintain the hormonal/histogenic stability, and improve rooting effectiveness [
19]. Using mT instead of benzyladenine (BA) usually generates plants of higher quality, such as with better coloring [
20], and reduces the risk of various types of deformations that do occurring during in vitro propagation [
13], Kőszeghi et al. [
13] who used mT in the propagation of basil (
Ocimum basilicum L.) and produced fewer deformed plants than with BA.
It is well known that tissue culture has the capacity to induce somaclonal variation [
21]. In some circumstances it may be of value as new genetic variation but in commercial propagation, it is critical that the product be identical to the original material and uniform. As somaclonal variation occurs at the DNA level, molecular tools are best suited for its detection [
22]. Among a range of such tools, random amplification of polymorphic DNA (RAPD) and intersample sequence repeat (ISSR) markers have been used in such species as
Platanus acerifolia Willd. [
23],
Morus alba L. [
24], or
Ziziphus spina-christi (L.) Willd. [
25]. To the best of our knowledge, no studies have ever been performed on
Daphne.
The study aimed to compare the effects of the commonly used BA with its new alternative mT on the regeneration efficiency of Daphne mezereum on the levels of various components important in regeneration, and to test the genetic uniformity of regenerated shoots.
2. Materials and Methods
All tests were done on February daphne (
Daphne mezereum L.). The material for the initiation of cultures was 5–8 cm fragments of young shoots that came from 3-year-old mother shrub from nursery. The initial material used in described experiments, in the stage of proliferation, was obtained from the 8–10-week-old in vitro daphne cultures growing on the MS medium (All macronutrients used for media preparation were obtained from POCH S.A. (Gliwice, Poland); while micronutrients and vitamins were obtained from Sigma-Aldrich
®.) supplemented with 2.5 mg·L
−1 BA (Sigma-Aldrich
®) and 0.1 mg·L
−1 NAA (Sigma-Aldrich
®). Only apical microshoots fragments, roughly 0.8–1 cm in length, were collected to research. Two different media were tested: MS [
8] and a modified WPM [
9] where the levels of microelements and vitamins corresponded to those of MS. Each medium was supplemented by 0.1 mg·L
−1 NAA, and either cytokinin BA or mT (Sigma-Aldrich
®), each in the concentration of 1 mg·L
−1 (based on previous studies, this appears to be the optimal cytokinin concentration for
D. mezereum). MS and WPM without growth regulators served as controls and in one control group, the NAA auxin was added. Altogether, 8 different media combinations were tested. Media were solidified with agar 8.0 g/L Bacto™ (Becton, Dickinson and Company, Sparks, MD, USA). Media pH were adjusted to 5.7 with either 1.0 M sodium hydroxide (NaOH) or 1.0 M hydrochloric acid (HCl) and 50 mL of each medium were placed in 450 mL jars, autoclaved for 20 min at 121 °C and 110 kPa. As the carbon source, 3% sucrose (Diamant, Pfeifer & Langen Polska S.A., Gostyń, Poland) was used. Ten trials for each medium combination were conducted with 6 explants per jar. Jars with explants were placed in the phytotron at 23 ± 1 °C, 16 h photoperiod, with light provided by fluorescent lamps at 35 µmol m
−2 s
−1 intensity.
2.1. Evaluation of Explants
Six weeks after placing explants on the medium, the percentages of regenerating explants, the lengths of regenerated shoots, and the numbers of shoots per explant were evaluated.
2.2. Biochemical Analysis
After six weeks on the media, samples were collected for measurements of various compounds. The materials was finely chopped, mixed, and 0.25 g samples were used for extraction. Triplicate extracts were prepared for each analysis and three measurements were done for each extract. The total chlorophyll content (chlorophyll a + b) was analyzed according to Lichtenthaler and Wellburn [
26]. Total soluble sugars were determined by the colorimetric method according to Dubois et al. [
27]. Protein levels were determined according to Bradford [
28], free amino acids by the method of Rosen [
29] and the hydrogen peroxide content was measured according to Pick and Keisari [
30]. The catalase activity was analyzed according to Góth [
31].
2.3. Statistical Analysis
All results were subjected to the analysis of variance using Statgraphics Centurion XVI (Statgraphics Technologies, Inc., The Plains, VA, USA), after the Shapiro–Wilk test. Arcsine transformation was performed for all experimental data taken in percentages before subjecting them to statistical analysis. Experimental data were subjected to two-way analysis of variance and then to Tukey’s multiple range test to separate the means at the significance level of
p ≤ 0.05 [
32].
2.4. Molecular Analyses
Eight week old microshoots from 10 randomly selected plants from each combination were collected and 100 mg of the tissue were ground in liquid nitrogen and stored at −80 °C. Genomic DNA was extracted using the GeneMATRIX™ Plant and Fungi DNA Purification Kit (EURX® Molecular Biology Products, Gdańsk, Poland).
