Evaluation of Different Crop Models for Simulating Rice Development and Yield in the U.S. Mississippi Delta
Abstract
1. Introduction
2. Materials and Methods
2.1. Observed Data
2.2. CERES-Rice Model
2.2.1. Development
2.2.2. Biomass Growth
2.3. ORYZA Model
2.3.1. Development
2.3.2. Biomass Growth
2.4. Model Input Data and Calibration
2.5. Model Simulations and Data Analysis
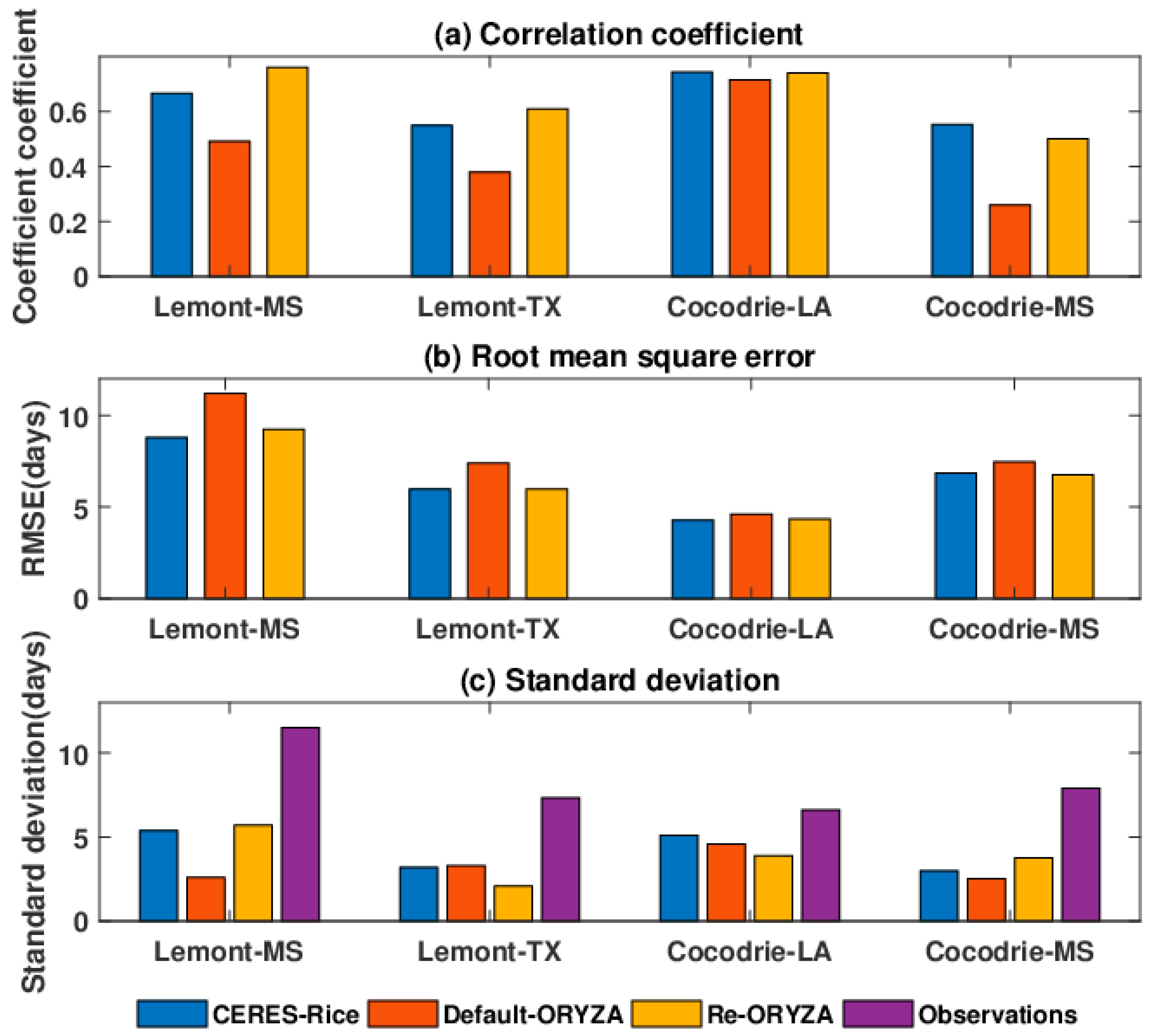
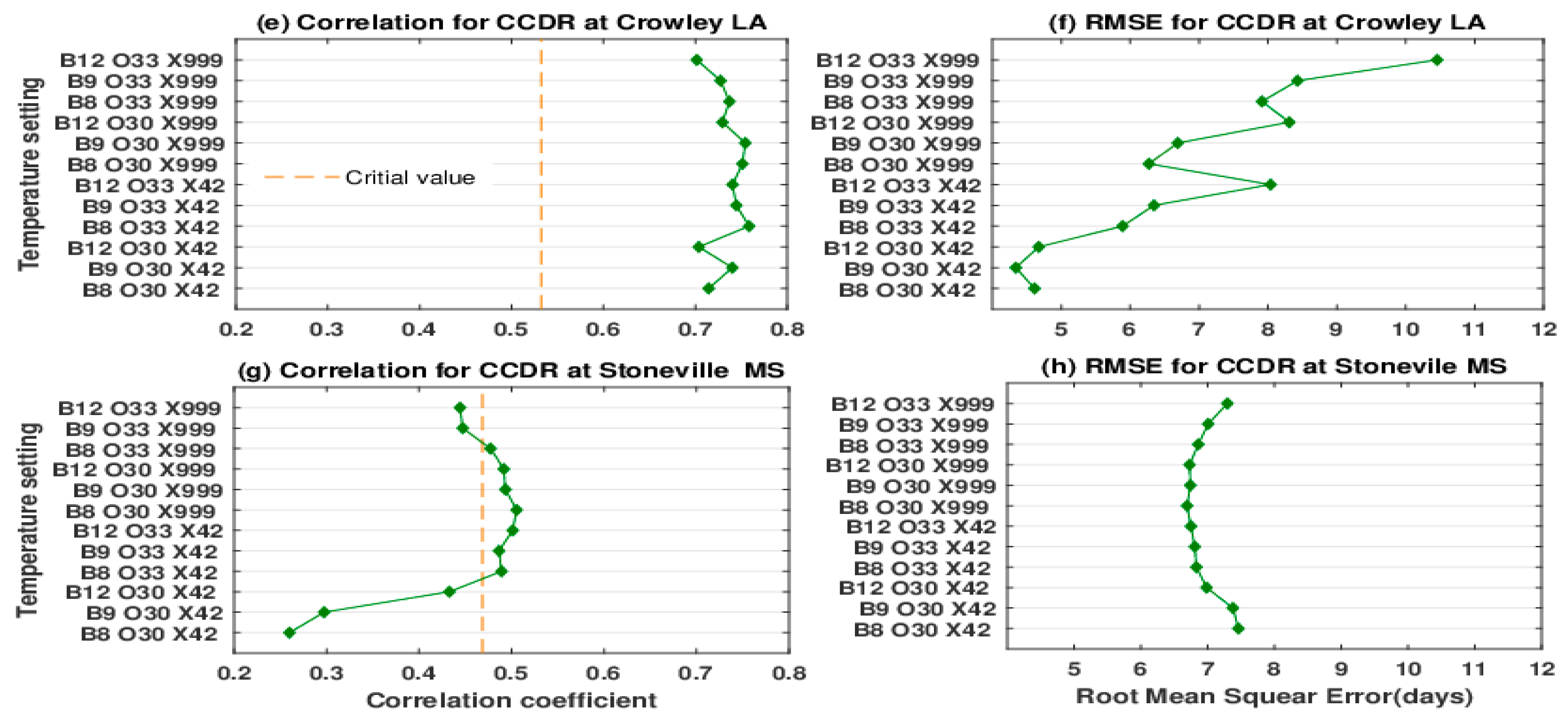
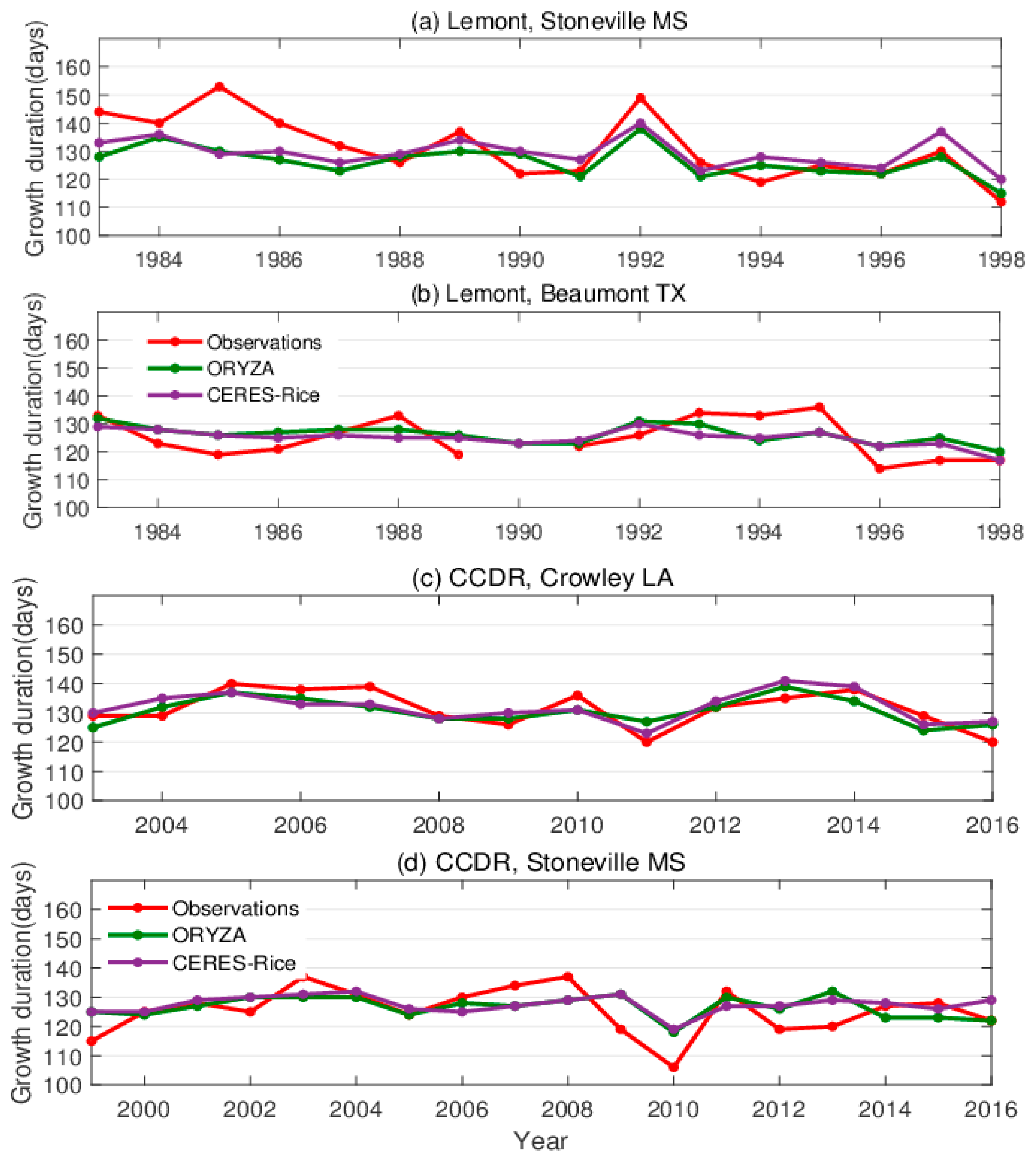
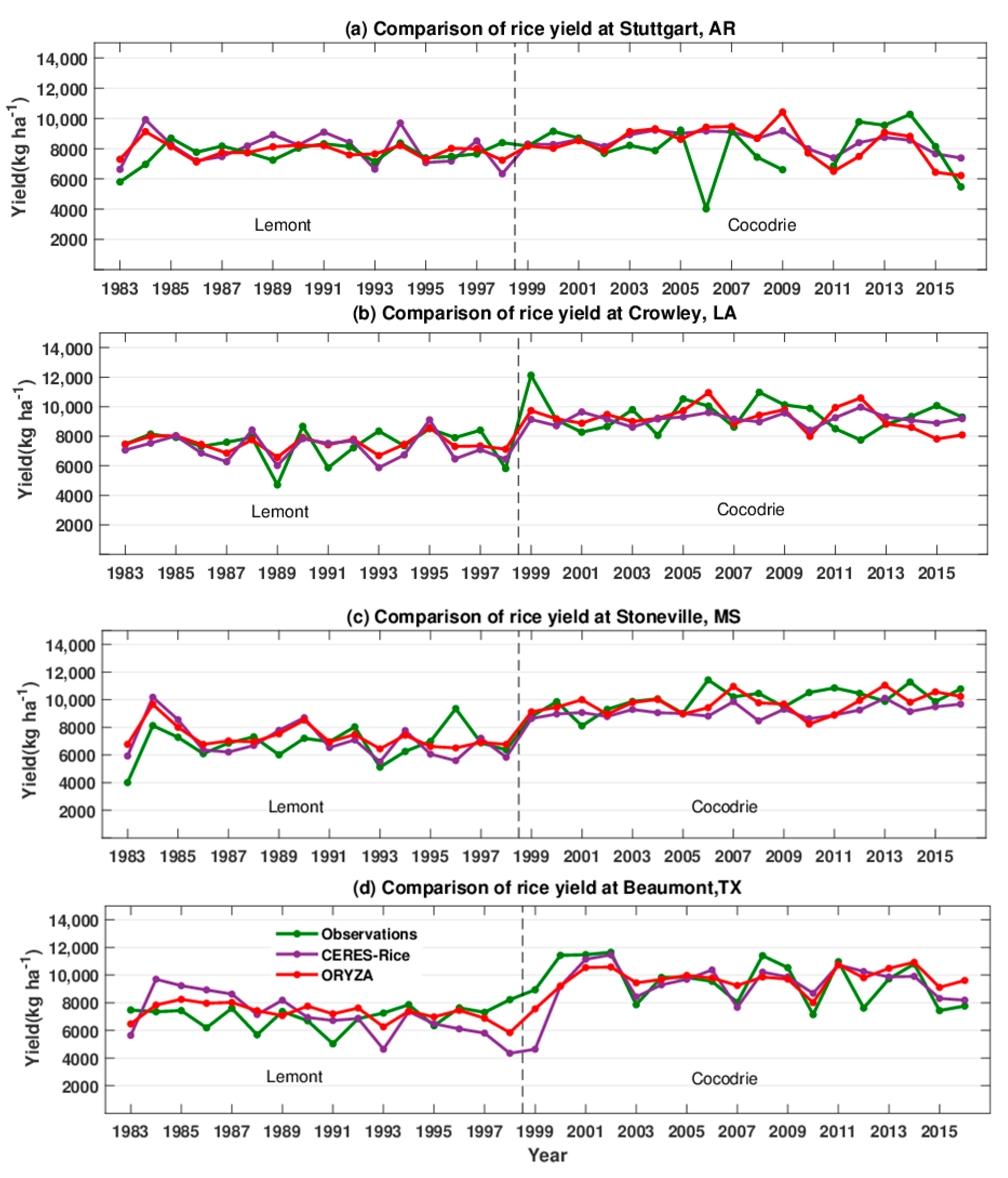
3. Results
3.1. Comparison of Growth Duration
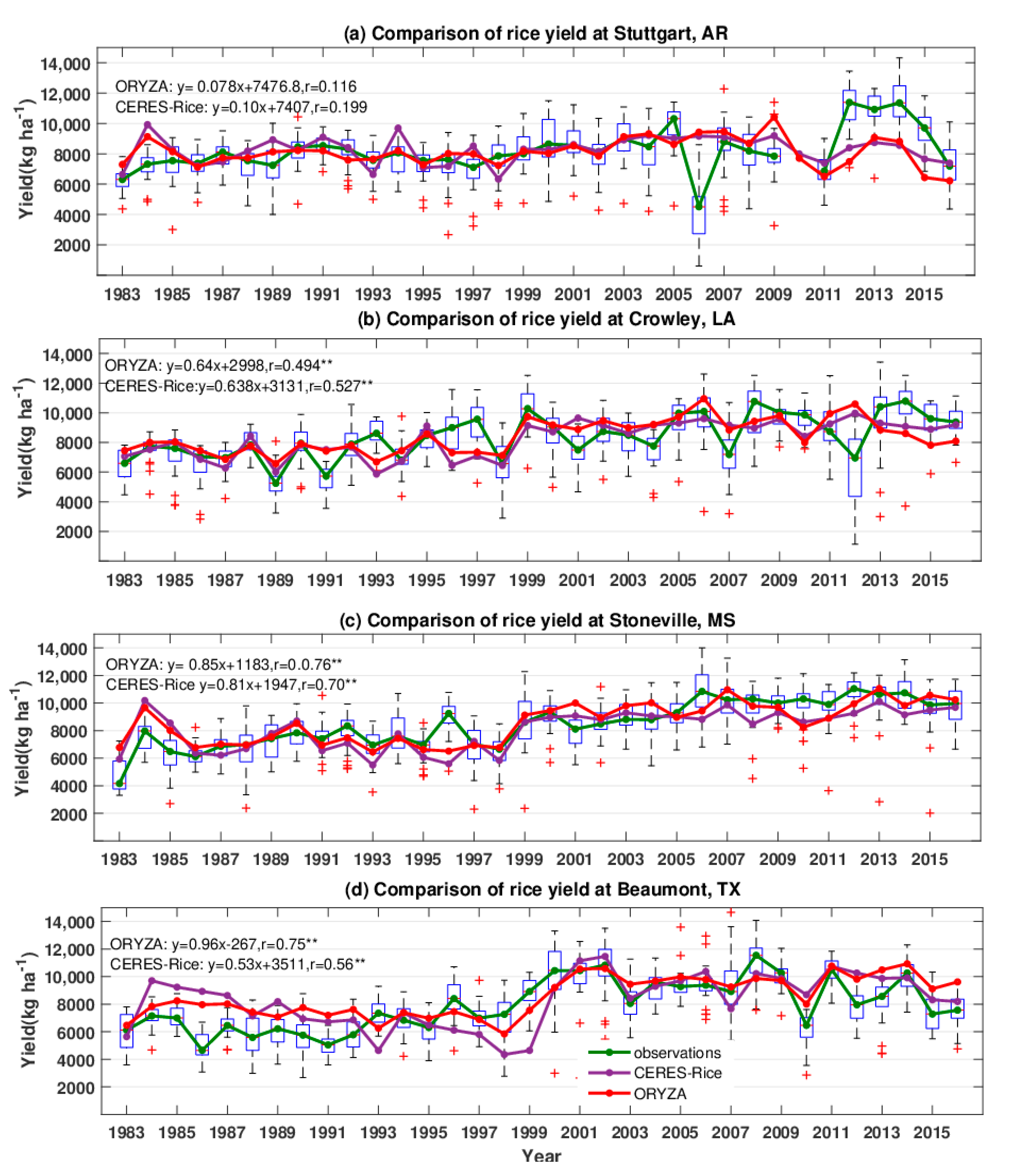
3.2. Comparison of Observed and Simulated Yields
3.3. Model Skill in Simulating 50% Percentile Yield of 101 Varieties
4. Discussion
4.1. Simulated Rice Responses
4.2. Model Knowledge Gaps
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Disclosure Statement
References
- Sandhu, N.; Kumar, A. Bridging the rice yield gaps under drought: QTLs, genes, and their use in breeding programs. Agronomy 2017, 7, 27. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Foley, A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Solut. Cultiv. Planet Nat. 2011, 478, 337–342. [Google Scholar]
- Reba, M.L.; Daniels, M.; Chen, Y.; Sharpley, A.; Bouldin, J.; Teague, T.G.; Daniel, P.; Henry, C.G. A statewide network for monitoring agricultural water quality and water quantity in Arkansas. J. Soil Water Conserv. 2013, 68, 45A–49A. [Google Scholar] [CrossRef]
- McClung, A.M.; Rohila, J.S.; Henry, C.G.; Lorence, A. Response of U.S. rice cultivars grown under non-flooded irrigation management. Agronomy 2020, 10, 55. [Google Scholar]
- Baker, J.T. Yield responses of southern US rice cultivars to CO2 and temperature. Agric. For. Meteorol. 2004, 122, 129–137. [Google Scholar] [CrossRef]
- Morita, S.; Wada, H.; Matsue, Y. Countermeasures for heat damage in rice grain quality under climate change. Plant. Prod. Sci. 2016, 19, 1–11. [Google Scholar]
- Shi, W.; Yin, X.; Struik, P.C.; Solis, C.; Xie, F.; Schmidt, R.C.; Huang, M.; Zou, Y.; Ye, C.; Jagadish, S.V.K. High day- and night-time temperatures affect grain growth dynamics in contrasting rice genotypes. J. Exp. Bot. 2017, 68, 5233–5245. [Google Scholar] [CrossRef]
- Food & Agriculture Organization (FAO). FAO Trade Yearbook; FAO: Rome, Italy, 2010. [Google Scholar]
- FAOSTAT. Production Quantities by Country. Available online: http://faostat3.fao.org/ (accessed on 1 March 2016).
- Cogato, A.; Meggio, F.; De Antoni Migliorati, M.; Marinello, F. Extreme Weather Events in Agriculture: A Systematic Review. Sustainability 2019, 11, 2547. [Google Scholar]
- Panagopoulos, Y.; Gassman, P.W.; Arritt, R.W.; Herzmann, D.E.; Campbell, T.D.; Jha, M.K.; Kling, C.L.; Srinivasan, R.; White, M.; Arnold, J.G. Surface water quality and cropping systems sustainability under a changing climate in the Upper Mississippi River Basin. J. Soil Water Conserv. 2014, 69, 483–494. [Google Scholar]
