Using Proximal Hyperspectral Sensing to Predict Herbage Nutritive Value for Dairy Farming
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Canopy Spectral Measurements
2.3. Herbage Cuts
2.4. Determination of the Nutritive Value of Herbage Samples
2.5. Outlier Detection
2.6. Spectral Data Pre-Treatment
2.7. Calibration Model Development
2.8. Model Accuracy Assessment
3. Results
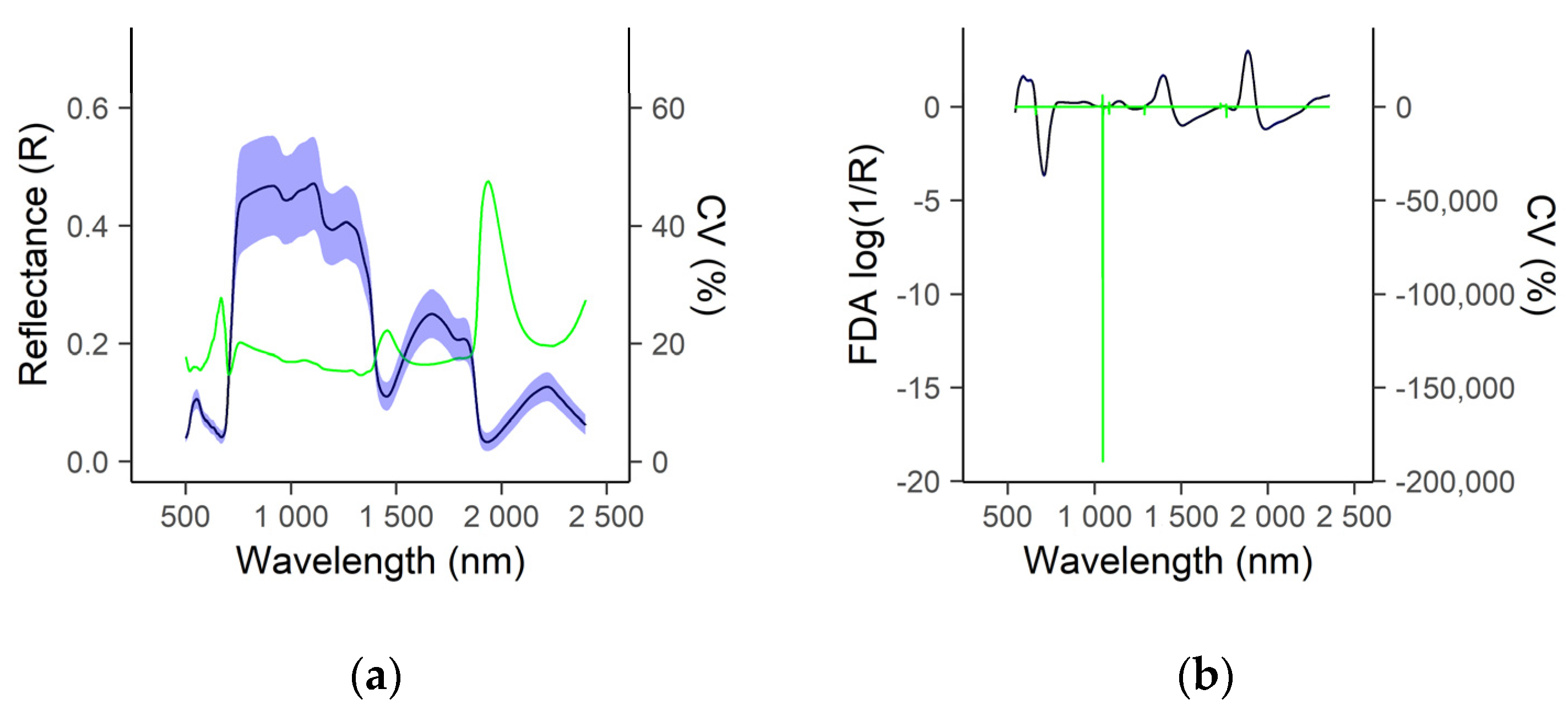
3.1. Descriptive Statistics of Reference Nutritive Values and Spectral Data
3.2. Prediction of Herbage Nutritive Value Using Proximal Canopy Spectra
3.2.1. Model Accuracy
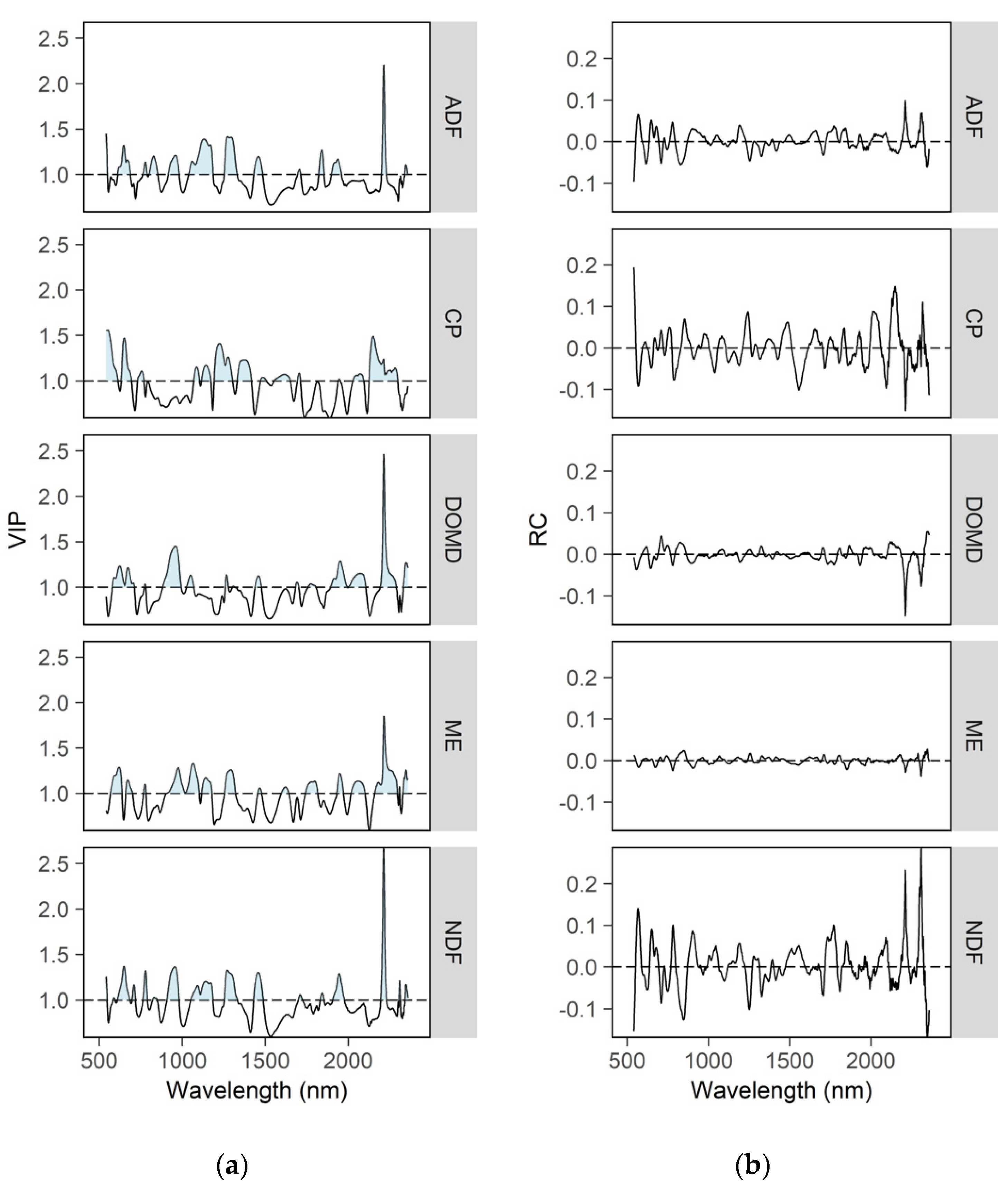
3.2.2. Wavelength Contribution to the Predictive Capability of the Calibration Models
4. Discussion
4.1. Representativeness of the Data Used for Building the Calibration Models
4.2. Predictive Capability of Calibration Models
4.3. Wavelength Contribution for Predicting Herbage Nutritive Value
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beukes, P.C.; McCarthy, S.; Wims, C.M.; Romera, A.J. Regular estimates of paddock pasture mass can improve profitability on New Zealand dairy farms. J. N. Z. Grassl. 