RAPD analysis: the polymerase chain reaction (PCR) was performed in a volume of 25 μL containing 1 μL template DNA, 2.5 μL 10× Taq buffer with KCl, 200 μM of each dNTP, 1.5 mM MgCl
2, 0.8 μM primer, and 0.125 U Taq DNA Polymerase (EURX
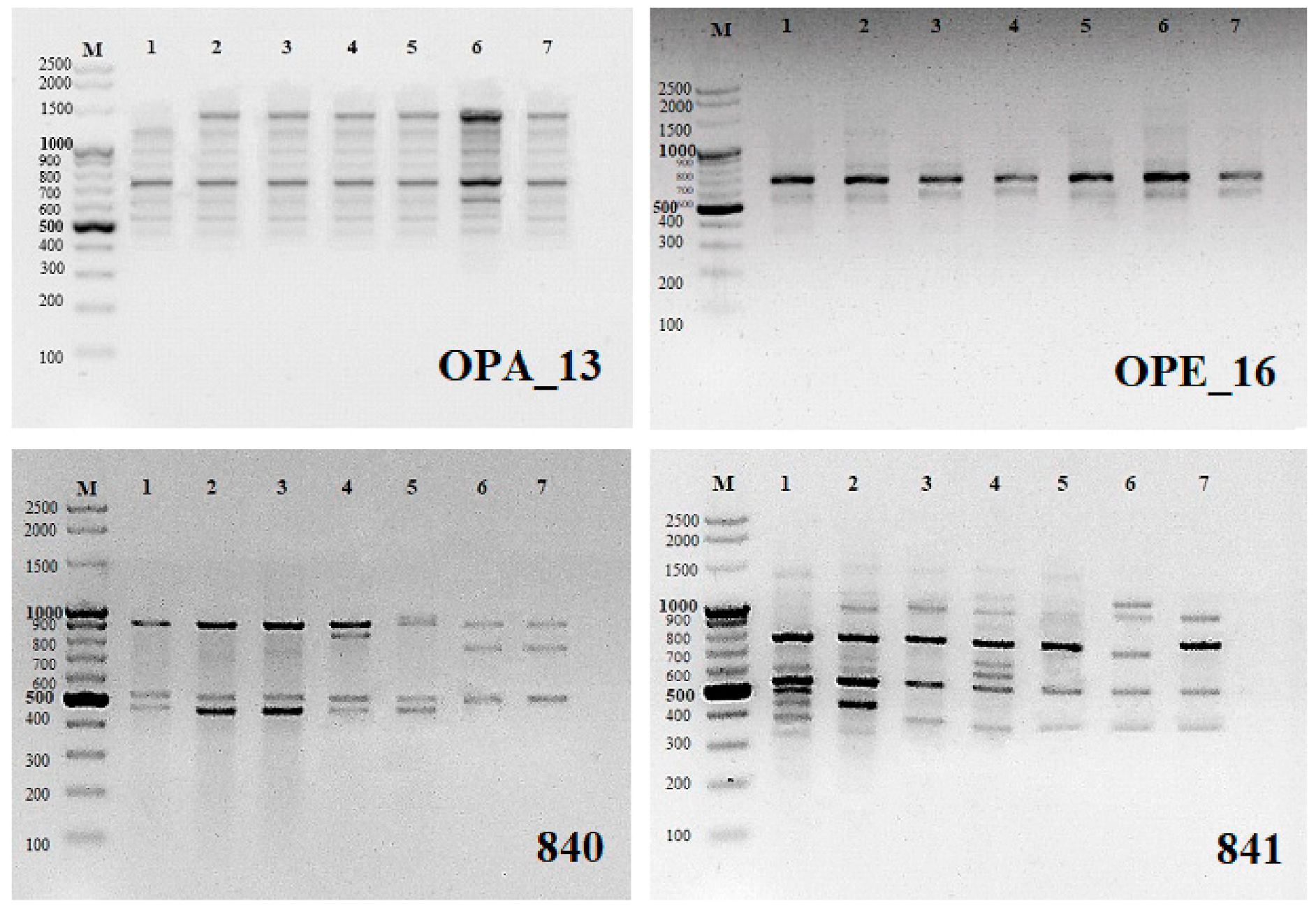
® Molecular Biology Products, Gdańsk, Poland). A total of 48 arbitrary RAPD primers (Operon Technologies, Alameda, CA, USA) were tested for PCR amplification (OPA-01 to OPA-20, OPB-01, OPC-02, OPC-06, OPC-11, OPC-16, OPC-18, OPC-19, OPD-05, OPD-10, OPD-13, OPD-15, OPD-18 to OPD-20, OPE-01, OPE-03, OPE-09, OPE-16, OPE-17, OPF-02 to OPF-06, OPF-09, OPF-10, OPF-20). From these, 10 (
Table 1) were chosen for the analysis as they produced highly readable and reproducible bands. The reaction was cycled 35 times at 94 °C for 40 s for denaturation of the template DNA, 35 °C for 1 min for primer annealing, and 72 °C for 2 min for primer extension in a thermal cycler (Mastercycler
® nexus gradient; Eppendorf, Hamburg, Germany). The final extension cycle allowed an additional incubation for 10 min at 72 °C. The samples were stored at 4 °C until electrophoresis.