- Panagopoulos, Y.; Gassman, P.W.; Arritt, R.W.; Herzmann, D.E.; Campbell, T.D.; Valcu, A.; Jha, M.K.; Kling, C.L.; Srinivasan, R.; White, M.; et al. Impacts of climate change on hydrology, water quality and crop productivity in the Ohio-Tennessee River Basin. Int. J. Agric. Biol. Eng. 2015, 8, 36–53. [Google Scholar]
- Jones, J.W.; Antle, J.M.; Basso, B.; Boote, K.J.; Conant, R.T.; Foster, I.; Godfray, H.C.J.; Herrero, M.; Howitt, R.E.; Janssen, S.; et al. Brief history of agricultural systems modeling. Agric. Syst. 2017, 155, 240–254. [Google Scholar] [CrossRef]
- Loomis, R.S.; Ragginage, R.; Ng, E. Explanatory models in crop physiology. Ann. Rev. Plant. Physiol. 1979, 30, 339–367. [Google Scholar] [CrossRef]
- Gupta, R.; Mishra, A. Climate change induced impact and uncertainty of rice yield of agro-ecological zones of India. Agric. Syst. 2019, 173, 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Niu, Z.; Zhang, M.; Li, C.; Li, J. The effects of projected climate change and extreme climate on maize and rice in the Yangtze River Basin, China. Agric. For. Meteorol. 2020, 282–283, 107867. [Google Scholar] [CrossRef]
- Boonwichai, S.; Shrestha, S.; Babel, M.S.; Weesakul, S.; Datta, A. Climate change impacts on irrigation water requirement, crop water productivity and rice yield in the Songkhram River Basin, Thailand. J. Clean. Prod. 2018, 198, 1157–1164. [Google Scholar] [CrossRef]
- Ziska, L.H.; Fleisher, D.H.; Linscombe, S. Ratooning as an adaptive management tool for climatic change in rice systems along a north-south transect in the southern Mississippi valley. Agric. For. Meteorol. 2018, 263, 409–416. [Google Scholar] [CrossRef]
- Espe, M.B.; Yang, H.; Cassman, K.G.; Guilpart, N.; Sharifi, H.; Linquist, B.A. Estimating yield potential in temperate high-yielding, direct-seeded US rice production systems. Field Crops Res. 2016, 193, 123–132. [Google Scholar] [CrossRef]
- Li, T.; Hasegawa, T.; Yin, X.; Zhu, Y.; Boote, K.; Adam, M.; Bregaglio, S.; Buis, S.; Confalonieri, R.; Fumoto, T.; et al. Uncertainties in predicting rice yield by current crop models under a wide range of climatic conditions. Glob. Chang. Biol. 2015, 21, 1328–1341. [Google Scholar] [CrossRef]
- Fleisher, D.H.; Condori, B.; Quiroz, R.; Alva, A.; Asseng, S.; Barreda, C.; Bindi, M.; Boote, K.J.; Ferrise, R.; Franke, A.C.; et al. A potato model inter-comparison across varying climates and productivity levels. Glob. Chang. Biol. 2017, 23, 1258–1281. [Google Scholar] [CrossRef]
- Boote, K.J.; Jones, J.W.; White, J.W.; Asseng, S.; Lizaso, J.I. Putting mechanisms into crop production models. Plant. Cell Environ. 2013, 36, 1658–1672. [Google Scholar] [CrossRef]
- Kanter, D.G.; Theodore, C.M.; Joe, E.S. Mississippi Rice Variety Trials; Mississippi Agricultural and Forestry Experiment Station: Bost North, MS, USA, 1998–2016. [Google Scholar]
- Bouman, B.A.M.; Kropff, M.J.; Tuong, T.P.; Wopereis, M.C.S.; ten Berge, H.F.M.; van Laar, H.H. ORYZA2000: Modeling Lowland Rice; IRRI: Los Banos, Philippines, 2001. [Google Scholar]
