Abstract
Studies on crop response to light quality (red (R) to far-red (FR) light ratio) often recommend early weed removal to reduce the effects of shade avoidance responses on crop yield. However, it is unclear whether crops are able to distinguish reflected light quality of kin from that of nonkin. We evaluated the response of sugar beet (Beta vulgaris L.) to reflected FR light from sugar beet, common lambsquarters (Chenopodium album L.), Kentucky bluegrass (Poa pratensis L.), alfalfa (Medicago sativa L.), and bare soil (control) under outdoor conditions in 2016 and 2017. Treatments were completely randomized with 10 replications per treatment. The study methods ensured there was no direct resource competition. The reflected R:FR of plant species ranged from 0.06 (common lambsquarters) to 0.24 (sugar beet) compared to 0.7 for the bare soil. In 2016 and 2017, there were 2 to 4 more leaves in the sugar beet surrounded by soil compared to sugar beet surrounded by neighboring species. There was up to 47, 57, 43, and 23% reduction in sugar beet leaf area, shoot dry weight, root diameter, and root dry weight, respectively, due to reflected R:FR light from neighboring species. Sugar beet did not respond differently to reflected light quality of kin compared to nonkin.
1. Introduction
Competition among plants arises due to the limited availability of nutrients, water, and light [1]. Plants, though sessile, are not passive. Immobility has made plants evolve mechanisms for detecting neighbor proximity and initiate responses to enable them to compete for resources [2]. Proximity detection is particularly important in the competition for light because light serves as the primary source of energy for photosynthesis in plants. Through photoreceptors (e.g., phytochromes and cryptochromes), plants are able to sense the quality of light (particularly red (R) to far-red (FR) ratio) and use this as a cue to perceive the proximity of neighbors and impending competition for light [3]. Green vegetation absorbs R and reflects much of incoming FR light and thus, neighbor proximity often reduces R:FR. In response to reduced R:FR, plants tend to elongate their hypocotyl, stem, and petioles; decrease rate of leaf expansion; and flower early [3]. These responses are collectively referred to as the shade avoidance syndrome [3,4].
This phenomenon has been widely studied as it affects the growth and yield of crops [5,6,7,8,9]. Most studies on shade avoidance evaluated the effects of reflected light quality from heterospecifics (different species) on crop growth and yield [5,9,10]. Since reflected light from all green vegetation has a reduced R:FR, it will be of interest to know if crops are able to distinguish reflected light quality of kin (kin recognition) from that of nonkin such as weeds. Kin recognition, or the ability to “discriminate between related and unrelated individuals” [11], has been relatively less studied in plants compared to animals. Most kin recognition studies in plants focused on root interactions and resource competition or volatile compound signals [12,13,14,15,16]. In addition, results from photoreceptor-mediated kin recognition studies are not consistent. For example, Crepy and Casal [17,18] observed that Arabidopsis thaliana (L.) Heynh. recognized kin and reoriented leaves more horizontally when grown with kin than when grown with nonkin. They elucidated that the response involved the perception of R:FR and blue light. However, de Wit et al. [19] reported that in rosette-forming plants such as A. thaliana, physical touching of leaf tips might precede R:FR sensing for neighbor detection. Thus, neighboring species architecture may influence shade avoidance responses.
Robson et al. [20] stated that rapid stem expansion often observed at high crop densities is an “agriculturally wasteful allocation of assimilates to stem growth.” Photoreceptor-mediated kin recognition is important because studies on shade avoidance recommend early weed removal as a management strategy for reducing the effects of shade avoidance on crop yield [7,21,22]. Thus, in the absence of weeds, it could be useful for plants to be able to distinguish light quality of conspecifics from that of heterospecifics to prevent yield loss due to shade avoidance. The specific question we seek to answer is, can sugar beet differentiate reflected light quality of kin (sugar beet) from nonkin (not sugar beet)? In a recent review, it was argued that current environmental and land-use conditions may necessitate planting crops at higher densities to increase crop yields [3]. In fact, it was posited that optimizing shade avoidance responses may be important for increasing crop yields at high planting densities [23]. Since kin recognition may be density-dependent [24], understanding crop response to reflected light quality from conspecifics and heterospecifics is important. This study, therefore, evaluated the response of sugar beet to reflected light quality from kin and nonkin.