ISSR analysis: 15 microsatellite primers (UBC, University of British Columbia, Vancouver, BC, Canada) were randomly selected and tested (#807, 808, 809, 810, 811, 812, 816, 818, 819, 825, 827, 836, 840, 841, 856). Twelve of them (
Table 2) produced highly readable and reproducible bands and were selected for the analysis. PCR for ISSR amplification was performed in a volume of 25 μL with the same concentrations and reaction volumes as for RAPDs. The initial denaturation was 5 min at 94 °C, followed by 35 cycles of 45 s denaturation at 94 °C, 1 min annealing at the temperature shown in
Table 2, and 2 min extension at 72 °C, with a final extension at 72 °C for 7 min. Samples were stored at 4 °C. RAPD and ISSR amplifications were performed at least three times and only the reproducible PCR products were scored.
Amplification products for all samples were resolved on 1.5% (w/v) agarose basic LE (Prona®, ABO Sp. z o.o, Gdańsk, Poland) gels in 1× TAE buffer and stained with ethidium bromide (Sigma-Aldrich®). Images were recorded using a gel documentation system (Syngen Imagine®, Syngen Imagine 1.1 program, Syngen Biotech, Wrocław, Poland). The size of each amplicon was estimated by comparing it with the GeneRuler™ 100 bp Plus DNA ladder (Thermo Scientific®, Waltham, MA, USA).
2.5. Molecular Data Analysis
All reactions were repeated two or three times. Banding profiles of the unambiguous and reproducible bands generated by RAPD and ISSR primers were scored. The data were pooled into a binary matrix based on the presence (1) or absence (0) of the selected bands. Differences in band intensity were ignored. Genetic similarity was estimated using the Jaccard’s coefficient [
33]. These were used to construct a dendrogram (for RAPD primers, ISSR primers, and also connected RAPD and ISSR) through the XLStat 2018.6 program software (Addinsoft Inc., New York, NY, USA) using unweighted pair-group method with arithmetic mean (UPGMA) method.
3. Results
Two-factor variance analysis of the percentage of regenerated explants of Daphne mezereum has shown that only the effects of the growth regulators used were statistically significant at both stages of evaluation.
Six weeks after the study began, almost 89% of shoots fragments on the medium “0” regenerated. In comparison with the control groups, the best effects were observed on the medium with mT—over 99% of regenerated explants. The type of medium used had no effect on the percentage of regenerated shoots. There were no significant differences among various combinations of the MS medium. The lowest percentage of regenerated explants, 88%, was on the medium with BA, and the highest was on the medium with mT. In WPM, the control group—medium without growth regulators—had the lowest percentage of regenerated explant—the average of almost 83%. Addition of any growth regulator to the WPM, even the auxin, increased regeneration rate, ranging 95.3% (with BA) to 100% (with mT) (
Table 3).
There were clear effects of growth regulators on proliferation, expressed as the average number of shoots per regenerated explant while the type of medium used had no significant role. The lowest number of shoots was in the control group. Supplementation with NAA or with BA (1 mg·L
−1 BA + 0.1 mg·L
−1 NAA) led to a roughly 24% increase in the number of new shoots relative to the “0” combination (
Table 4). Again, the highest regeneration rate was observed in the presence of mT—over 5.85 of shoots per explant, or ca. three times higher than in controls.
On the MS medium, the lowest number of new shoots was observed in the “0” combination—1 shoot per explant. Auxin (0.1 mg·L
−1 NAA) almost doubled that number relative to controls. Again, the best effect on the regeneration rate was on media supplemented with mT - more than four times as many shoots relative to the “0” combination. On WPM + mT, the number of shoots increased even further, with 2.6 to 3.4 more shoots than in other combinations of the same medium (
Table 4).
BA in the media inhibited shoot elongation, producing shoots with the average length of 0.68 cm (
Table 5). This combination produced the shortest shoots. Medium without any growth regulators, as well as a medium with 0.1 mg·L
−1 NAA produced significantly longer shoots. Once again, the best results were observed on media combined with mT. The MS medium produced longer shoots, by ca. 0.21 cm, to WPM.
On MS, all shoots were of similar length regardless of the growth regulator used. No combination of MS with any growth regulator improved the growth rate of shoots. On the WPM, the shortest shoots were in combination with BA (ca. 0.59 cm, 0.19 cm shorter than those with the BA). In the WPM, control shoots reached ca. 0.7 cm, significantly less than on the MS medium. The addition of mT to the WPM with auxin produced shoots almost 1 cm in length (
Table 5).