- Li, T.; Angeles, O.; Marcaida, M.; Manalo, E.; Manalili, M.P.; Radanielson, A.; Mohanty, S. From ORYZA2000 to ORYZA(v3): An improved simulation model for rice in drought and nitrogen deficient environments. Agric. For. Meteorol. 2017, 237, 246–256. [Google Scholar] [CrossRef]
- Jones, J.; Hoogenboom, G.; Porter, C.; Boote, K.; Batchelor, W.; Hunt, L.; Wilkens, P.; Singh, U.; Gijsman, A.; Ritchie, J. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, A.; Tojo Soler, C.M.; Ali, H.; Zia-Ul-Haq, M.; Anothai, J.; Hussain, A.; Hoogenboom, G.; Hasanuzzaman, M. Application of the CSM-CERES-Rice model for evaluation of plant density and nitrogen management of fine transplanted rice for an irrigated semiarid environment. Precis. Agric. 2012, 13, 200–218. [Google Scholar] [CrossRef]
- Asadi, M.E.; Clement, R.S. Evaluation of CERES-Maize of DSSAT model to simulate nitrate leaching, yield and soil moisture content under tropical conditions. J. Food Agri. Eng. Res. 2003, 1, 270–276. [Google Scholar]
- Timsina, J.; Humphreys, E. Performance of CERES- Rice and CERES-Wheat models in rice-wheat systems: A review. Agric. Syst. 2006, 90, 5–31. [Google Scholar] [CrossRef]
- Timsina, J.; Humphreys, E. Performance and Application of CERES and SWAGMAN Destiny Models for Rice Wheat Cropping Systems of Asia and Australia—A Review; Technical Report 16/03; CSIRO Land and Water: Griffith, NSW, Australia, 2003. [Google Scholar]
- Kerdsuk, V. Application of Crop. Modeling and GIS for Agroclimatic of KDML105 in Tung Samrit; Suranaree University of Technology: Nakhon Rachasima, Thailand, 2002. [Google Scholar]
- Cheyglinted, S.; Ranamukhaarachchi, S.L.; Singh, G. Assessment of the CERES-Rice model for rice production in the Central Plain of Thailand. J. Agric. Sci. 2001, 137, 289–298. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; van Laar, H.H. Description and evaluation of the rice growth model ORYZA2000 under nitrogen-limited conditions. Agric. Syst. 2006, 87, 249–273. [Google Scholar] [CrossRef]
- Amiri, E.; Rezaee, M.E. Valuation of water-nitrogen schemes for rice in Iran, using ORYZA2000 model. Commun. Soil Sci. Plant. Anal. 2013, 41, 2459–2477. [Google Scholar] [CrossRef]
- Jing, Q.; Bouman, B.A.M.; Hengsdijk, H.; Keulen, H.; Cao, W. Exploring options to combine high yields with high nitrogen use efficiencies in irrigated rice in China. Eur. J. Agron. 2007, 26, 166–177. [Google Scholar] [CrossRef]
- Feng, L.; Bouman, B.A.M.; Tuong, T.P.; Cabangon, R.J.; Li, Y.; Lu, G.; Feng, Y. Exploring options to grow rice using less water in northern China using a modelling approach. I: Field experiments and model evaluation. Agric. Water Manag. 2007, 88, 1–13. [Google Scholar] [CrossRef]
- Aggarwal, P.K.; Mall, R.K. Climate change and rice yields in diverse agroenvironments of India. ii. effects of uncertainties in scenarios and crop models on impact assessment. Clim. Chang. 2002, 52, 331–343. [Google Scholar]
- Sudhir-Yadav; Li, T.; Humphreys, E.; Gill, G.; Kukal, S.S. Evaluation and application of ORYZA2000 for irrigation scheduling of puddled transplanted rice in northwest India. Field Crops Res. 2011, 122, 104–117. [Google Scholar]