2015, 77, 29–34. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Clark, D.A. Feeding value of pastures for ruminants. N. Z. Vet. J. 2004, 52, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Poppi, D.P. Predictions of food intake in ruminants from analyses of food composition. Aust. J. Agric. Res. 1996, 47, 489–504. [Google Scholar] [CrossRef]
- Dillon, P. Achieving high dry-matter intake from pasture with grazing dairy cows. In Fresh Herbage for Dairy Cattle, The Key to a Sustainable Food Chain; Elgersma, A., Dijkstra, J., Tamminga, S., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 18, pp. 1–26. [Google Scholar]
- Ganche, E.; Delaby, L.; O’Donovan, M.; Boland, T.M.; Galvin, N.; Kennedy, E. Post-grazing sward height imposed during the first 10 weeks of lactation: Influence on early and total lactation dairy cow production, and spring and annual sward characteristics. Livest. Sci. 2013, 157, 299–311. [Google Scholar] [CrossRef]
- Lee, J.M.; Donaghy, D.J.; Roche, J.R. The effect of grazing severity and fertiliser application during winter on herbage regrowth and quality of perennial ryegrass (Lolium perenne L.). Anim. Prod. Sci. 2007, 47, 825–832. [Google Scholar] [CrossRef]
- Macdonald, K.A.; Glassey, C.B.; Rawnsley, R.P. The emergence, development and effectiveness of decision rules for pasture based dairy systems. In Proceedings of the 4th Australasian Dairy Science Symposium, Lincoln, New Zealand, 31 August–2 September 2010; Caxton Press: Christchurch, New Zealand; pp. 199–209. [Google Scholar]
- Shalloo, L.; O’Donovan, M.; Leso, L.; Werner, J.; Ruelle, E.; Geoghegan, A.; Delaby, L.; O’Leary, N. Grass-based dairy systems, data and precision technologies. Animal 2018, 12, 262–271. [Google Scholar] [CrossRef]
- Cosgrove, G.P.; Betteridge, K.; Thomas, V.J.; Corson, D.C. A sampling strategy for estimating dairy pasture quality. In Proceedings of the New Zealand Society of Animal Production, Palmerston North, New Zealand, 22–25 June 1998; Volume 58, pp. 25–28. [Google Scholar]
- Marten, G.C.; Shenk, J.; Barton, F. Near Infrared Reflectance Spectroscopy (NIRS): Analysis of Forage Quality; USDA Handbook 643; USDA: Washington, DC, USA, 1989.