3.1. Biochemical Analysis
Both the media and growth regulators tested in the study had important effects on the levels of organic compounds present in the shoots. Relative to controls, a ca. twofold increase in the chlorophyll a + b content was observed on media supplemented with NAA and mT. However, the highest chlorophyll levels in the shoots were media with only NAA (
Table 6). Relative to the control, addition of NAA to MS resulted in a 42% increase in the chlorophyll levels. In WPM, the chlorophyll levels were over four times higher than in controls. Regardless of the medium, the addition of BA had no significant effect on the chlorophyll content. However, with WPM, but in comparison with the MS medium with auxin, there was a 23% increase. WPM with BA had lower chlorophyll levels. The effects of the mT was missed: there was an increase in the chlorophyll level on WPM, but none on the MS (
Table 6).
Among media without growth regulators, the levels of the total soluble sugars were lower on the MS medium (
Table 7) while the highest were found in medium with the addition of NAA. Supplementation with mT did not affect the amount of the total soluble sugars. These were higher in all plants on the MS plus any of the growth regulators relative to controls. MS plus auxin produced an over 40% increase in total soluble sugars, further addition of cytokinins led to a further increase. In WPM, growth regulators did not lead to increases in sugars (
Table 7).
Plants growing on the MS medium with no growth regulators contained more protein than those on WPM. Enhancing the MS medium in any way led to an increase in the protein levels. The highest levels in the plants growing on MS + mT—143% of the amount in the control group. This cytokinin significantly increased the soluble protein levels (
Table 8).
Plants growing on the MS medium contained twice as much amino acids as those growing on the WPM. The effects of cytokinins were different in different media. Relative to the NAA supplementation, BA significantly reduced the amino acid levels. All of the plants on MS + mT had higher amino acid levels than those on MS + BA (
Table 9).
Two-factor analysis of variance shows the effects of various concentrations of growth regulators and the medium type on the levels of hydrogen peroxide present in the tissues. These levels were significantly higher on the MS than on the WPM when no growth regulators were present. In both cases, addition of the auxin led to a significant increase H
2O
2 levels. High levels of H
2O
2 were also found in shoots growing on the media with the auxin and BA. The addition of mT reduced those levels (
Table 10).
Catalase activity was higher in shoots growing on the WPM than on the MS, and the effect of growth regulators depended on the medium. The auxin had only a minor effect. Further supplementation of the MS with cytokinins increased the catalase activity. On the WPM, the highest catalase activity was when BA was added—15% higher in a combination with the auxin and almost 30% higher with mT (
Table 11).
3.2. Molecular Analysis
The RAPD and ISSR markers used produced a total of 24 and 28 monomorphic products, respectively (54.5% and 31.5% of all products On average, a single RAPD marker generated 4.4 bands, ranging in length from ca. 300 to 2000 bp (
Table 1,
Figure 1). An average ISSR marker generated of 7.4 bands with lengths ranging from ca. 200 to 2000 bp (
Table 2). The RAPD profile generated with only two primers (OPD-13 and OPE-16) showed prominent monomorphic banding pattern indicating 100% genetic identity among microcuttings regenerating on media with different cytokinins. Remaining RAPD primers showed polymorphic banding in the range from 14.3% (OPA-03) to even 100% (OPC-16 and OPD-18). ISSR markers showed a greater number of polymorphic bands. Three ISSR primers gave 100% polymorphic products (UBC 811, 816 and 836) and the rest 12.5% (UBC 827) to 88.9% (UBC 841) polymorphic bands (
Table 2,
Figure 1).