- Horie, T.; Nakagawa, M.; Ohnishi, M.; Nakno, J. Rice Production in Japan under 8 Current and Future Climates. In Modeling the Impact of Climate Change on Rice Production in Asia; Matthews, R.B., Kropff, M.J., Bachelet, D., Laar, H.H., Eds.; IRRI/CAB International: Wallingford, UK, 1995; pp. 143–164. [Google Scholar]
- Meinke, H. Agricultural impacts: Europe’s diminishing bread basket. Nat. Clim. Chang. 2014, 3, 541–542. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, J.; Yang, X. Non-stationary thermal time accumulation reduces the predictability of climate change effects on agriculture. Agric. For. Meteorol. 2008, 148, 1412–1418. [Google Scholar] [CrossRef]
- Kropff, M.J.; Van Laar, H.H.; Matthews, R.B. ORYZA1: An Ecophysiological Model for Irrigated Rice Production (SARP Research Proceedings); International Rice Research Institute: Manila, Philippines, 1994; p. 110. [Google Scholar]
- Ten Berge, H.F.M.; Kropff, M.J. Founding a systems research network for rice. In Eco-Regional Approaches for Sustainable Land Use and Food Production; Bouma, J., Kuyvenhoven, A., Bouman, B.A.M., Luyten, J.C., Zandstra, H.G., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 263–282. [Google Scholar]
- Van Oort, P.A.J.; Zhang, T.; de Vries, M.E.; Heinemann, A.B.; Meinke, H. Correlation between temperature and phenology prediction error in rice (Oryza sativa L.). Agric. For. Meteorol. 2011, 151, 1545–1555. [Google Scholar] [CrossRef]
- Tsvetsinskaya, E.A.; Mearns, L.O.; Mavromatis, T.; Gao, W.; McDaniel, L.; Downton, M.W. The effect of spatial scale of climatic change scenarios on simulated maize, winter wheat, and rice production in the southeastern United States. Clim. Chang. 2003, 60, 37–71. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. 2006: World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- FAO-UNESCO. FAO-UNESCO Soil Map of the World; UNESCO: Paris, France, 1974; Volume 1. [Google Scholar]
- Pachepsky, Y.; Park, Y. Saturated hydraulic conductivity of US soils grouped according to textural class and bulk density. Soil Sci. Soc. Am. J. 2015, 79, 1094–1100. [Google Scholar] [CrossRef]
- Ritchie, J.T.; Singh, U.; Godwin, D.; Bowen, W.T. Cereal Growth Development and Yield. In Understanding Options for Agricultural Production; Tsuji, G.Y., Hoogenboom, G., Thornton, P.K., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 79–97. [Google Scholar]
- Daniel, W.; Sarath, P.N.; Asha, S.K.; Weerakoon, W.M.W.; Peter, J.T.; Kenneth, J.B.; James, W.J. Accounting for both parameter and model structure uncertainty in crop model predictions of phenology: A case study on rice. Eur. J. Agron. 2017, 88, 53–62. [Google Scholar]
- Gao, L.; Jin, Z.; Huang, Y.; Zhang, L. Rice clock model—A computer model to simulate rice development. Agric. For. Meteorol. 1992, 60, 1–16. [Google Scholar] [CrossRef]
- Spitters, C.J.T.; Toussaint, H.A.J.M.; Goudriaan, J. Separating the diffuse and direct component of global radiation and its implications for modeling canopy photosynthesis. I. Components of incoming radiation. Agric. For. Meteorol. 1986, 38, 217–229. [Google Scholar] [CrossRef]