2. Materials and Methods
2.1. Site Description, Treatments, Experimental Design, and Data Collection
Studies were conducted in 2016 and 2017 at the University of Wyoming Laramie Research and Extension Center, Laramie, Wyoming, to evaluate the response of sugar beet to reflected light quality from different species. The experiment comprised four neighboring species (sugar beet, common lambsquarters (Chenopodium album L.), Kentucky bluegrass (Poa pratensis L.), alfalfa (Medicago sativa L.)) and a bare soil (untreated check) treatment arranged in a completely randomized design with 10 replicates. Species were selected to ensure that not all species were closely related to sugar beet while ensuring different functional groups were present. The study setup ensured there was no resource competition (Figure 1). Neighboring species were planted at high densities to ensure that there was full coverage of soil surface for maximal FR reflection. Three seeds of sugar beet (cultivar “BTS60RR27” and “RR014GEM50” in 2016 and 2017, respectively) were planted per pot and thinned to one seedling per pot immediately after emergence. The sugar beet cultivars used in 2016 and 2917 were both glyphosate-resistant (Roundup ready) and were obtained from the source. An observational conducted study in 2017 showed there were no differences between the cultivars Sugar beet was planted after neighboring species have emerged to ensure there was reduced R:FR at the time of sugar beet emergence. Sugar beet and neighboring species were drip irrigated daily to ensure water was not a limiting factor. Sugar beet was fertilized with 40 g of 14:14:14:5.5% (N:P:K:S) polymer-coated fertilizer at planting to ensure a slow and continuous release of nutrients throughout the growing season. The inner pail (Figure 1) was either raised or neighboring species clipped regularly to prevent direct shading and competition for sunlight.
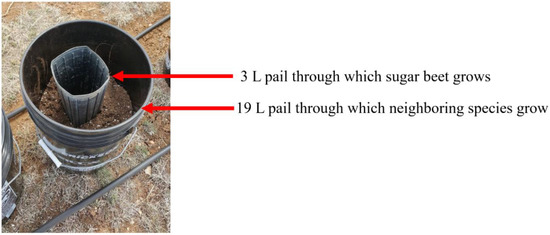
Figure 1.
Experiment setup ensuring there was no resource competition. This setup made it possible to raise the inner pail when necessary to prevent direct shading of sugar beet.
The spectral signature of the four species and the bare soil surface were measured only in 2016 using a FieldSpec4 standard resolution spectroradiometer (PANalytical (formerly ASD Inc.), Longmont, CO, USA). The spectral data were used to calculate the average reflectance of R (655–665 nm) and FR light (725–735 nm) and these were used to estimate the average R:FR of reflected light. Plant and soil reflectance were measured only in 2016 because we did not expect reflectance to differ between years and we are not aware of any studies demonstrating that year affects plant light reflectance.
Number of leaves and angle (from the horizontal) of the oldest, healthiest leaf were measured weekly after seedling emergence. Leaf angle was measured using a protractor. Plants were harvested at 63 and 47 days after planting (DAP) in 2016 and 2017, respectively. At harvest, number of leaves, root length, root diameter, root fresh weight, root dry weight, total leaf area, shoot fresh weight, and shoot dry weight were measured. Root diameter and leaf area were measured using a digital caliper and leaf area meter (LI-3100C) (LI-COR Inc., Lincoln, NE, USA), respectively.
2.2. Data Analysis
Nonlinear regression analysis was performed using the ”drc” package (v4.0-2) in the R statistical language (v 3.5.1) to quantify the effect of reflected light from neighboring species and soil surface on sugar beet leaf number over time [25,26]. A three-parameter Weibull model was used (Equation (1)), where Y is the number of sugar beet leaves at time x, d is the upper limit asymptote, x is time in days after planting, e is the value of x at the inflection point on the curve, and b is a slope parameter. The Weibull model provides appropriate statistical parameter estimates of plant growth and also has parameter estimates with meaningful biological interpretations [27]. The effect of reflected light from neighboring species on the number of sugar beet true leaves at final harvest, which corresponded with 63 and 47 d in 2016 and 2017, respectively, was estimated from the model, and 95% confidence intervals were calculated. Nonoverlapping 95% confidence intervals were used as a conservative estimate of statistical difference between treatments [28].