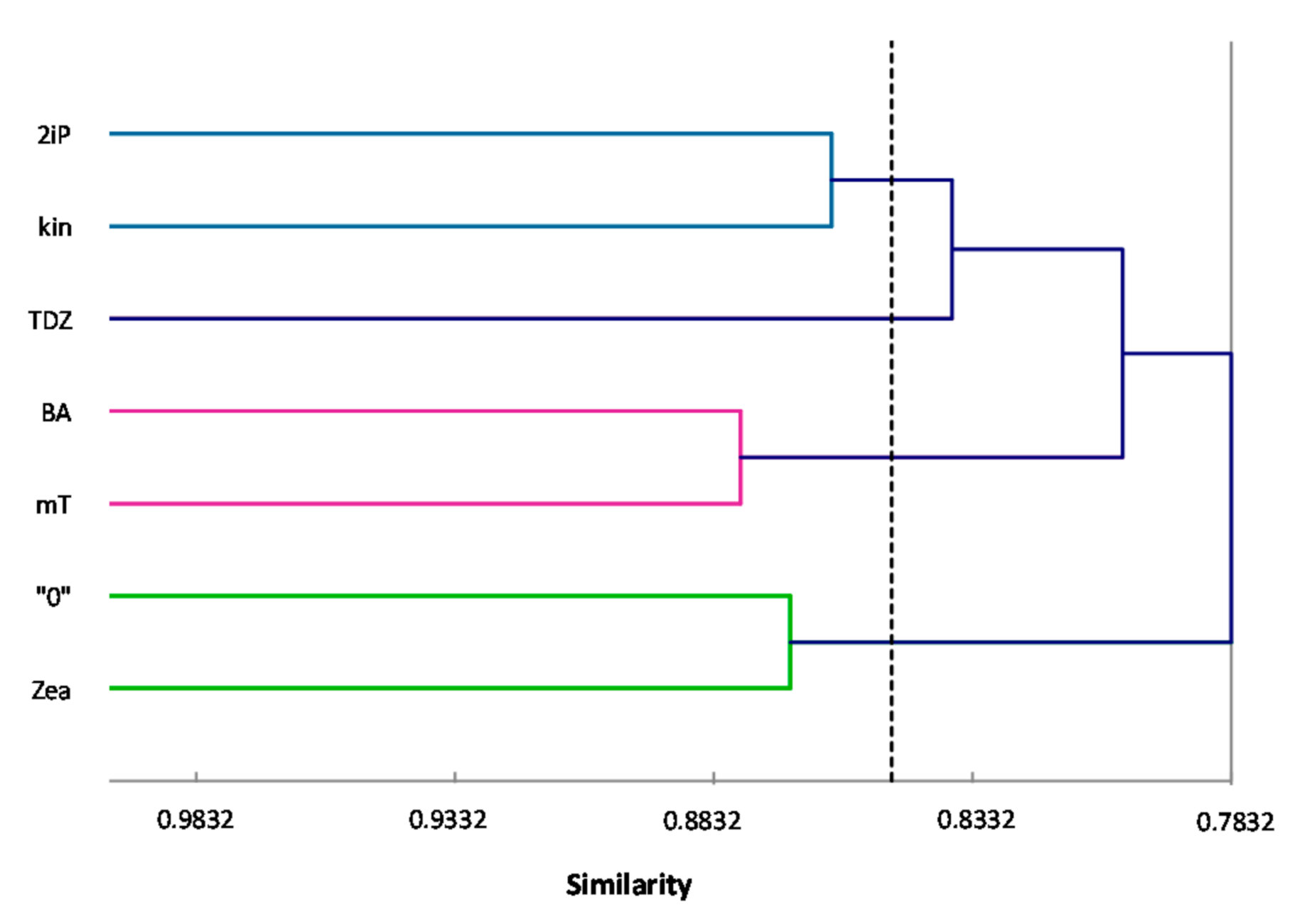
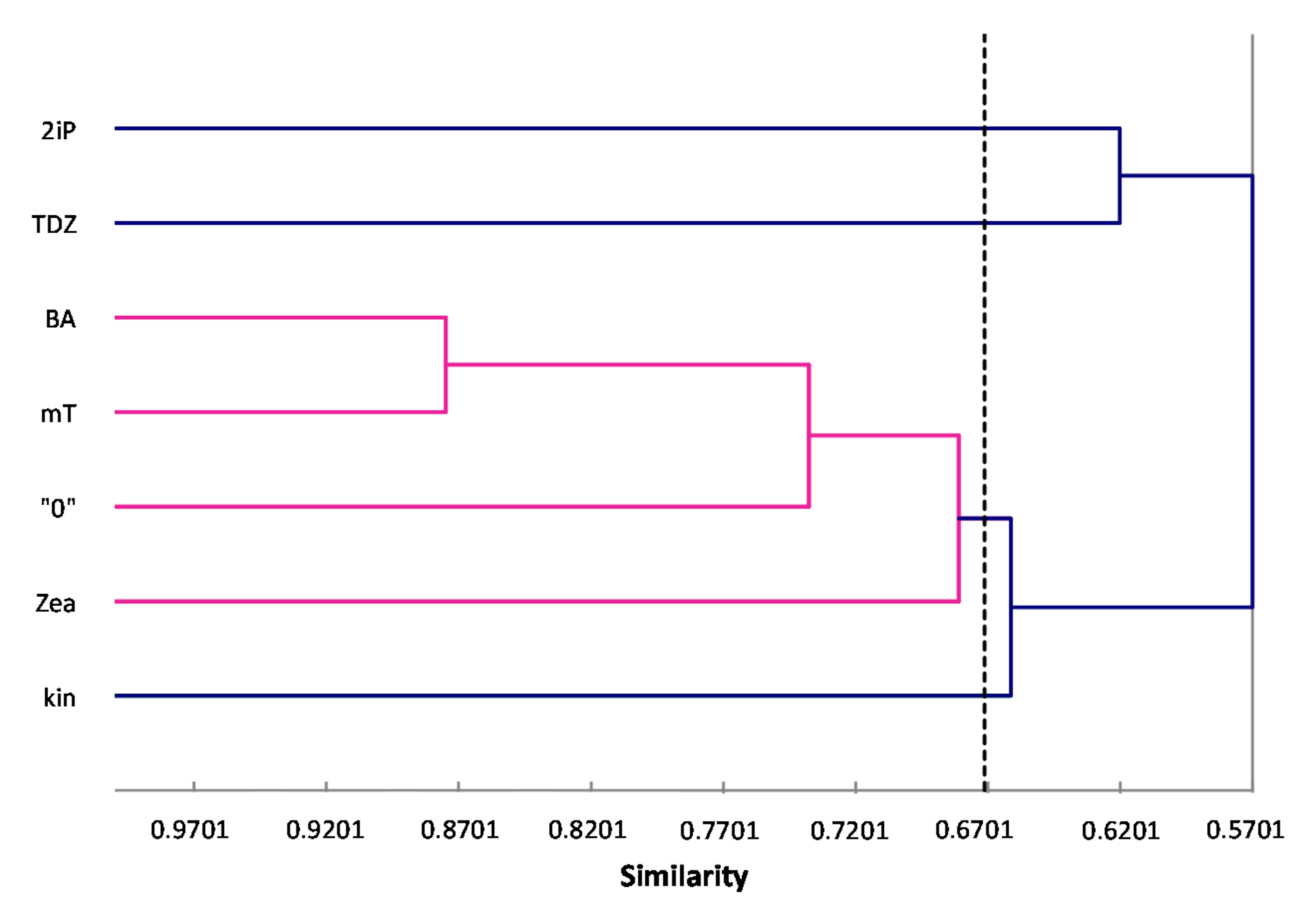
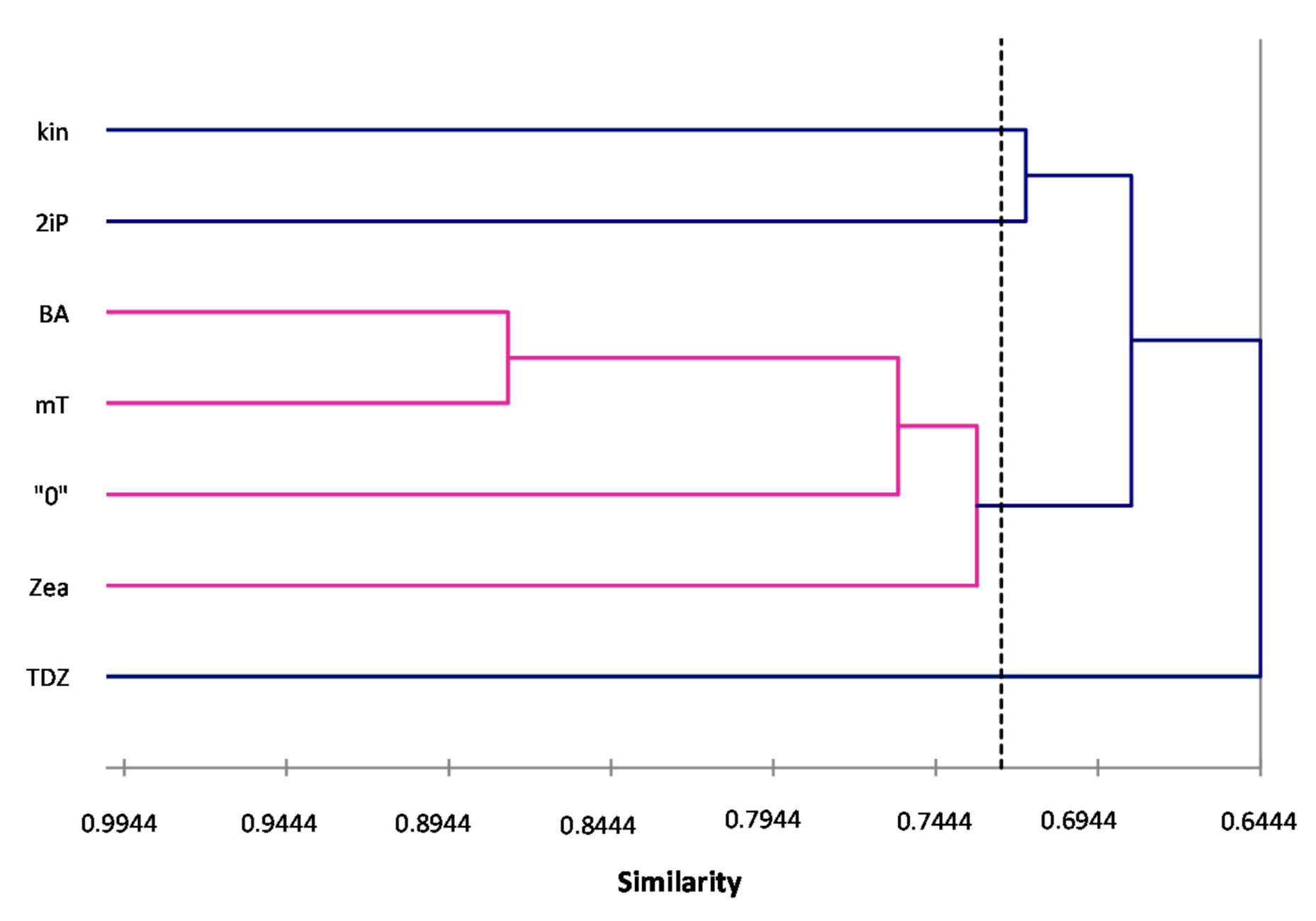
ISSR primers showed more polymorphic loci than RAPDs, which is confirmed by the mean value of similarity coefficients according to Jaccard [
33]. For RAPDs this coefficient stands at 0.8488, and for ISSR it is 0.6713. For the pooled data from both analysis, the genetic similarity between all microcuttings, was 0.724 (72%). There is also a significant difference between the lower limit of the Jaccard’s similarity coefficient value range, which for RAPDs started with 0.7832 (i.e., from 78% similarity), and ISSR only 0.5701 (57% similarity) (
Figure 2 and
Figure 3). The highest level of genetic similarity, regardless of the type of analysis (dendrogram for individual markers or pooled RAPD and ISSR), is in material from the combination of mT with BA (
Figure 1,
Figure 2,
Figure 3 and
Figure 4). Microcuttings from medium with mT or BA are always genetically closer to shoots regenerated on media without growth regulators. The most genetically distant group were shoots regenerated on media with TDZ compared to those with natural cytokinins (mT, Zea) (
Figure 2,
Figure 3 and
Figure 4).
4. Discussion
The focus of this study was on the propagation efficiency; a critical aspect of the in vitro production of plants. Two cytokinins in two different growth media formulations were tested. The two media are widely used at the propagation stage in the in vitro culture. The two cytokinins promote cell division and hence, lateral budding [
34,
35]. Of the two cytokinins, meta-Topolin (mT) in combination with an auxin (NAA) produced the best results.
Similar studies on
