Indoor Farming Marjoram Production—Quality, Resource Efficiency, and Potential of Application
Abstract
1. Introduction
2. Materials and Methods
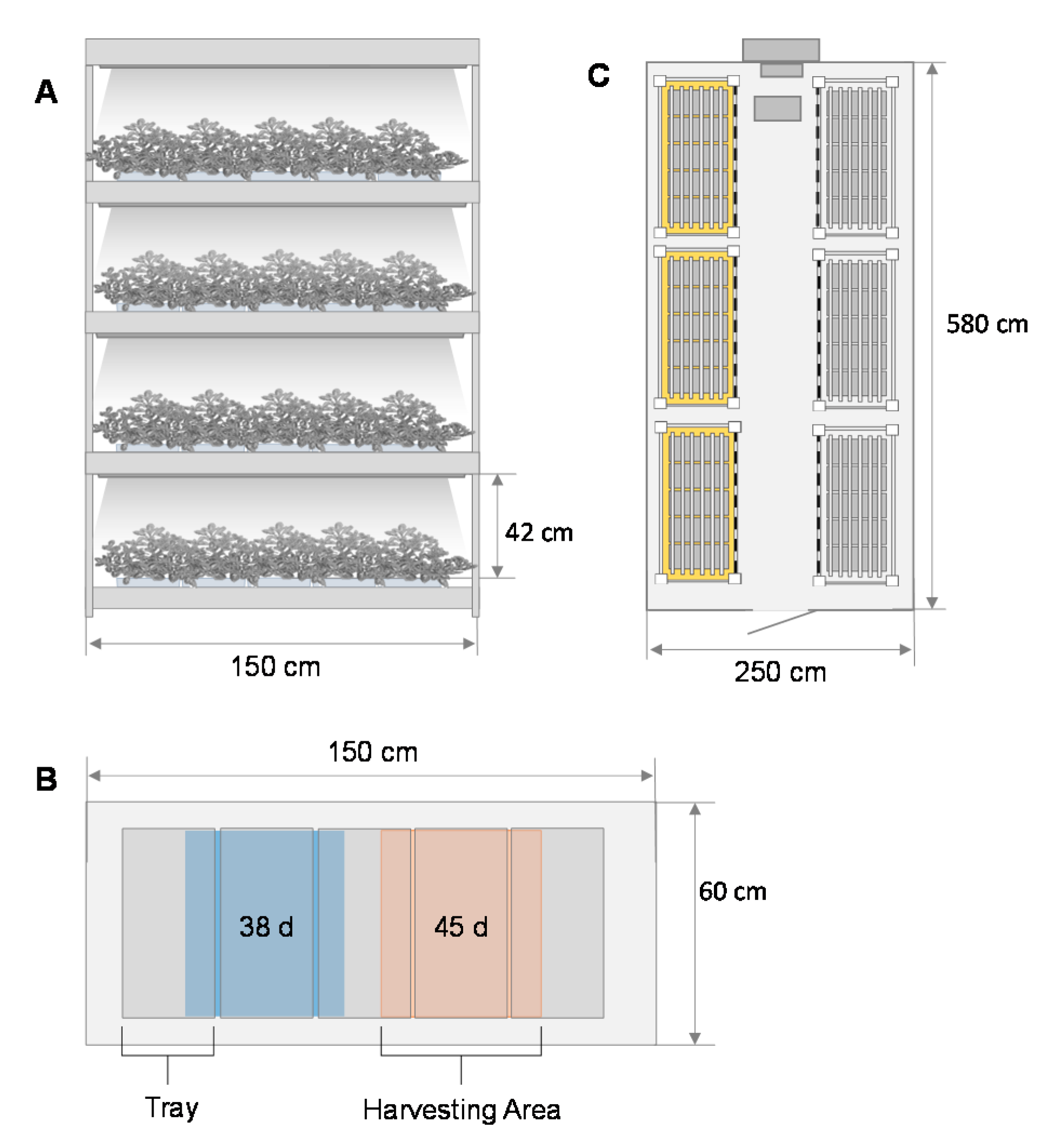
2.1. Plant Material and Culture Conditions
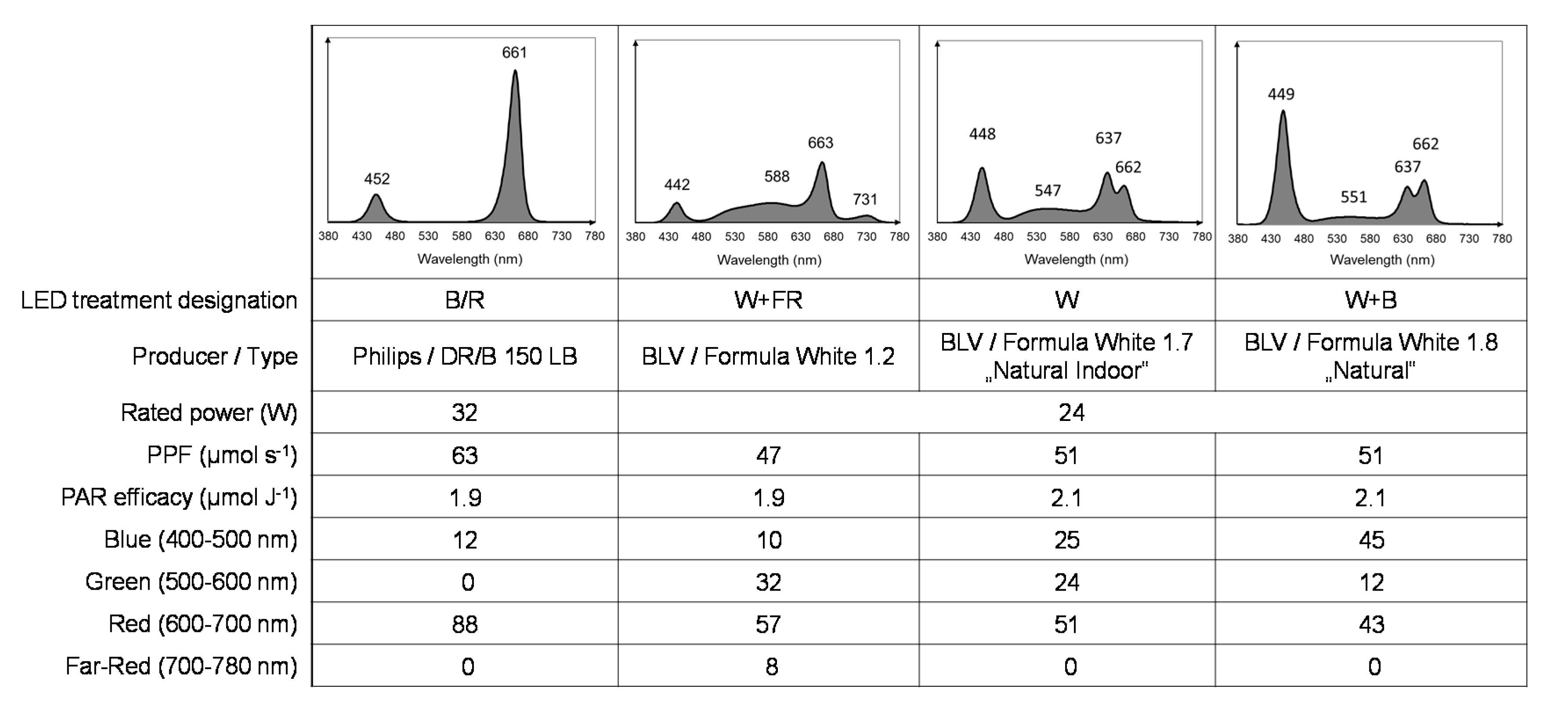