Y = d × −exp(b × (log(x) − e))
A repeated measures analysis with a Satterthwaite denominator degree of freedom approximation to account for correlated errors was performed on sugar beet leaf angle using the “lme4” package [29]. For leaf angle, Tukey-adjusted pairwise treatment comparisons were performed using the ”emmeans” package [30]. Analysis of variance was performed on reflectance and harvest data (total leaf area, root length, root diameter, shoot dry weight, and root dry weight). Treatments differences were compared at alpha of 0.05 using Fisher’s protected least significant difference (LSD).
3. Results and Discussion
3.1. Light Reflectance from Plant Canopies
The spectral signature of neighboring species and the bare soil showed that plant species reflected less than 20% of incoming R light and about 36 to 56% of incoming FR light (Figure 2). It is because photosynthetic pigments absorb more in the R region than the FR region of incoming solar radiation [31].
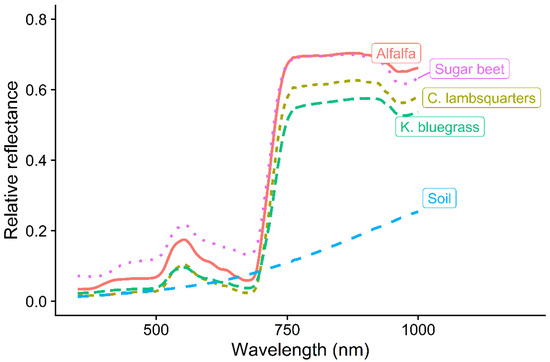
Figure 2.
Reflectance spectra of alfalfa, sugar beet, common lambsquarters, Kentucky bluegrass, and soil in 2016 (n = 10 for each species).
The high levels of FR light reflected from the leaf surfaces explains the reduction in R:FR ratio in crowded plant communities [32,33]. Reflected R and FR light from the surface of maize (Zea mays L.) leaves were reported to be approximately 5% and 40%, respectively [34]. There were differences in the relative amounts of R and FR light reflected by the plant species (Table 1). This resulted in differences (p < 0.0001) in R:FR (Table 1). This might be due to differences in the greenness (chlorophyll concentration) and leaf surface brightness of the plant species. For example, visual observation showed that alfalfa and sugar beet leaves were deep green in color compared to pale green color of common lambsquarters. The R:FR of plant species ranged from 0.06 (common lambsquarters) to 0.24 (sugar beet) (Figure 2). This was within the range of 0.05–1.15 reported by [35].

Table 1.
Relative reflectance of red and far-red light and red to far-red light ratio of neighboring species and bare soil surface in 2016, Laramie, Wyoming, USA.
The soil surface had the greatest R:FR (0.7; Table 1). The R:FR of gray-white and brick-red soil surfaces were reported to be 1.0 and 1.18, respectively [34]. The lesser ratio obtained in this study was due to the potting media used. The potting media was mostly peat, which has a dark brown to black color. Thus, most of the incoming radiation was absorbed by the media. Studies on light quality have shown that phytochrome photoequilibrium (the ratio of FR absorbing phytochrome to the sum of R and FR absorbing phytochrome) of daylight is about 0.81 [36] and stem extension was observed at phytochrome photoequilibrium of 0.63 (R:FR ~ 0.66) [37]. Although the results from Sager, Smith, Edwards, and Cyr [36] and Craig and Runkle [37] were based on photosynthetic photon flux rather than the relative reflectance reported in this study, their results suggest that plants responded to marginal reductions in light quality.