- Goudriaan, J.; van Laar, H.H. Simulation of Crop Growth Processes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; p. 238. [Google Scholar]
- Goudriaan, J. A simple and fast numerical method for the computation of daily totals of canopy photosynthesis. Agric. For. Meteorol. 1986, 43, 251–255. [Google Scholar]
- Batjes, N.H.A. World dataset of derived soil properties by FAO-UNESCO soil unit for global modelling. Soil Use Manag. 1997, 13, 9–16. [Google Scholar] [CrossRef]
- Sacks, W.J.; Deryng, D.; Foley, J.; Ramankutty, N. Crop planting dates: An analysis of global patterns. Glob. Ecol. Biogeogr. 2010, 19, 607–620. [Google Scholar] [CrossRef]
- Karen, M.; Paul, C.; Jarrod, H. Chapter 2 Rice Growth and Development. In Arkansas Rice Production Handbook-MP192; Division of Agriculture Cooperative Extension Service, University of Arkansas: Little Rock, AR, USA, 2018. [Google Scholar]
- Wilson, L.T.; Yang, Y.; Wang, J.; Arthur, F.H. Web-Based Information Delivery. In 2012 Texas Rice Production Guidelines; Way, M.O., Ed.; Texas AgriLife Research and Extension Center: Beaumon, TX, USA, 2012; pp. 64–73. [Google Scholar]
- Linscombe, S.D.; Sha, X.Y.; Bearb, K.F.; Conner, C.A.; Howard, A.M.; Theunissen, B.W.; Henry, B.J.; Hoffpauir, H.L. Rice Breeding. In 94th–108th Annual Research Report; Rice Research Station: Crowley, LA, USA, 2002–2016. [Google Scholar]
- University of Arkansas Cooperative Extension Service (CES). Arkansas Rice Performance Trials; University of Arkansas Division of Agriculture: Marion, AR, USA; U.S. Department of Agriculture and County Governments Cooperating: Little Rock, AR, USA, 2007–2015.
- Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Shelia, V.; Wilkens, P.W.; Singh, U.; White, J.W.; Asseng, S.; Lizaso, J.; Moreno, L.P.; et al. The DSSAT crop modeling ecosystem. In Advances in Crop Modeling for Sustainable Agriculture; Boote, K.J., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 173–216. [Google Scholar]
- Willmott, C.J. Some comments on the evaluation of model performance. Bull. Am. Meteor. 1982, 63, 1309–1313. [Google Scholar] [CrossRef]
- Boza, E.J.; Correll, J.C.; Lee, F.N.; Moldenhauer, K.A.K.; Gibbons, J.W. Screening of the Rice Breeder Germplasm (URRN’s, ARTP’s, and PRELIMS) to Seven Races of the Rice Blast Pathogen, Pyricularia grisea. In Rice Research Studies 2006; Norman, R.J., Meullenet, J.F., Moldenhauer, K.A.K., Eds.; Arkansas Agricultural Experiment Station, Division of Agriculture, University of Arkansas System: Fayetteville, AR, USA, 2006; Series 550. [Google Scholar]
- Heidmann, T.; Tofteng, C.; Abrahamsen, P.; Plauborg, F.; Hansen, S.; Battilani, A.; Coutinho, J.; Doležal, F.; Mazurczyk, W.; Ruiz, J.D.R.; et al. Calibration procedure for a potato crop growth model using information from across Europe. Ecol. Model. 2008, 211, 209–223. [Google Scholar] [CrossRef]
- Board, J.E.; Peterson, M.L.; Ng, E. Floret sterility in rice in a cool environment. Argon. J. 1980, 72, 483–487. [Google Scholar] [CrossRef]