Corylus colurna, compared the effects of both cytokinins, and also indicated that the mT performs better in micropropagation and ensures better condition of plants. The addition of 8.2 μM mT had a positive effect—the percentage of regenerated plants was higher than of those growing with 8.2 μM BA [
36]. Kőszeghi et al. [
13] compared the effects of the mT and benzyladenine on shoot regeneration in common basil (
Olicimum basilicum). Out of the five variants, each with a different concentration of mT in the MS medium, that using 1 mg·L
−1 produced the highest number of shoots. Similar results were observed in this study—nearly 6 shoots per explant were obtained with mT. In smoke bush shoots, Podwyszyńska et al. [
37] had better results with mT than with BA but in micropropagation of
Citrus sinensis ‘Hamlin’, BA produced better results. It significantly improved the reproduction ratio, resulting, on the average, in 2 new shoots per explant. However, high concentration of BA can inhibit shoot development [
38]. Here, the percentage of explants obtained when using a medium containing benzyladenine was above 90%. In
Citrus hystric Eng et al. [
39] and
Cestrum nocturnum Rasheed [
40] addition of BA resulted in 100% regeneration rate. Here, the same ultimate level was observed for the mT.
Addition of meta-Topolin to the regeneration medium also increases the levels of basic organic compounds, such as chlorophyll, soluble proteins and free amino acids. Photosynthetic pigments and growth regulators play a key role in plant development and morphogenesis [
41]. Here, the highest chlorophyll levels were observed in WPM with 0.1 mg·L
−1 NAA. In general, the chlorophyll’s levels were higher on the WPM than on the MS. A simple comparison between the BA and meta-Topolin shows that the latter significantly increases the chlorophyll levels just as in a study by Elayaraja et al. [
42]. Podwyszyńska et al. [