3.2. Sugar Beet Leaf Angle
There was no time by treatment interaction effect on sugar beet leaf angle in 2016 (p = 0.21) and 2017 (p = 0.15). However, sugar beet leaf angle was different in the presence of neighboring species in 2016 and 2017 (p < 0.001). Sugar beet grown with neighbors had more upright leaf angles (Figure 3). This modified morphology was initiated as early as 21 DAP in 2016 and 15 DAP in 2017 (data not presented). Leaf angle was lower in the soil treatment in both years (Figure 3). This shows that sugar beet responded to reduced R:FR ratio and not necessarily species identity. Contrary to our results, Crepy and Casal [17] observed that A. thaliana reorient leaves more horizontally when grown with kin than when grown with nonkin. They elucidated that the response involved the perception of R:FR and blue light. Mullen et al. [38] reported that rosette leaves of A. thaliana were more vertically oriented when exposed to low light levels or shaded by neighboring leaves. However, leaves reorient horizontally when returned to white light. Reduced R:FR also increased leaf angle in tobacco [39]. Ethylene, a gaseous phytohormone essential in shade avoidance responses [40] increased leaf angle in A. thaliana [41]. Sugar beet is rosette-forming in the first season of growth. Thus, the steeper leaf angles might be an adaptation for efficient light interception.
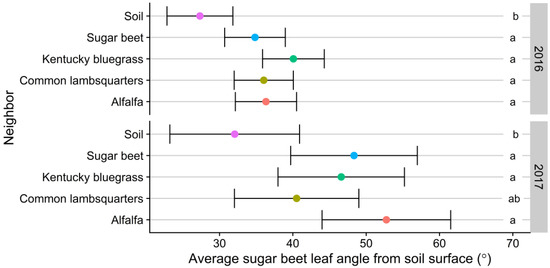
Figure 3.
Effect of reflected far-red light from neighboring species on sugar beet leaf angle (from the horizontal) in 2016 and 2017, Laramie, Wyoming, USA. Within years, estimated marginal means followed by the same letter are not significantly different according to Tukey-adjusted pairwise comparisons (alpha = 0.05). Bars indicate 95% confidence interval.
At any given radiation stream, leaf angle (to the horizontal) determines the flux of solar radiation per unit leaf area [42,43]. However, the relationship between leaf angle and light interception is largely influenced by solar inclination. At low sun angles such as in the morning and late afternoon, steeper leaf angles increased solar radiation interception [43,44]. For example, light interception increased with increasing leaf angle (leaf angles between 25 to 75° to the horizontal) at 15° and 30° solar inclination [43]. Based on the findings by Falster and Westoby [43], it is estimated that with an average sugar beet leaf angle of about 30° in the bare soil treatment and about 50° in the sugar beet (kin) treatments in 2017 (Figure 3), direct light interception could be reduced by 12% and 22% at solar angles of 45° and 60°, respectively. However, at the canopy level, steeper leaf angles may reduce mutual shading and thus maximize light interception [38]. Robson, McCormac, Irvine, and Smith [20] stated that increased allocation of photosynthates for rapid stem expansion in dense crop stands is agriculturally wasteful. Allocation of assimilates to petioles and steeper leaf orientation might be an important adaptation strategy in sugar beet, because of the rosette growth and horizontal leaf architecture, which make sugar beet leaves more prone to mutual shading. Thus, vertical leaf angles will be important when sugar beet is planted at high densities.
3.3. Sugar Beet Leaf Number
The number of sugar beet leaves was reduced by reflected FR light from neighboring species in both years of the study (Figure 4). Sugar beet leaf appearance was delayed by the presence of neighboring species in both years. At final harvest, there were 2 to 4 leaves in the sugar beet surrounded by bare soil, compared to sugar beet surrounded by neighboring species (Figure 5 and Table 2). Reflected FR from weeds was reported to have increased allocation to stem extension and reduced the number of visible leaf tips in maize [6,22,45]. Thus, the modified morphology (hyponasty) possibly resulting from an increased allocation to petiole growth might explain the reduced number of leaves observed in this study.