- Corson, D.C.; Waghorn, G.C.; Ulyatt, M.J.; Lee, J. NIRS: Forage analysis and livestock feeding. In Proceedings of the New Zealand Grassland Association, Napier, New Zealand, 5–7 October 1999; Volume 61, pp. 127–132. [Google Scholar]
- Fulkerson, W.J.; Donaghy, D.J. Plant-soluble carbohydrate reserves and senescence-key criteria for developing an effective grazing management system for ryegrass-based pastures: A review. Aust. J. Exp. Agric. 2001, 41, 261–275. [Google Scholar] [CrossRef]
- Chapman, D.F.; Lee, J.M.; Waghorn, G.C. Interaction between plant physiology and pasture feeding value: A review. Crop Pasture Sci. 2014, 65, 721–734. [Google Scholar] [CrossRef]
- Lemaire, G.; Belanger, G. Allometries in plants as drivers of forage nutritive value: A review. Agriculture 2020, 10, 5. [Google Scholar] [CrossRef]
- Govender, M.; Chetty, K.; Bulcock, H. A review of hyperspectral remote sensing and its application in vegetation and water resource studies. Water SA 2007, 33, 145–151. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Lyon, J.G. Hyperspectral Remote Sensing of Vegetation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Sims, D.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, U.; Merzlyak, M.N. Relation-ships between leaf chlorophyll content and spectral reflectance and algo-rithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Mutanga, O.; Skidmore, A.K.; Prins, H.H.T. Predicting in situ pasture quality in the Kruger National Park, South Africa, using continuum-removed absorption features. Remote Sens. Environ. 2004, 89, 393–408. [Google Scholar] [CrossRef]
- Pullanagari, R.R.; Yule, I.J.; Tuohy, M.P.; Hedley, M.J.; Dynes, R.A.; King, W.M. In-field hyperspectral proximal sensing for estimating quality parameters of mixed pasture. Precis. Agric. 2012, 13, 351–369. [Google Scholar] [CrossRef]
- Godinho, S.; Surovy, P.; Sousa, A.; Gil, A. Advances in remote-sensing applications in silvo-pastoral systems. Int. J. Remote Sens. 2018, 39, 4565–4571. [Google Scholar] [CrossRef]
- Yule, I.J.; Pullanagari, R.R.; Irwin, M.; McVeagh, P.; Kereszturi, G.; White, M.; Manning, M. Mapping nutrient concentration in pasture using hyperspectral imaging. J. N. Z. Grassl. 2015, 77, 47–50. [Google Scholar] [CrossRef]
- Ali, I.; Cawkwell, F.; Dwyer, E.; Barrett, B.; Green, S. Satellite remote sensing of grasslands: From observation to management. J. Plant Ecol. 2016, 9, 649–671. [Google Scholar] [CrossRef]
- Kawamura, K.; Lim, J.; Kurokawa, Y.; Obitsu, T.; Yayota, M.; Ogura, S. Monitoring spatial heterogeneity of pasture within paddock scale using a small unanned aerial vehicle (sUAV). J. Integr. Field Sci. 2017, 14, 61–66. [Google Scholar]
- Von Bueren, S.K.; Burkart, A.; Hueni, A.; Rascher, U.; Tuohy, M.P.; Yule, I.J. Deploying four optical UAV-based sensors over grassland: Challenges and limitations. Biogeosciences 2015, 12, 163–175. [Google Scholar] [CrossRef]
- Gebbers, R.; Adamchuk, V.I. Precision agriculture and food security. Science 2010, 327, 828–831. [Google Scholar] [CrossRef]
- Sanches, I.D. Hyperspectral Proximal Sensing of the Botanical Composition and Nutrient Content of New Zealand Pastures. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2009. [Google Scholar]
- Holland, K.H.; Lamb, D.W.; Schepers, J.S. Radiometry of proximal active optical sensors (AOS) for agricultural sensing. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 1793–1802. [Google Scholar] [CrossRef]
- Roberts, J.; Suarez Infiesta, A.; Schäbitz, B.; Fourie, J.; Werner, A. Active optical sensing of canopies in pasture management. J. N. Z. Grassl. 2015, 77, 35–40. [Google Scholar] [CrossRef]