2.2. Light Treatment
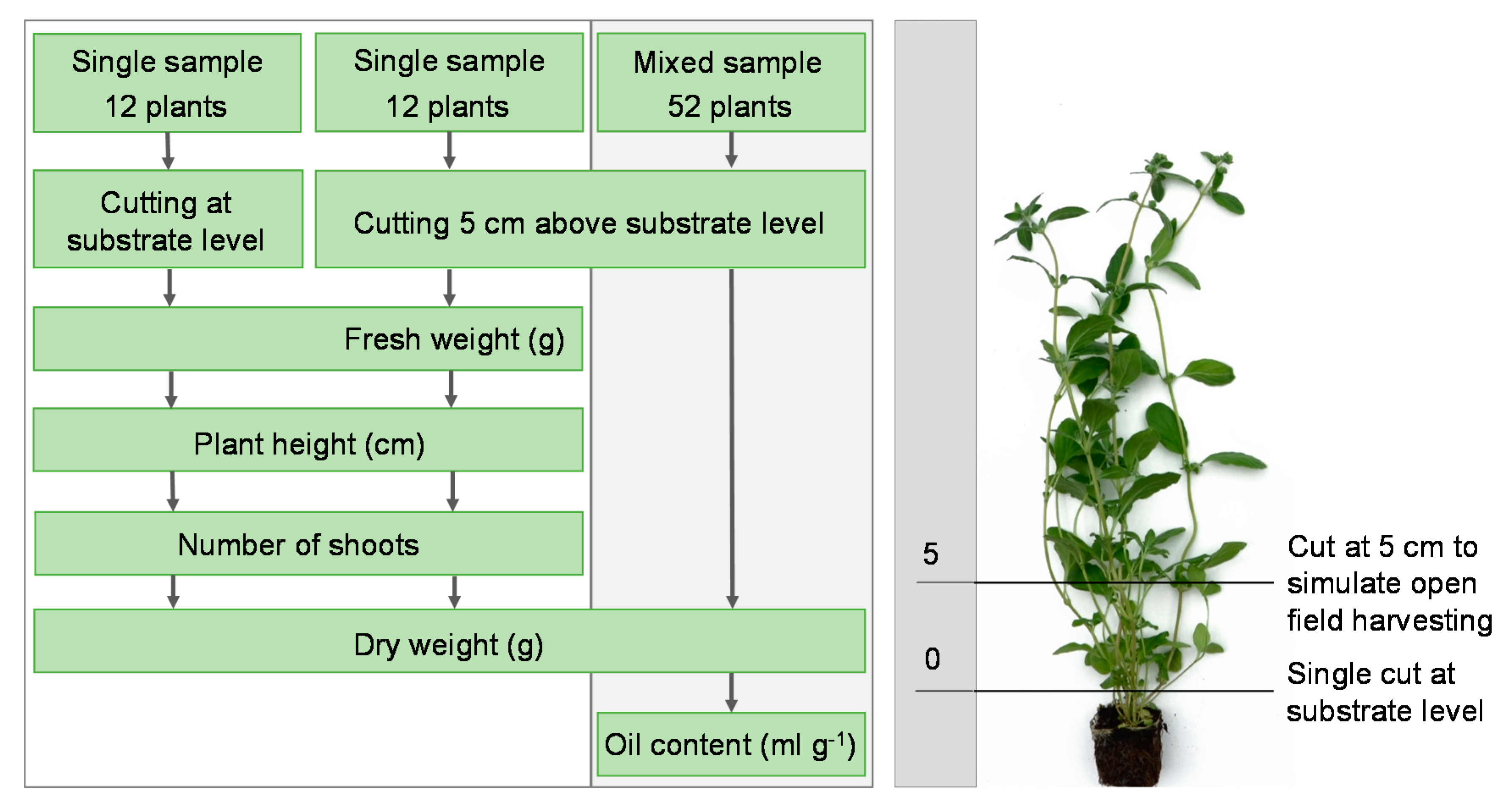
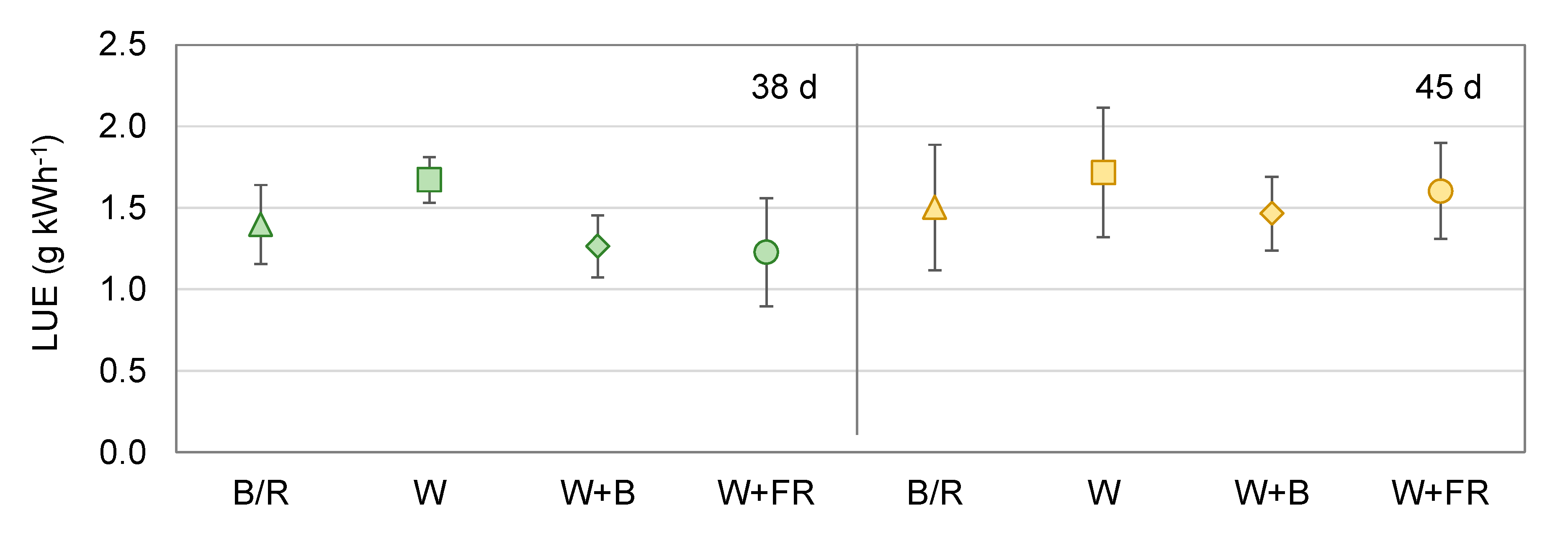
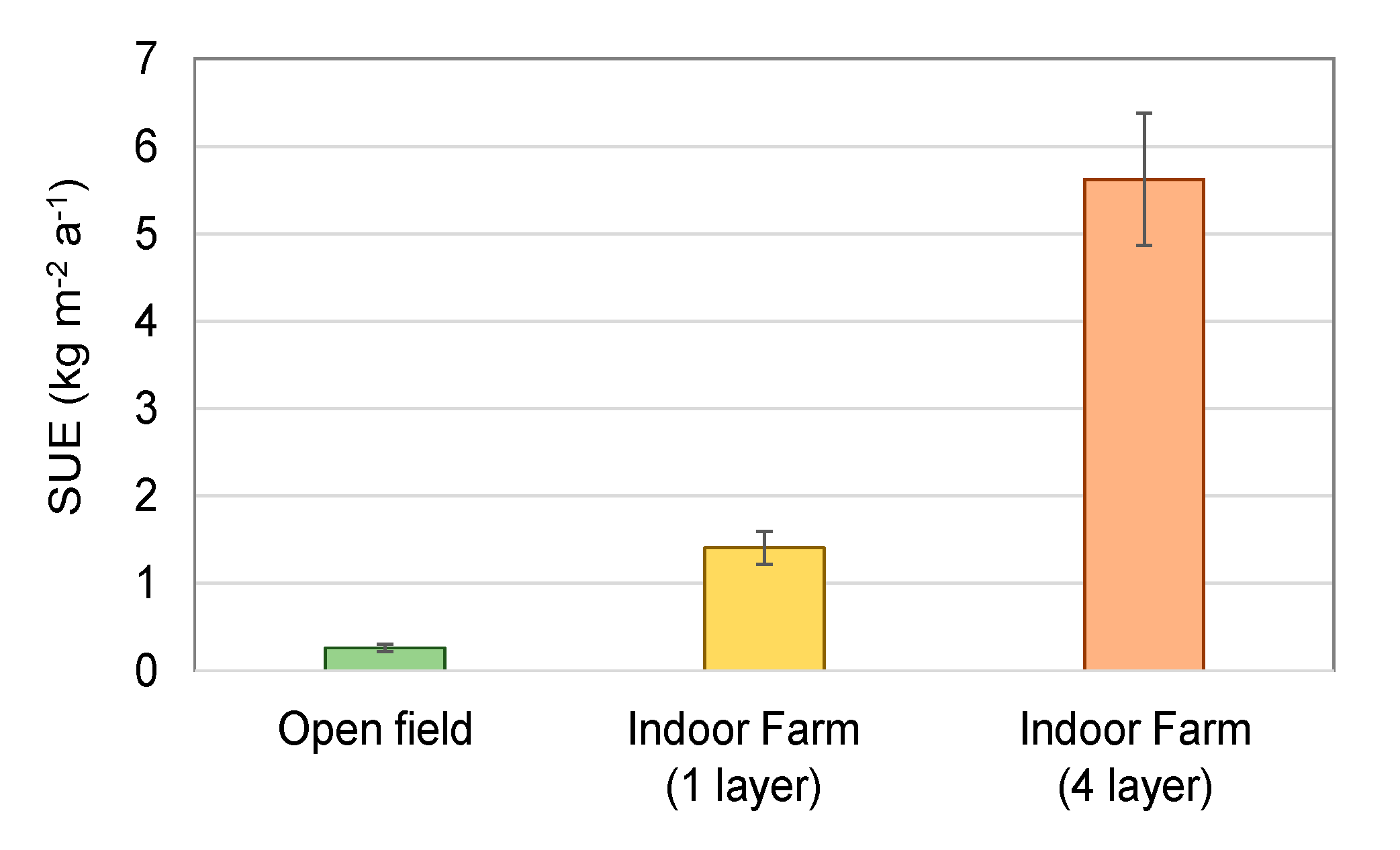
2.3. Cultivation Period, Growth Characteristics, and Resource Efficiency
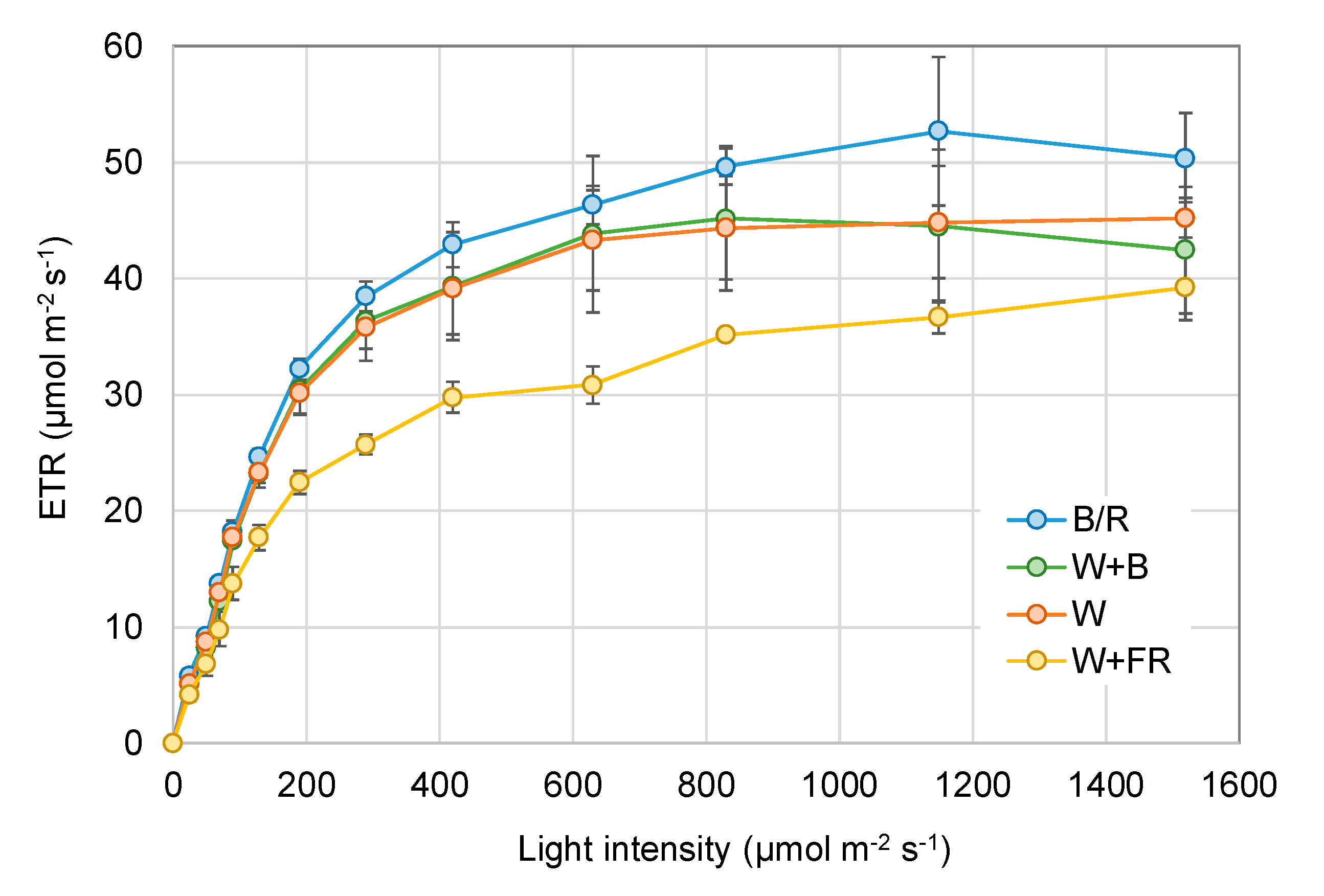