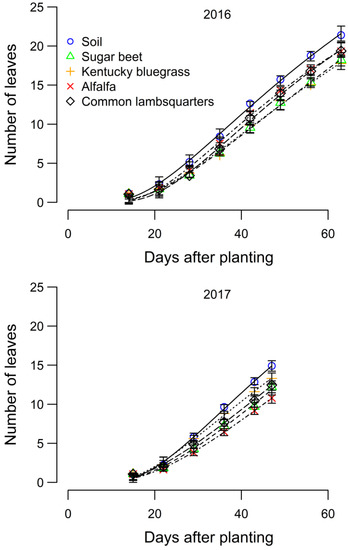
Figure 4.
Effect of reflected far-red light from alfalfa, common lambsquarters, Kentucky bluegrass, sugar beet, and bare soil on sugar beet leaf number in 2016 and 2017, Laramie, WY, USA. Bars indicate 95% confidence interval.
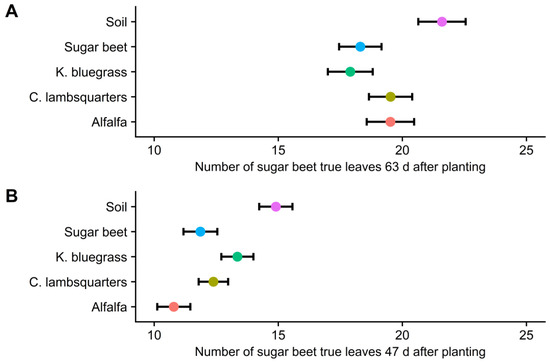
Figure 5.
Effect of reflected far-red light from alfalfa, common lambsquarters, Kentucky bluegrass, sugar beet, and bare soil on sugar beet leaf number at 63 days in 2016 (A) and 43 days in 2017 (B), Laramie, Wyoming, USA. Bars indicate 95% confidence interval, and nonoverlapping confidence intervals were used as a conservative estimate of statistical difference among treatments [28].

Table 2.
Parameter estimates describing sugar beet leaf number following the three-parameter Weibull model for 2016 and 2017 experiments, Laramie, WY, USA.
3.4. Sugar Beet Shoot and Root Growth
The presence of neighboring species reduced sugar beet root diameter, leaf area, and shoot dry weight in 2016 (Table 3). However, sugar beet root dry weight, root length, and shoot-to-root dry weight ratio were not affected by treatments. This was likely due to the late harvest in 2016 (63 d) that resulted in root-bound conditions. This is because sugar beet in the bare soil treatment generally grew the fastest. Thus, a root bound condition early in the season would likely stall growth in the control treatment while other treatments have enough space for increased root growth. On the contrary, only root length and shoot-to-root dry weight ratio were not affected by neighboring species presence in 2017 (Table 3).

Table 3.
Effect of reflected far-red light from neighboring species on sugar beet growth at 63 days after planting (DAP) in 2016 and 47 DAP in 2017, Laramie, WY, USA.
Shade avoidance was reported to have affected sugar beet growth and development [9]. Schambow, Adjesiwor, Lorent, and Kniss [9] demonstrated that in the absence of resource competition, reflected FR light from Kentucky bluegrass canopies reduced sugar beet leaf biomass, total leaf area, and root fresh weight by 30, 63, and 70%, respectively, when plants were harvested 90 DAP. Studies have also demonstrated that reflected FR light reduced leaf area and leaf biomass in maize [6,22], root and total biomass in maize [45]; leaf area, leaf, root, and total biomass in soybean [8]. The reduced sugar beet leaf area and shoot dry weight (Table 3) was due to fewer leaves (Figure 4 and Figure 5). Since there was no below-ground resource competition, differences in root length due to treatments was not expected. However, root diameter was expected to be more correlated with root weight. Thus, reduced allocation to roots due to reflected FR light from neighboring species reduced root diameter in both years (Table 3). In both years and for response variables where differences were observed, sugar beet growth was greater when surrounded by soil than when surrounded by sugar beet. These observations suggest that sugar beet could not discriminate reflected FR light of kin from that of nonkin, and this answered our research question. Sugar beet responded to reduced light quality rather than absolute reductions in light quality. Previous studies have shown that Sorghum vulgare Pers. [46], Cycas micronesica K.D. Hill [15], Cakile edentula (Bigelow) Hook. [47], Ambrosia artemisiifolia L. [48], and Trifolium repens L. [24] were able to discriminate between kin and nonkin. However, there were root interactions in those studies, suggesting that direct root interaction may be required for kin recognition in some plants. This is because root exudates mediate neighbor recognition in most plants [49]. Aside from root interactions, volatile compound signals may also be involved in kin recognition, and this has been found in Artemisia tridentata Nutt. ssp. arbuscula (Nutt.) H.M. Hall and Clem. [50]. Thus, the lack of kin recognition observed in this study could be due to either the absence of root interaction or volatiles. Although results from this study suggest that reflected FR light from neighboring sugar beets might affect growth, development, and yield of sugar beet, this would largely depend on plant spacing and planting density. Thus, at low planting densities, it is likely that the effect of reflected light on sugar beet growth might be minimal until canopy closure. It is concluded that sugar beet could not differentiate reflected light quality of kin from that of nonkin.