- Pullanagari, R.R.; Yule, I.J.; Hedley, M.J.; Tuohy, M.P.; Dynes, R.A.; King, W.M. Multi-spectral radiometry to estimate pasture quality components. Precis. Agric. 2012, 13, 442–456. [Google Scholar] [CrossRef]
- Pullanagari, R.R.; Yule, I.J.; Tuohy, M.P.; Hedley, M.J.; Dynes, R.A.; King, W.M. Proximal sensing of the seasonal variability of pasture nutritive value using multispectral radiometry. Grass Forage Sci. 2013, 68, 110–119. [Google Scholar] [CrossRef]
- Kawamura, K.; Watanabe, N.; Sakanoue, S.; Inoue, Y. Estimating forage biomass and quality in a mixed sown pasture based on partial least squares regression with waveband selection. Grassl. Sci. 2008, 54, 131–145. [Google Scholar] [CrossRef]
- Kawamura, K.; Betteridge, K.; Sanches, I.D.; Tuohy, M.P.; Costall, D.; Inoue, Y. Field radiometer with canopy pasture probe as a potential tool to estimate and map pasture biomass and mineral components: A case study in the Lake Taupo catchment, New Zealand. N. Z. J. Agric. Res. 2009, 52, 417–434. [Google Scholar] [CrossRef]
- Adjorlolo, C.; Mutanga, O.; Cho, M.A. Predicting C3 and C4 grass nutrient variability using in situ canopy reflectance and partial least squares regression. Int. J. Remote Sens. 2015, 36, 1743–1761. [Google Scholar] [CrossRef]
- Delagarde, R.; Peyraud, J.L.; Delaby, L.; Faverdin, P. Vertical distribution of biomass, chemical composition and pepsin-cellulase digestibility in a perennial ryegrass sward: Interaction with month of year, regrowth age and time of day. Anim. Feed Sci. Technol. 2000, 84, 49–68. [Google Scholar] [CrossRef]
- Nave, R.L.; Sulc, R.M.; Barker, D.J.; St-Pierre, N. Changes in forage nutritive value among vertical strata of a cool-season grass canopy. Crop Sci. 2014, 54, 2837–2845. [Google Scholar] [CrossRef]
- Asner, G.P. Biophysical and biochemical sources of variability in canopy reflectance. Remote Sens. Environ. 1998, 64, 234–253. [Google Scholar] [CrossRef]
- NIWA. CliFlo: NIWA’s National Climate Database on the Web. Palmerston North Ews Station. Available online: https://cliflo.niwa.co.nz/ (accessed on 10 August 2018).
- Stevens, A.; Ramirez-Lopez, L. An Introduction to the Prospectr Package. R Package Vignette. 2013. Available online: https://cran.r-project.org/web/packages/prospectr/vignettes/prospectr.html (accessed on 10 August 2018).
- Burger, J.; Geladi, P. Spectral pre-treatments of hyperspectral near infrared images: Analysis of diffuse reflectance scattering. J. Near Infrared. Spec. 2007, 15, 29–37. [Google Scholar] [CrossRef]
- Barnes, R.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Jobson, J.D. Applied Multivariate Data Analysis: Regression and Experimental Design; Springer Media: New York, NY, USA, 1991. [Google Scholar] [CrossRef]
- Wold, S.; Johansson, E.; Cocchi, M. PLS-partial least squares projections to latent structures. In 3D-QSAR in Drug Design; Theory Methods and Applications; Kubinyi, H., Ed.; ESCOM Science Publishers: Leiden, Germany, 1993; pp. 523–550. [Google Scholar]
- Haaland, D.M.; Thomas, E.V. Partial least-squares methods for spectral analyses 1. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal. Chem. 1988, 60, 1193–1202. [Google Scholar] [CrossRef]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemometr. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- DairyNZ. Facts and Figures: A Quick Reference Guide for New Zealand Dairy Farmers; DairyNZ: Hamilton, New Zealand, 2017; Available online: https://www.dairynz.co.nz/publications/dairy-industry/facts-and-figures/ (accessed on 10 August 2018).