37] found that smoke bush cuttings on a medium with meta-methoxy-Topolin had 60% more chlorophyll than the control group.
The levels of sugars present in microcuttings may indicate their general condition. Polysaccharides, such as starch, are broken down into monosaccharides when the concentration of the latter falls. This reaction also occurs the direction, when polysaccharides are accumulated due to high levels of monosaccharides. High sugar content reduces the photosynthesis efficiency, affects the management of reserve materials and carbohydrate storage [
43]. Here, cytokinins and auxins in the WPM reduced the total sugar levels. Different reactions were observed on the MS medium. Higher levels of sugars are beneficial because carbohydrates are an important source of energy during growth and development, they are also a direct indicator of the photosynthetic intensity [
44].
The highest levels of proteins and free amino acids were observed on media with mT. Compared to other growth regulators, mT resulted in a better regeneration of shoots. This may be related to the role of amino acids—they are the main substrate for the formation of new structures. It is also believed that their higher content, together with a high concentration of soluble proteins, indicates that the plant is in the juvenile phase [
45], and that means a higher regenerative capacity. High protein levels may also suggest that plants are subjected to stress, while the high levels of free amino acids suggest the plants have a higher tolerance to stress factors [
46,
47]. Numerous studies confirm that CKs induce the activity of antioxidant enzymes. Hönig et al. [
48] showed that plants with higher levels of cytokinins (CKs) showed increased activity of antioxidant enzymes, as well as increased levels of chlorophyll and proteins.