- Board, J.E.; Peterson, M.L. Rice sterility varies with variety and area. Calif. Agric. 1982, January–February, 6–7. [Google Scholar]
- Uchijima, T. Some aspects of relation between low air temperature and sterile spikelets in rice plants. J. Agric. Meteorol. 1976, 31, 199–202. [Google Scholar] [CrossRef]
- Timsina, J.; Singh, U.; Godwin, D.; Humphreys, E. Modelling Chilling Injury in Rice Using CSM-Ceres-Rice; CRC for Sustainable Rice Production: Canberra, Australia, 2004. [Google Scholar]
- Farrell, T.C.; Fox, K.M.; Williams, R.L.; Fukai, S. Genotypic variation for cold tolerance during reproductive development in rice: Screening with cold air and cold water. Field Crops Res. 2006, 98, 178–194. [Google Scholar] [CrossRef]
- Yang, P.; Chen, C.L.; Zou, G.X.; Peng, Z.Q.; Wu, Y.S.; Huang, Y.P.; Xiong, Y.H.; Yin, J.H. Research progress in relevant theories of increasing breeding level of direct-seeding rice. Acta Agric. Jiangxi 2015, 27, 33–35. [Google Scholar]
- Satake, T.; Yoshida, S. High temperature-induced sterility in Indica rice at flowering. Jpn. J. Crop. Sci. 1978, 47, 6–17. [Google Scholar] [CrossRef]
- Matsui, T.; Horie, T. Effect of elevated CO2 and high temperature on growth and yield of rice. 2. Sensitive period and pollen germination rate in high temperature sterility of rice spikelets at flowering. Jpn. J. Crop. Sci. 1992, 61, 148–149. [Google Scholar]
- Jagadish, S.V.K.; Craufurd, P.; Wheeler, T.R. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef]
- van Oort, P.A.J.; de Vries, M.E.; Yoshida, H.; Saito, K. Improved climate risk simulations for Rice in arid environments. PLoS ONE 2015, 10, e0118114. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Kobayasi, K.; Yoshimoto, M. Rice Flower Opening Time in Southern U.S. Cultivars. In Proceedings of the ASA and CSSA Meeting, Baltimore, MD, USA, 4–7 November 2018. [Google Scholar]
- Mohammed, A.R.; Tarpley, L. Impact of high nighttime temperature on respiration, membrane stability, antioxidant capacity, and yield of rice plants. Crop. Sci. 2009, 49, 313–322. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. High nighttime temperatures affect rice productivity through altered pollen germination and spikelet fertility. Agric. For. Meteorol. 2009, 14, 999–1008. [Google Scholar] [CrossRef]
- Long, S.P. Modification of the response of photosynthetic 841 productivity to rising temperature by atmospheric CO2 concentrations: Has its importance been underestimated? Plant. Cell Environ. 1991, 14, 729–739. [Google Scholar] [CrossRef]
- Amthor, J.S. The McCree-de Wit -Penning De Vrie -Thornley respiration paradigms: 3 years later. Ann. Bot. 2000, 86, 1–20. [Google Scholar] [CrossRef]
- Monteith, J.L. Climatic variation and the growth of crops. Q. J. R. Meteorol. Soc. 1981, 107, 749–774. [Google Scholar] [CrossRef]

| Site | Beaumont, TX | Crowley, LA | Stuttgart, AR | Stoneville, MS |
|---|---|---|---|---|
| Latitude, Longitude | 30.07, −94.29 | 30.24, −92.38 | 34.5, −91.55 | 33.43, −94.28 |