- Holmes, C.W. Milk Production from Pasture, 2nd ed.; Massey University: Palmerston North, New Zealand, 2002. [Google Scholar]
- Moller, S.N. An Evaluation of Major Nutrients in Dairy Pasture in New Zealand and Their Effects on Milk Production and Herd Reproductive Performance. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 1997. [Google Scholar]
- Curran, P.J. Imaging spectrometry. Prog. Phys. Geog. 1994, 18, 247–266. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F. Chemometrics applied to plant spectral analysis. In Comprehensive Analytical Chemistry: Vibrational Spectroscopy for Plant Varieties and Cultivars Caraterization; Lopes, J., Sousa, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 69–104. [Google Scholar] [CrossRef]
- Eastwood, C.R.; Dela Rue, B.T. Identification of operational performance attributes for pasture measuring devices. J. N. Z. Grassl. 2017, 79, 211–216. [Google Scholar] [CrossRef]
- Malley, D.F.; Martin, P.D.; Ben-Dor, E. Near Infrared Spectroscopy in Agriculture. In Application in Analysis of Soils; Roberts, C.A., Workman, J., Jr., Reeves, J.B., III, Eds.; Soil Science Society of America: Madison, WI, USA, 2004; pp. 729–784. [Google Scholar] [CrossRef]
- Biewer, S.; Fricke, T.; Wachendorf, M. Development of canopy reflectance models to predict forage quality of legume–grass mixtures. Crop Sci. 2009, 49, 1917–1926. [Google Scholar] [CrossRef]
- Clark, D.H.; Lamb, R.C. Near infrared reflectance spectroscopy: A survey of wavelength selection to determine dry matter digestibility. J. Dairy Sci. 1991, 74, 2200–2205. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Clark, R.N. Spectroscopic determination of leaf biochemistry using band-depth analysis of absorption features and stepwise multiple linear regression. Remote Sens. Environ. 1999, 67, 267–287. [Google Scholar] [CrossRef]
- Pettai, H.; Oja, V.; Freiberg, A.; Laisk, A. Photosynthetic activity of far-red light in green plants. Biochim. Biophys. Acta 2005, 1708, 311–321. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C.; Corsi, F.; Cho, M. LAI and chlorophyll estimation for a heterogeneous grassland using hyperspectral measurements. ISPRS J. Photogramm. Remote Sens. 2008, 63, 409–426. [Google Scholar] [CrossRef]
- Kumar, L.; Schmidt, K.; Dury, S.; Skidmore, A. Imaging spectrometry and vegetation science. In Imaging Spectrometry; Meer, F.D., Jong, S.M.D., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 111–115. [Google Scholar] [CrossRef]
| NV Trait | R2 | RMSE | RPE | Bias | RPD |
|---|---|---|---|---|---|
| ME | 0.92 | 0.43 | 4.04 | 0.02 | 3.46 |
| CP | 0.94 | 1.13 | 6.34 | 0.08 | 4.27 |
| NDF | 0.87 | 2.95 | 5.76 | 0.70 | 2.86 |
| ADF | 0.76 | 2.27 | 8.24 | –0.03 | 1.84 |
| DOMD | 0.95 | 1.68 | 2.93 | –0.25 | 4.55 |
| Metric | Equation | |
|---|---|---|
| Coefficient of determination | (2) | |
| Root mean square error | (3) | |
| Relative prediction error | (4) | |
| Bias | (5) | |