2.4. Chlorophyllfluorescence Measurements
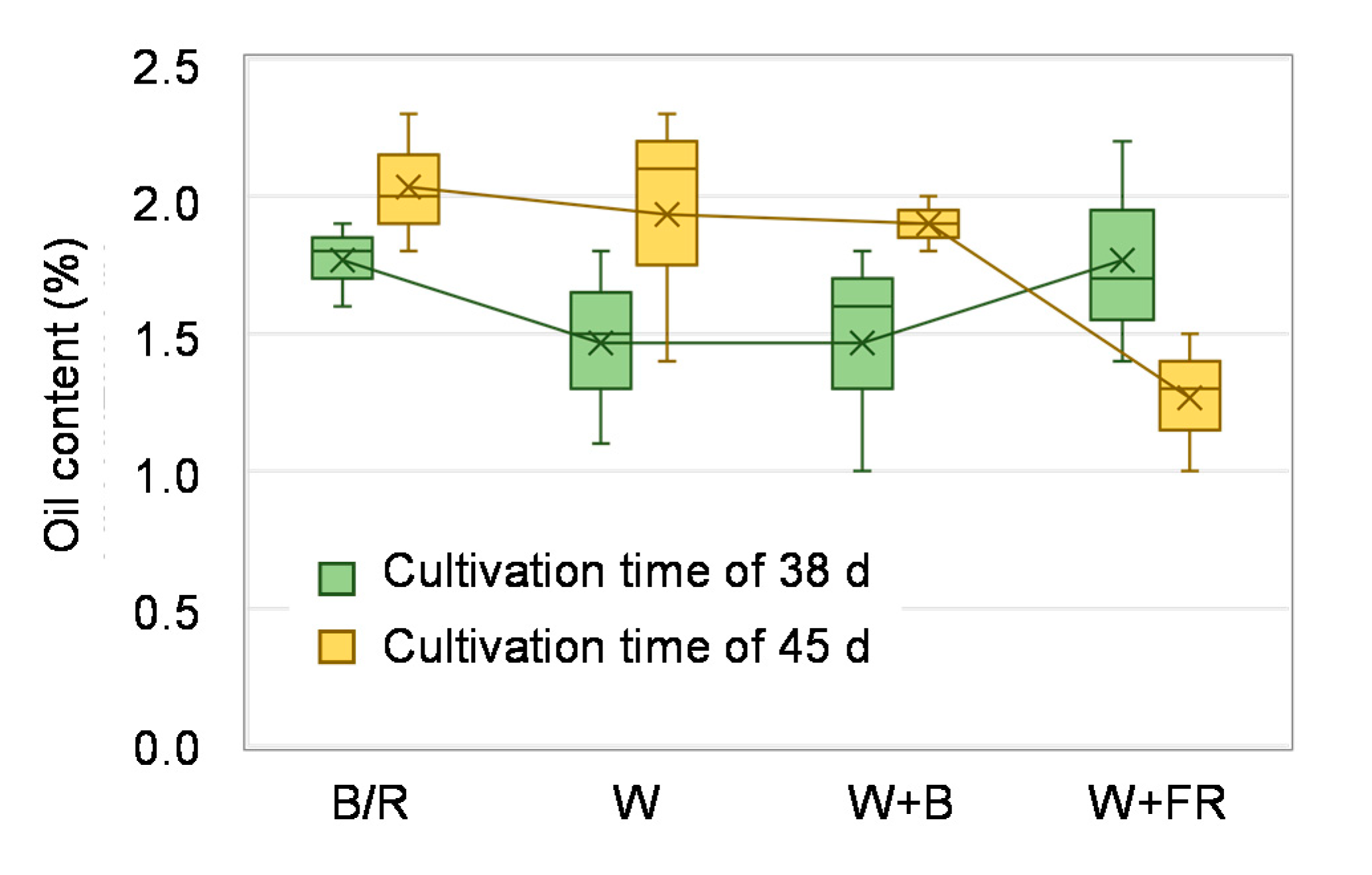
2.5. Essential Oil Content
2.6. Statistical Analysis
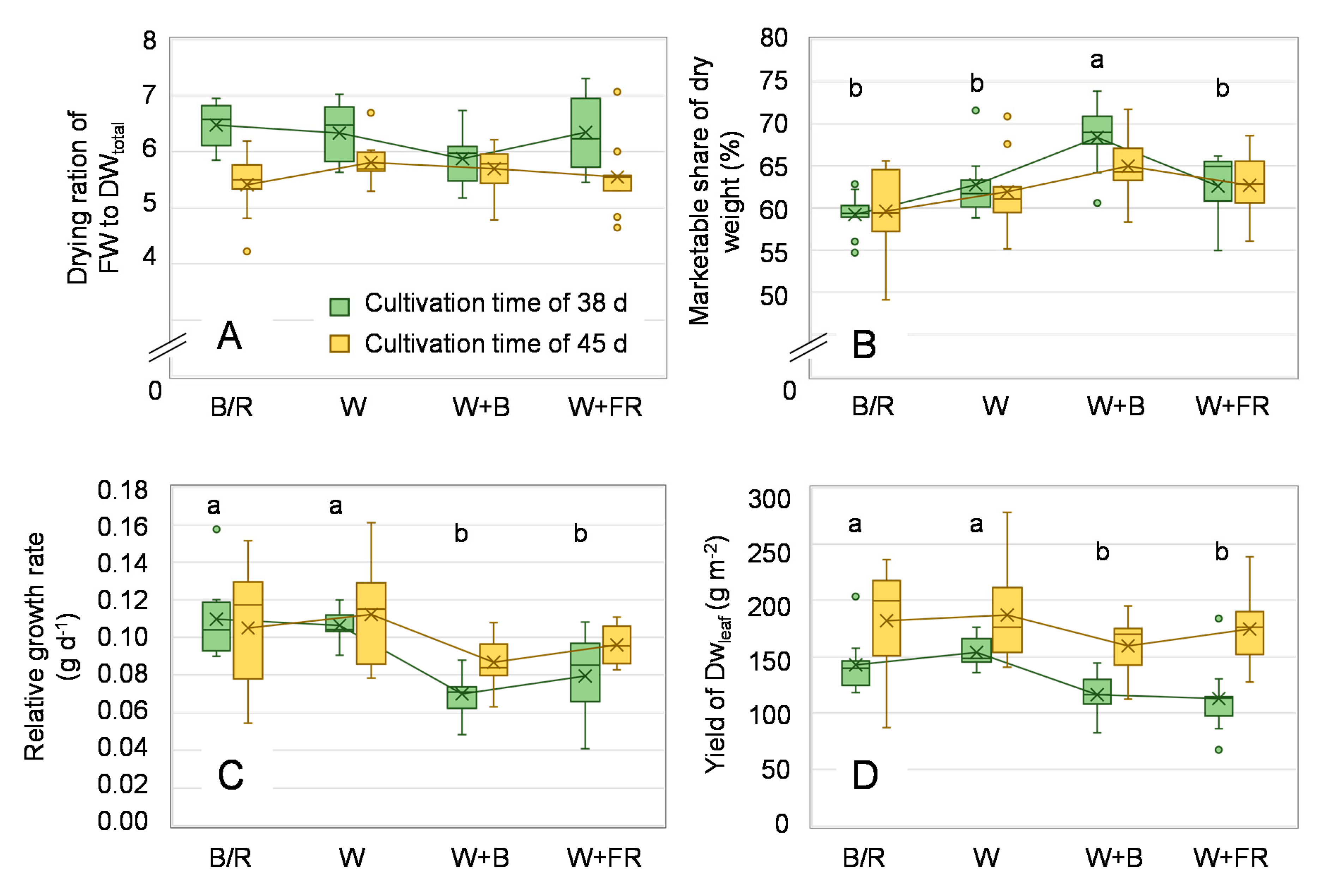
3. Results
4. Discussion
4.1. Growth Characteristics and Achievable Yields
4.2. Essential oil Content and Composition
4.3. Ressource Use Efficiency
4.4. Application
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nurzyńska-Wierdak, R.; Dzida, K. Influence of plant density and term of harvest on yield and chemical composition of sweet marjoram (Origanum majorana L.). Acta Sci. Pol. Hortorum Cultus 2009, 8, 51–61. [Google Scholar]
- Zawiślak, G.; Dzida, K. Yield and quality of sweet marjoram herb depending on harvest time. Acta Sci. Pol. Hortorum Cultus 2010, 9, 65–72. [Google Scholar]
- Zawiślak, G. Dependence on harvest date and yielding of Marjoram (Origanum majorana L.) cv. ‘Miraż’ cultivated from a seedling. Acta Sci. Pol. Hortorum Cultus 2008, 7, 7. [Google Scholar]
- Anbauverfahren Majoran. Available online: https://publikationen.sachsen.de/bdb/artikel/13856 (accessed on 5 April 2020).