Author Contributions
Conceptualization, A.T.A. and A.R.K.; methodology, A.T.A. and A.R.K.; software, A.T.A.; validation, A.R.K.; formal analysis, A.T.A.; investigation, A.T.A. and A.R.K.; resources, A.R.K.; data curation, A.T.A.; writing—original draft preparation, A.T.A.; writing—review and editing, A.T.A. and A.R.K.; visualization, A.T.A.; supervision, A.R.K.; project administration, A.R.K.; funding acquisition, A.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by the United States Department of Agriculture—National Institute of Food and Agriculture grant 2016-67013-24912, and by the Western Sugar Cooperative–Grower Joint Research Committee.
Acknowledgments
The authors thank David Claypool who helped with the study establishment, harvesting, and processing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Depuydt, S. Arguments for and against self and non-self root recognition in plants. Front. Plant Sci. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Biedrzycki, M.L.; Bais, H.P. Kin recognition in plants: A mysterious behaviour unsolved. J. Exp. Bot. 2010, 61, 4123–4128. [Google Scholar] [CrossRef] [PubMed]
- Roig-Villanova, I.; Martínez-García, J.F. Plant Responses to Vegetation Proximity: A Whole Life Avoiding Shade. Front. Plant Sci. 2016, 7, 236. [Google Scholar] [CrossRef]
- Smith, H. The ecological functions of the phytochrome family. clues to a transgenic programme of crop improvement. Photochem. Photobiol. 1992, 56, 815–822. [Google Scholar] [CrossRef]
- McKenzie-Gopsill, A.G.; Lukens, L.; Lee, E.; Swanton, C.J. Does the presence of neighbouring weeds alter the expression of adaptive plasticity to subsequent drought stress in soybean? Field Crop. Res. 2016, 192, 144–153. [Google Scholar] [CrossRef]
- Page, E.R.; Tollenaar, M.; Lee, A.E.; Lukens, L.; Swanton, C.J. Shade avoidance: An integral component of crop-weed competition. Weed Res. 2010, 50, 281–288. [Google Scholar] [CrossRef]
- Rajcan, I.; Chandler, K.J.; Swanton, C.J. Red–far-red ratio of reflected light: A hypothesis of why early-season weed control is important in corn. Weed Sci. 2004, 52, 774–778. [Google Scholar] [CrossRef]
- Green-Tracewicz, E.; Page, E.R.; Swanton, C.J. Shade Avoidance in Soybean Reduces Branching and Increases Plant-to-Plant Variability in Biomass and Yield Per Plant. Weed Sci. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Schambow, T.J.; Adjesiwor, A.T.; Lorent, L.; Kniss, A.R. Shade avoidance cues reduce Beta vulgaris growth. Weed Sci. 2019, 67, 311–317. [Google Scholar] [CrossRef]
- Liu, J.G.; Mahoney, K.J.; Sikkema, P.H.; Swanton, C.J. The importance of light quality in crop-weed competition. Weed Res. 2009, 49, 217–224. [Google Scholar] [CrossRef]
- Mehlis, M.; Bakker, T.C.; Frommen, J.G. Smells like sib spirit: Kin recognition in three-spined sticklebacks (Gasterosteus aculeatus) is mediated by olfactory cues. Anim. Cogn. 2008, 11, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Dudley, S.A.; Murphy, G.P.; File, A.L. Kin recognition and competition in plants. Funct. Ecol. 2013, 27, 898–906. [Google Scholar] [CrossRef]