| Tmean (°C) | 25.7 ± 0.64 | 25.5 ± 0.6 | 25.1 ± 0.76 | 25.7 ± 0.79 |
| Rainfall (mm) | 1033 ± 288 | 744 ± 372 | 424 ± 132 | 467 ± 134 |
| Mean yield (kg ha-1) | 7919 ± 1849 | 8388 ± 1463 | 8195 ± 1387 | 8509 ± 1646 |
| Trend in Tmax (°C decade−1) | 0.01 | 0.01 | 0.1 | 0.44 (**) |
| Trend in Tmin (°C decade−1) | 0.60 (**) | 0.43 (**) | 0.41 (**) | 0.33 (**) |
| Trend in Yield (kg year−1) | 116 (**) | 93 (**) | 63 (**) | 144 (**) |
| Model | Input Type | Variables | Data Source |
|---|---|---|---|
| CERES-Rice | Weather * | Tmin, Tmax, SRAD, rainfall | iAIMS Climate data (1) |
| Soil | soil characterization data such as physical, chemical, and morphological properties (2) | [56] | |
| Crop | cultivar coefficients for Lemont and Cocodrie | Calibrated by Genetic Coefficient Estimator | |
| Management | planting date, row, and plant spacing | [24,57] | |
| Calibration inputs | yield, grain weight, flowering, and harvest dates | [24] | |
| ORYZA | Weather | Tmin, Tmax, SRAD, rainfall, VP, WD | iAIMS Climate data (1) |
| Soil | soil texture and hydrological properties (2) | [48,49] | |
| Crop | phenology development rate fraction of total dry matter partitioned to organs over growth stages specific leaf area index | Calibrated by DRATES Standard crop file from ORYZA v3 | |
| Management | dates of emergence | [24] | |
| Calibration inputs | dates of emergence, panicle initiation, flowering, and maturity | [24,58] | |
| Both models | Planting dates | TX: April 1–27 | [59] |
| MS: April 23–May 19 | [24] | ||
| LA: March 5–28 | [60] | ||
| AR: April 12–May 13 | [61] |
| Model | Parameter 1 | Value | |
|---|---|---|---|
| Lemont | Cocodrie | ||
| CERES-Rice | P1 | 604.3 | 366.9 |
| P2O | 10.36 | 48.41 | |
| P2R | 49.60 | 75.62 | |
| P5 | 517.4 | 730.1 | |
| G1 | 45.26 | 10.54 | |
| G2 | 0.0250 | 0.0250 | |
| G3 | 1.00 | 1.00 | |
| G4 | 1.00 | 1.00 | |
| ORYZA | DVRJ | 0.000903 | 0.000784 |
| DVRI | 0.000758 | 0.000758 | |
| DVRP | 0.000839 | 0.001247 | |
| DVRR | 0.001832 | 0.001538 | |
| Model | Varieties | Sites | r | d | RMSE (kg ha−1) | SRMSE | S/ob | MAPE | Ob Std (kg ha−1) | S Std (kg ha−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| CERES-Rice | Lemont | Stuttgart | 0.24 | 0.54 | 1153 | 0.15 | 1.04 | 4% | 716 | 1098 |
| Crowley | 0.41 | 0.65 | 1094 | 0.15 | 0.96 | 4% | 1103 | 899 | ||
| Stoneville | 0.30 | 0.57 | 1307 | 0.22 | 1.03 | 3% | 1232 | 1273 | ||
| Beaumont | –0.13 | 0.25 | 1575 | 0.26 | 1.00 | 0% | 842 | 1575 | ||
| Cocodrie | Stuttgart | 0.12 | 0.42 | 1607 | 0.21 | 1.06 | 6% | 1607 | 594 | |
| Crowley | –0.32 | 0.18 | 1282 | 0.14 | 0.97 | 3% | 1115 | 383 | ||
| Stoneville | 0.04 | 0.45 | 1272 | 0.13 | 0.91 | 9% | 860 | 438 | ||
| Beaumont | 0.56 * | 0.75 | 1456 | 0.15 | 0.98 | 2% | 1584 | 1572 | ||
| 50% percentile yield | Stuttgart | 0.20 | 0.46 | 1476 | 0.18 | 1.00 | 0% | 1387 | 903 | |
| Crowley | 0.53 ** | 0.73 | 1326 | 0.16 | 0.98 | 2% | 1463 | 1209 | ||
| Stoneville | 0.70 ** | 0.81 | 1315 | 0.16 | 0.96 | 4% | 1646 | 1430 | ||
| Beaumont | 0.56 ** | 0.74 | 1786 | 0.21 | 1.04 | 4% | 1849 | 1934 | ||