Hydrogen peroxide (H
2O
2) is one of the most common and most active forms of oxygen. It indirectly effects cell damage, lipid breakdown and protein degradation in plants [
49], has a strong bactericidal and fungicidal effect and can be accumulated in plant vessels or stomata [
50]. H
2O
2 is an important signal molecule capable of traveling within the cell and between plant tissues. There are many links between the levels of hydrogen peroxide and the activity of growth regulators, and these links affect stomata [
51,
52]. Hydrogen peroxide in plant tissues is often associated with the response to stress, mainly to abiotic stress factors such as water shortage, rapid loss of turgor, inadequate temperature, etc. [
53]. In this study the highest levels of hydrogen peroxide were observed on media (MS or WPM) with 1 mg·L
−1 BA + 0.1 mg·L
−1 NAA. The same effect was observed in
Sesamum indicum L. where shoots obtained on a medium with BA showed significantly higher H
2O
2 content than those with mT [
42]. On the other hand, Pacholczak et al. [
54] observed that hydrogen peroxide levels dropped in ‘Red Baron’ cuttings treated with an aqueous solution of the IBA auxin. It is well known that H
2O
2 directly reduces plant growth and, similarly to stress factors (e.g., increased levels of heavy metals in the medium), inhibits the activity of antioxidant enzymes [
55]. These reports explain why, relative to mT, supplementation with BA weakened the growth rate of microcuttings and produced fewer shoots.
It is now known that Meta-Topolin has a more positive effect on the overall condition of plants. It mediates stress associated with micropropagation and culture, delays aging, increases the levels of photosynthetic pigments, and affects the activity of antioxidant enzymes [
56,
57]. The same is true in this study. We confirm these assumptions as the lowest levels of hydrogen peroxide were observed in the microcuttings regenerating on the control MS medium and WPM with the addition of auxin and 1 mg·L
−1 cytokinin mT. Meta-Topolin probably efficiently removes and detoxifies stress-related compounds by the antioxidant mechanism of the plant, of which the oxidoreductase group enzymes are an important element, e.g., the catalase analyzed in this paper. An increased activity of these enzymes is a result of stress factors, e.g., mechanical stimuli, such as cutting off a fragment from the plant. Catalase is responsible mainly for the decomposition of the hydrogen peroxide molecules into water and oxygen [
58,
59], so it would appear that the higher the H
2O
2 level, the higher the catalase activity should be to neutralize them. On the other hand, however, too high levels of hydrogen peroxide are likely to inhibit the catalase activity [
55]. The lowest average catalase activity was observed here shoots on the “0” combination (medium with no growth regulators) or that with auxin (0.1 mg·L
−1 NAA). Generally, the higher the levels of hydrogen peroxide and the greater catalase activity were observed on media combined with BA. To control intracellular levels of ROS and to avoid oxidative damage, plants increase the level of elements of the antioxidative defense system [
60,
61,
62], which shows that due to the greater activity of catalase, WPM medium is better for the general condition of daphe’s microcuttings.
RAPD and ISSR markers are a tool to analyze genetic variation occurring during micropropagation. For daphne, there are no studies on the genetic stability of conventionally or in vitro propagated plants. Here, RAPD and ISSR markers were randomly chosen as no genomic information is available for the species. Some of these markers worked well and can now be used for further research on this valuable plant. Regardless of the system/approach used (RAPD, ISSR or pooled RAPD-ISSR), microcuttings from media with mT or BA always grouped together indicating that the two compound used did not differ in their ability/tendency to generate random DNA changes. Similar results have been shown by Bairu et al. [
63] who showed BA, mT and mTR had no significant effect on genetic variation generated during micropropagation of ‘Williams’ banana. The same was reported by Elayaraja et al. [
42] also obtained similar results using the RAPD and SCoT primers (BA or mT showed similar banding pattern) during micropropagation of sesame.
While cytokinins are essential during shoot proliferation of woody plants, their high concentrations or prolonged exposure can lead to stress conditions that limit micropropagation. Mutation triggers in tissue culture had been attributed to various stress factors [
64,
65]. In this study, the level of DNA polymorphism detected was relatively high but it may reflect the genetic nature of the starting material. As mentioned earlier, daphne bushes are now generated mostly from seed [
3,
4], and the seed most likely is a consequence of cross pollination. In cross pollinating species the level of heterozygosity is high, and there may not be many identical individuals. This explanation of the levels of DNA polymorphism detected in this study appear plausible, given that in the reproduction of blueberry cultivars, normally reproduced only by cloning, hence genetically identical, no genetic variation was discovered when RAPDs were used to screen for possible somaclonal variation [
22].