- Abakumova, M.; Zobel, K.; Lepik, A.; Semchenko, M. Plasticity in plant functional traits is shaped by variability in neighbourhood species composition. N. Phytol. 2016, 211, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.V.; Khandelwal, A.; Dudley, S.A. Kin recognition, not competitive interactions, predicts root allocation in young Cakile edentula seedling pairs. N. Phytol. 2010, 189, 1135–1142. [Google Scholar] [CrossRef]
- Marler, T.E.; Dongol, N.; Cruz, G.N. Plastic responses mediated by identity recognition in below-ground competition in Cycas micronesica KD Hill. Trop. Conserv. Sci. 2016, 9, 648–657. [Google Scholar]
- Karban, R.; Shiojiri, K.; Ishizaki, S.; Wetzel, W.C.; Evans, R.Y. Kin recognition affects plant communication and defence. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123062. [Google Scholar] [CrossRef]
- Crepy, M.A.; Casal, J.J. Photoreceptor-mediated kin recognition in plants. N. Phytol. 2014, 205, 329–338. [Google Scholar] [CrossRef]
- Crepy, M.A.; Casal, J.J. Kin recognition by self-referent phenotype matching in plants. N. Phytol. 2015, 209, 15–16. [Google Scholar] [CrossRef]
- De Wit, M.; Kegge, W.; Evers, J.B.; Eijk, M.H.V.-V.; Gankema, P.; Voesenek, L.A.C.J.; Pierik, R. Plant neighbor detection through touching leaf tips precedes phytochrome signals. Proc. Natl. Acad. Sci. USA 2012, 109, 14705–14710. [Google Scholar] [CrossRef]
- Robson, P.R.; McCormac, A.C.; Irvine, A.S.; Smith, H. Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nat. Biotechnol. 1996, 14, 995–998. [Google Scholar] [CrossRef]
- Page, E.R.; Cerrudo, D.; Westra, P.; Loux, M.; Smith, K.; Foresman, C.; Wright, H.; Swanton, C.J. Why Early Season Weed Control Is Important in Maize. Weed Sci. 2012, 60, 423–430. [Google Scholar] [CrossRef]
- Page, E.R.; Tollenaar, M.; Lee, A.E.; Lukens, L.; Swanton, C.J. Does the shade avoidance response contribute to the critical period for weed control in maize (Zea mays)? Weed Res. 2009, 49, 563–571. [Google Scholar] [CrossRef]
- Wang, H.; Wu, G.; Zhao, B.; Wang, B.; Lang, Z.-H.; Zhang, C.; Wang, H. Regulatory modules controlling early shade avoidance response in maize seedlings. BMC Genom. 2016, 17, 269. [Google Scholar] [CrossRef] [PubMed]
- Lepik, A.; Abakumova, M.; Zobel, K.; Semchenko, M. Kin recognition is density-dependent and uncommon among temperate grassland plants. Funct. Ecol. 2012, 26, 1214–1220. [Google Scholar] [CrossRef]
- R. Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.r-project.org/ (accessed on 13 July 2020).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Shafii, B.; Price, W.J.; Swensen, J.B.; Murray, G.A. Nonlinear estimation of growth curve models for germination data analysis. In Proceedings of the 3rd Annual Conference Proceedings, Manhattan, KS, USA, 28–30 April 1991. [Google Scholar]
- Austin, P.C.; Hux, J.E. A brief note on overlapping confidence intervals. J. Vasc. Surg. 2002, 36, 194–195. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.; Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.3.4. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 8 July 2020).