| Re-ORYZA | Lemont | Stuttgart | 0.08 | 0.44 | 831 | 0.11 | 1.02 | 2% | 716 | 511 |
| Crowley | 0.52 * | 0.62 | 914 | 0.12 | 1.00 | 0% | 1103 | 527 | ||
| Stoneville | 0.32 | 0.50 | 1299 | 0.19 | 1.07 | 7% | 1232 | 848 | ||
| Beaumont | –0.23 | 0.26 | 1179 | 0.17 | 1.04 | 4% | 842 | 670 | ||
| Cocodrie | Stuttgart | 0.07 | 0.46 | 1896 | 0.23 | 1.04 | 4% | 1607 | 1145 | |
| Crowley | 0.01 | 0.35 | 1367 | 0.14 | 0.98 | 2% | 1115 | 861 | ||
| Stoneville | 0.03 | 0.39 | 1122 | 0.11 | 0.97 | 3% | 860 | 732 | ||
| Beaumont | 0.58 * | 0.69 | 1257 | 0.13 | 1.01 | 1% | 1584 | 881 | ||
| 50% percentile yield | Stuttgart | 0.12 | 0.44 | 1558 | 0.19 | 0.99 | 1% | 1387 | 923 | |
| Crowley | 0.49 ** | 0.71 | 1315 | 0.16 | 1.00 | 0% | 1463 | 1127 | ||
| Stoneville | 0.76 ** | 0.87 | 1077 | 0.12 | 1.01 | 1% | 1646 | 1461 | ||
| Beaumont | 0.75 ** | 0.81 | 1365 | 0.17 | 1.08 | 8% | 1849 | 1448 |
| Sites | Variables | CERES-Rice | ORYZA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LMNT | CCDR | LMNT | CCDR | ||||||
| r | Slope | r | Slope | r | Slope | r | Slope | ||
| Stuttgart | T | −0.70 ** | −863 | −0.59 * | −478 | −0.50 * | −290 | −0.61 ** | −958 |
| SRAD | −0.25 | −514 | 0.61 ** | 413 | −0.23 | −222 | 0.54 * | 706 | |
| P | 0.59 ** | 4.8 | 0.15 | 0.04 | 0.55 * | 2.11 | 0.31 | 2.48 | |
| Crowley | T | 0.23 | 85 | −0.38 | −271 | 0.16 | 158 | −0.25 | −381 |
| SRAD | 0.61 * | 945 | 0.44 | 334 | 0.78 ** | 619 | 0.64 ** | 1074 | |
| P | −0.28 | 0.70 | 0.17 | 0.42 | −0.33 | −0.88 | −0.43 | −2.3 | |
| Stoneville | T | −0.66 ** | −1010 | −0.36 | −223 | −0.62 * | −614 | −0.54 * | −553 |
| SRAD | 0.17 | 381 | 0.76 ** | 411 | 0.23 | 329 | 0.49 * | 448 | |
| P | −0.07 | −0.69 | 0.15 | 0.50 | −0.14 | −0.94 | 0.50 * | 2.7 | |
| Beaumont | T | −0.34 | −1012 | −0.28 | −1086 | −0.28 | −357 | −0.48 * | −1082 |
| SRAD | 0.81 ** | 706 | 0.93 ** | 852 | 0.76 ** | 284 | 0.68 ** | 348 | |
| P | 0.08 | 0.43 | 0.17 | 1.2 | 0.006 | 0.01 | 0.10 | 0.40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Fleisher, D.; Timlin, D.; Reddy, V.R.; Wang, Z.; McClung, A. Evaluation of Different Crop Models for Simulating Rice Development and Yield in the U.S. Mississippi Delta. Agronomy 2020, 10, 1905. https://doi.org/10.3390/agronomy10121905
Li S, Fleisher D, Timlin D, Reddy VR, Wang Z, McClung A. Evaluation of Different Crop Models for Simulating Rice Development and Yield in the U.S. Mississippi Delta. Agronomy. 2020; 10(12):1905. https://doi.org/10.3390/agronomy10121905
Chicago/Turabian StyleLi, Sanai, David Fleisher, Dennis Timlin, Vangimalla R. Reddy, Zhuangji Wang, and Anna McClung. 2020. "Evaluation of Different Crop Models for Simulating Rice Development and Yield in the U.S. Mississippi Delta" Agronomy 10, no. 12: 1905. https://doi.org/10.3390/agronomy10121905
APA StyleLi, S., Fleisher, D., Timlin, D., Reddy, V. R., Wang, Z., & McClung, A. (2020). Evaluation of Different Crop Models for Simulating Rice Development and Yield in the U.S. Mississippi Delta. Agronomy, 10(12), 1905. https://doi.org/10.3390/agronomy10121905