| Ratio of prediction to deviation | (6) |
| NV Trait | Dataset | N | Range | Mean | SD | CV |
|---|---|---|---|---|---|---|
| ME | Training | 220 | 8.27–12.07 | 10.72 | 0.74 | 0.07 |
| Validation | 52 | 7.66–11.99 | 10.72 | 0.82 | 0.08 | |
| CP | Training | 220 | 5.58–25.65 | 17.88 | 3.69 | 0.21 |
| Validation | 52 | 7.04–23.88 | 17.52 | 4.50 | 0.26 | |
| NDF | Training | 220 | 30.28–55.52 | 40.42 | 4.77 | 0.12 |
| Validation | 52 | 31.14–57.93 | 40.39 | 5.38 | 0.13 | |
| ADF | Training | 220 | 14.81–28.66 | 21.32 | 2.69 | 0.12 |
| Validation | 52 | 15.57–31.51 | 21.17 | 3.03 | 0.14 | |
| DOMD | Training | 220 | 55.21–69.86 | 64.80 | 2.54 | 0.04 |
| Validation | 52 | 53.37–69.21 | 64.78 | 3.01 | 0.05 |
| Training | Validation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NV Trait | ME | CP | NDF | ADF | DOMD | ME | CP | NDF | ADF | DOMD |
| ME | 1.00 | 1.00 | ||||||||
| CP | 0.31 *** | 1.00 | 0.33 * | 1.00 | ||||||
| NDF | −0.76 *** | −0.28 *** | 1.00 | −0.86 *** | −0.43 ** | 1.00 | ||||
| ADF | −0.75 *** | −0.21 *** | 0.90 *** | 1.00 | −0.83 *** | −0.35 * | 0.88 *** | 1.00 | ||
| DOMD | 0.75 *** | 0.30 *** | −0.87 *** | −0.77 *** | 1.00 | 0.87 *** | −0.32 * | −0.93 *** | −0.83 *** | 1.00 |
| Dataset | NV Trait | R2 | RMSE | RPE | Bias | RPD |
|---|---|---|---|---|---|---|
| Training | ME | 0.67 | 0.42 | 3.96 | 6.90 × 10−16 | 1.89 |
| CP | 0.78 | 1.76 | 9.87 | −5.21 × 10−16 | 2.43 | |
| NDF | 0.54 | 3.33 | 8.25 | −3.44 × 10−15 | 1.54 | |
| ADF | 0.55 | 1.80 | 8.46 | 3.15 × 10−16 | 1.48 | |
| DOMD | 0.62 | 1.65 | 2.55 | −4.68 × 10−15 | 1.64 | |
| Validation | ME | 0.59 | 0.52 | 4.88 | 4.13 × 10−2 | 1.46 |
| CP | 0.77 | 2.05 | 11.73 | 4.41 × 10−1 | 1.84 | |
| NDF | 0.55 | 3.23 | 7.96 | −2.15 × 10−1 | 1.50 | |
| ADF | 0.56 | 1.98 | 9.35 | −1.13 × 10−1 | 1.27 | |
| DOMD | 0.58 | 1.60 | 2.47 | 1.72 × 10−1 | 1.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duranovich, F.N.; Yule, I.J.; Lopez-Villalobos, N.; Shadbolt, N.M.; Draganova, I.; Morris, S.T. Using Proximal Hyperspectral Sensing to Predict Herbage Nutritive Value for Dairy Farming. Agronomy 2020, 10, 1826. https://doi.org/10.3390/agronomy10111826
Duranovich FN, Yule IJ, Lopez-Villalobos N, Shadbolt NM, Draganova I, Morris ST. Using Proximal Hyperspectral Sensing to Predict Herbage Nutritive Value for Dairy Farming. Agronomy. 2020; 10(11):1826. https://doi.org/10.3390/agronomy10111826
Chicago/Turabian StyleDuranovich, Federico N., Ian J. Yule, Nicolas Lopez-Villalobos, Nicola M. Shadbolt, Ina Draganova, and Stephen T. Morris. 2020. "Using Proximal Hyperspectral Sensing to Predict Herbage Nutritive Value for Dairy Farming" Agronomy 10, no. 11: 1826. https://doi.org/10.3390/agronomy10111826
APA StyleDuranovich, F. N., Yule, I. J., Lopez-Villalobos, N., Shadbolt, N. M., Draganova, I., & Morris, S. T. (2020). Using Proximal Hyperspectral Sensing to Predict Herbage Nutritive Value for Dairy Farming. Agronomy, 10(11), 1826. https://doi.org/10.3390/agronomy10111826