- Martínez-García, J.F.; Galstyan, A.; Salla-Martret, M.; Cifuentes-Esquivel, N.; Gallemi, M.; Bou-Torrent, J. Regulatory Components of Shade Avoidance Syndrome. Adv. Bot. Res. 2010, 53, 65–116. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor Signaling Networks in Plant Responses to Shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Mizuno, T.; Oka, H.; Yoshimura, F.; Ishida, K.; Yamashino, T. Insight into the mechanism of end-of-day far-red light (EODFR)-induced shade avoidance responses in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2015, 79, 1987–1994. [Google Scholar] [CrossRef]
- Kasperbauer, M.J.; Karlen, D.L. Plant Spacing and Reflected Far-Red Light Effects on Phytochrome-Regulated Photosynthate Allocation in Corn Seedlings. Crop. Sci. 1994, 34, 1564–1569. [Google Scholar] [CrossRef]
- Neff, M.M.; Fankhauser, C.; Chory, J. Light: An indicator of time and place. Genes Dev. 2000, 14, 257–271. [Google Scholar] [PubMed]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic Efficiency and Phytochrome Photoequilibria Determination Using Spectral Data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Craig, D.S.; Runkle, E.S. A Moderate to High Red to Far-red Light Ratio from Light-emitting Diodes Controls Flowering of Short-day Plants. J. Am. Soc. Hortic. Sci. 2013, 138, 167–172. [Google Scholar] [CrossRef]
- Mullen, J.L.; Weinig, C.; Hangarter, R.P. Shade avoidance and the regulation of leaf inclination in Arabidopsis. Plant Cell Environ. 2006, 29, 1099–1106. [Google Scholar] [CrossRef]
- Pierik, R.; Cuppens, M.L.; Voesenek, L.A.; Visser, E.J.W. Interactions between Ethylene and Gibberellins in Phytochrome-Mediated Shade Avoidance Responses in Tobacco1. Plant Physiol. 2004, 136, 2928–2936. [Google Scholar] [CrossRef]
- Pierik, R.; Visser, E.J.W.; De Kroon, J.; Voesenek, L.A. Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ. 2003, 26, 1229–1234. [Google Scholar] [CrossRef]
- Millenaar, F.F.; Cox, M.C.; van Berkel, Y.E.D.J.; Welschen, R.A.; Pierik, R.; Voesenek, L.A.; Peeters, A.J. Ethylene-Induced Differential Growth of Petioles in Arabidopsis. Analyzing Natural Variation, Response Kinetics, and Regulation1. Plant Physiol. 2005, 137, 998–1008. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Cescatti, A.; Christian, R. Constraints on light interception efficiency due to shoot architecture in broad-leaved Nothofagus species. Tree Physiol. 2004, 24, 617–630. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf size and angle vary widely across species: What consequences for light interception? N. Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- James, S.A.; Bell, D.T. Leaf orientation, light interception and stomatal conductance of Eucalyptus globulus ssp. globulus leaves. Tree Physiol. 2000, 20, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Page, E.R.; Liu, W.; Cerrudo, D.; Lee, E.A.; Swanton, C.J. Shade Avoidance Influences Stress Tolerance in Maize. Weed Sci. 2011, 59, 326–334. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Tian, Y.; Xu, X.; Ouyang, H. Kin selection or resource partitioning for growing with siblings: Implications from measurements of nitrogen uptake. Plant Soil 2015, 398, 79–86. [Google Scholar] [CrossRef]
- Dudley, S.A.; File, A.L. Kin recognition in an annual plant. Biol. Lett. 2007, 3, 435–438. [Google Scholar] [CrossRef] [PubMed]
- File, A.L.; Klironomos, J.; Maherali, H.; Dudley, S.A. Plant Kin Recognition Enhances Abundance of Symbiotic Microbial Partner. PLoS ONE 2012, 7, e45648. [Google Scholar] [CrossRef]
- Semchenko, M.; Saar, S.; Lepik, A. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. N. Phytol. 2014, 204, 631–637. [Google Scholar] [CrossRef]
- Karban, R.; Wetzel, W.C.; Shiojiri, K.; Ishizaki, S.; Ramirez, S.R.; Blande, J.D. Deciphering the language of plant communication: Volatile chemotypes of sagebrush. N. Phytol. 2014, 204, 380–385. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).