- Estévez, M.; Ventanas, S.; Ramírez, R.; Cava, R. Influence of the addition of rosemary essential oil on the volatiles pattern of porcine frankfurters. J. Agric. Food Chem. 2005, 53, 8317–8324. [Google Scholar] [CrossRef]
- Banchio, E.; Bogino, P.C.; Zygadlo, J.; Giordano, W. Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 2008, 36, 766–771. [Google Scholar] [CrossRef]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind. Crop. Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Vági, E.; Simándi, B.; Suhajda, A.; Hethelyi, E. Essential oil composition and antimicrobial activity of Origanum majorana L. extracts obtained with ethyl alcohol and supercritical carbon dioxide. Food Res. Int. 2005, 38, 51–57. [Google Scholar] [CrossRef]
- Vera, R.R.; Chane-Ming, J. Chemical composition of the essential oil of marjoram (Origanum majorana L.) from Reunion Island. Food Chem. 1999, 66, 143–145. [Google Scholar] [CrossRef]
- Trivino, M.G.; Johnson, C.B. Season has a Major Effect on the Essential oil Yield response to Nutrient Supply in Origanum Majorana. J. Hortic. Sci. Biotechnol. 2000, 75, 520–527. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Nieto-Garibay, A.; López-Aguilar, R.; Troyo-Diéguez, E.; Rueda-Puente, E.O.; Flores-Hernández, A.; Ruiz-Espinoza, F.H. Physiological, morphometric characteristics and yield of Origanum vulgare L. and Thymus vulgaris L. exposed to open-field and shade-enclosure. Ind. Crop. Prod. 2013, 49, 659–667. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. (Eds.) Plant Factory. An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Academic Press, an Imprint of Elsevier: London, UK, 2020. [Google Scholar]
- Graamans, L.; Baeza, E.; van den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant factories versus greenhouses: Comparison of resource use efficiency. Agric. Syst. 2018, 160, 31–43. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red: Blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Yamaguchi, J.; Enjoji, A. Resource Utilization Efficiencies in a Closed System with Artificial Lighting during Continuous Lettuce Production. Agronomy 2020, 10, 723. [Google Scholar] [CrossRef]
- Nguyen, D.T.P.; Kitayama, M.; Lu, N.; Takagaki, M. Improving secondary metabolite accumulation, mineral content, and growth of coriander (Coriandrum sativum L.) by regulating light quality in a plant factory. J. Hortic. Sci. Biotechnol. 2020, 95, 356–363. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Aldarkazali, M.; Mohamed, S.J.; McMulkin, N.B.; Jbara, M.H.; Fuller, M.P. A Novel New Light Recipe Significantly Increases the Growth and Yield of Sweet Basil (Ocimum basilicum) Grown in Plant Factory System. Agronomy 2020, 10, 934. [Google Scholar] [CrossRef]
- Kalantari, F.; Mohd Tahir, O.; Mahmoudi Lahijani, A.; Kalantari, S. A Review of Vertical Farming Technology: A Guide for Implementation of Building Integrated Agriculture in Cities. AEF Adv. Eng. Forum 2017, 24, 76–91. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C. Unraveling the role of red: Blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Schwieterman, M.L.; Abrahan, C.E.; Colquhoun, T.A.; Folta, K.M. Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Front. Plant Sci. 2016, 7, 1328. [Google Scholar] [CrossRef]
- Schwend, T.; Prucker, D.; Peisl, S.; Nitsopoulos, A.; Mempel, H. The rosmarinic acid content of basil and borage correlates with the ratio of red and far-red light. Eur. J. Hortic. Sci. 2016, 81, 243–247. [Google Scholar] [CrossRef]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2000, 23, 1397–1405. [Google Scholar] [CrossRef]
- DIN EN ISO 6571. Gewürze, Würzende Zutaten und Kräuter—Bestimmung des Ätherischen Ölgehalts (Wasserdampfdestillationsverfahren) (ISO 6571:2008 + Amd 1:2017); Deutsche Fassung EN ISO 6571:2009 + A1:2017; DIN Deutsches Institut für Normung e.V.: Berlin, Germany, 2018. [Google Scholar]
- Galambosi, B.; Galambosi, Z.; Pessala, R.; Hupila, I.; Aflatuni, A.; Repcak, M.; Svoboda, P.K. Yield and Quality of Selected Herb Cultivars in Finland. Acta Hortic. 2002, 139–149. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. Horts 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Seidler-Łożykowska, K.; Golcz, A.; Wójcik, J. Yield and quality of sweet basil, savory, marjoram and thyme raw materials from organic cultivation on the composted manure. J. Res. Appl. Agric. Eng. 2008, 53, 63–66. [Google Scholar]
- Nurzyñska-Wierdak, R.; Zawislak, G.; Kowalski, R. The Content and Composition of Essential Oil of Origanum majorana L. Grown in Poland Depending on Harvest Tme and Method of Raw Material Preparation. J. Essent. Oil Bear. Plants 2015, 18, 1482–1489. [Google Scholar] [CrossRef]
- Circella, G.; Franz, C.; Novak, J.; Resch, H. Influence of day length and leaf insertion on the composition of marjoram essential oil. Flavour Fragr. J. 1995, 10, 371–374. [Google Scholar] [CrossRef]
- Davidenco, V.; Argüello, J.A.; Piccardi, M.B.; Vega, C.R.C. Day length modulates precocity and productivity through its effect on developmental rate in Origanum vulgare ssp. Sci. Hortic. 2017, 218, 164–170. [Google Scholar] [CrossRef]
- Kizil, S.; Toncer, O. Influence of different harvest times on the yield and oil composition of spearmint (Mentha spicata L. var. spicata). J. Food Agric. Environ. 2006, 4, 135. [Google Scholar]
- Saeb, K.; Gholamrezaee, S. Variation of essential oil composition of Melissa officinalis L. leaves during different stages of plant growth. Asian Pac. J. Trop. Biomed. 2012, 2, S547–S549. [Google Scholar] [CrossRef]
- Yosr, Z.; Hnia, C.; Rim, T.; Mohamed, B. Changes in essential oil composition and phenolic fraction in Rosmarinus officinalis L. var. typicus Batt. organs during growth and incidence on the antioxidant activity. Ind. Crop. Prod. 2013, 43, 412–419. [Google Scholar] [CrossRef]
- Alizadeh, A.; Khosh-khui, M.; Javidnia, K.; Firuzi, O.; Jokar, S.M. Chemical Composition of the Essential Oil, Total Phenolic Content and Antioxidant Activity in Origanum majorana L. (Lamiaceae) Cultivated in Iran. Adv. Environ. Biol. 2011, 5, 2326–2331. [Google Scholar]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Montesano, F.F.; van Iersel, M.W.; Boari, F.; Cantore, V.; D’Amato, G.; Parente, A. Sensor-based irrigation management of soilless basil using a new smart irrigation system: Effects of set-point on plant physiological responses and crop performance. Agric. Water Manag. 2018, 203, 20–29. [Google Scholar] [CrossRef]
- Ekren, S.; Sönmez, Ç.; Özçakal, E.; Kurttaş, Y.S.K.; Bayram, E.; Gürgülü, H. The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). Agric. Water Manag. 2012, 109, 155–161. [Google Scholar] [CrossRef]
- Mempel, H.; Jüttner, I.; Wittmann, S. The potentials of Indoor Farming for plant production. In at-Automatisierungstechnik; De Gruyter: Berlin, Germany, 2021; accepted. [Google Scholar]
- CUBES Circle. Available online: https://www.cubescircle.de/ (accessed on 31 October 2020).

| Treatment Code | Cultivation Time (d) | Shoot Length (cm) | FW (g plant−1) | DWtotal (g plant−1) | DWleaf (g plant−1) | DWstem (g plant−1) |
|---|---|---|---|---|---|---|
| B/R | 38 | 32.3 ± 3.1 a | 4.16 ± 0.81 a | 0.64 ± 0.09 a | 0.38 ± 0.07 ab | 0.26 ± 0.03 a |
| W | 31.2 ± 3.1 ab | 4.03 ± 0.35 a | 0.65 ± 0.04 a | 0.41 ± 0.03 a | 0.25 ± 0.03 a | |
| W+B | 22.9 ± 2.5 c | 2.65 ± 0.46 b | 0.45 ± 0.07 b | 0.30 ± 0.04 b | 0.14 ± 0.03 b | |
| W+FR | 28.0 ± 4.4 b | 3.01 ± 0.83 b | 0.48 ± 0.14 b | 0.30 ± 0.08 b | 0.18 ± 0.06 b | |
| B/R | 45 | 34.3 ± 6.1 A | 4.40 ± 1.38 A | 0.82 ± 0.22 A | 0.48 ± 0.13 A | 0.34 ± 0.10 A |
| W | 34.5 ± 4.2 A | 4.70 ± 1.20 A | 0.81 ± 0.20 A | 0.49 ± 0.12 A | 0.31 ± 0.09 A | |
| W+B | 27.4 ± 3.1 B | 3.64 ± 0.63 A | 0.66 ± 0.12 A | 0.42 ± 0.06 A | 0.24 ± 0.06 A | |
| W+FR | 32.9 ± 3.9 AB | 4.02 ± 0.43 A | 0.74 ± 0.11 A | 0.46 ± 0.09 A | 0.27 ± 0.04 A |
| Treatment Code | Flowering Stage after 38 d | Flowering Stage after 45 d |
|---|---|---|
| B/R | Budding stage | First flowers open |
| W | Budding stage | First flowers open |
| W+B | Budding stage | First flowers open |
| W+FR | First flowers open | Full-flowering stage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittmann, S.; Jüttner, I.; Mempel, H. Indoor Farming Marjoram Production—Quality, Resource Efficiency, and Potential of Application. Agronomy 2020, 10, 1769. https://doi.org/10.3390/agronomy10111769
Wittmann S, Jüttner I, Mempel H. Indoor Farming Marjoram Production—Quality, Resource Efficiency, and Potential of Application. Agronomy. 2020; 10(11):1769. https://doi.org/10.3390/agronomy10111769
Chicago/Turabian StyleWittmann, Sabine, Ivonne Jüttner, and Heike Mempel. 2020. "Indoor Farming Marjoram Production—Quality, Resource Efficiency, and Potential of Application" Agronomy 10, no. 11: 1769. https://doi.org/10.3390/agronomy10111769
APA StyleWittmann, S., Jüttner, I., & Mempel, H. (2020). Indoor Farming Marjoram Production—Quality, Resource Efficiency, and Potential of Application. Agronomy, 10(11), 1769. https://doi.org/10.3390/agronomy10111769






