The Use of Red Shade Nets Improves Growth in Salinized Pepper (Capsicum annuum L.) Plants by Regulating Their Ion Homeostasis and Hormone Balance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Treatments
2.2. Plant Growth-Related Determinations
2.3. Gas Exchange Measurements
2.4. Leaf Water Potential, Osmotic Potential and Relative Water Content
2.5. Chlorophyll Analysis
2.6. Ion Determinations
2.7. Hormone Extraction and Analysis
2.8. Statistical Analysis
3. Results
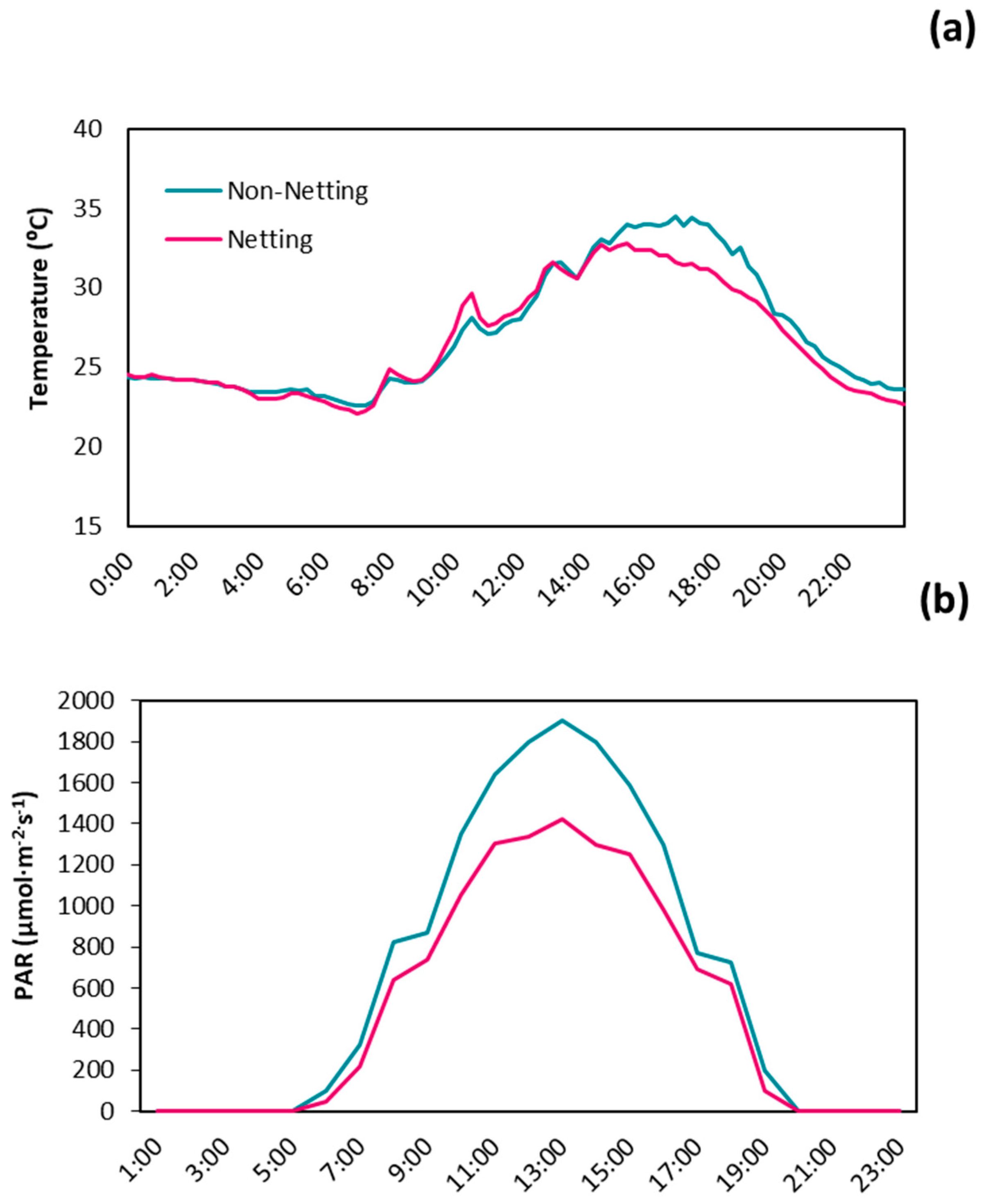
3.1. Microclimate Conditions
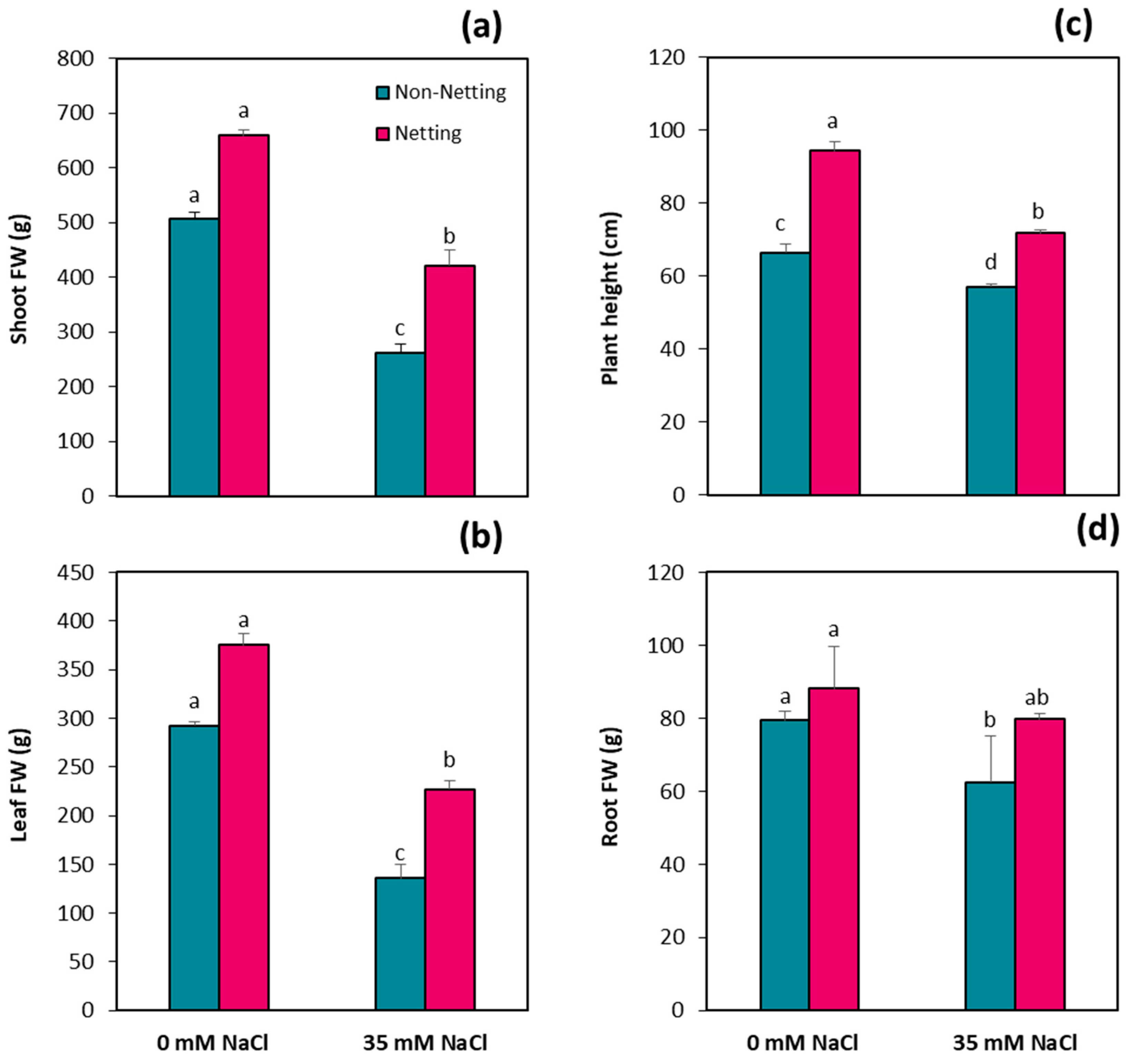
3.2. Plant Growth Parameters
3.3. Leaf Gas Exchange Parameters
3.4. Plant Water Relations
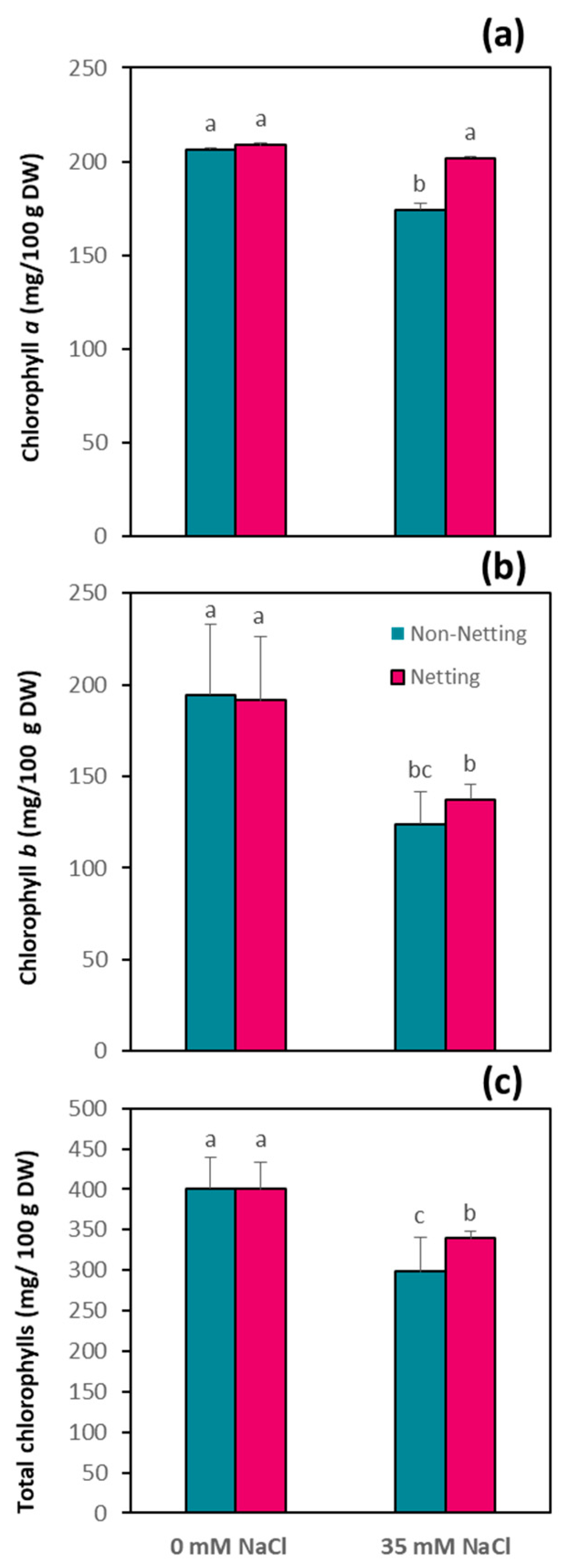
3.5. Chlorophyll Content
3.6. Mineral Composition
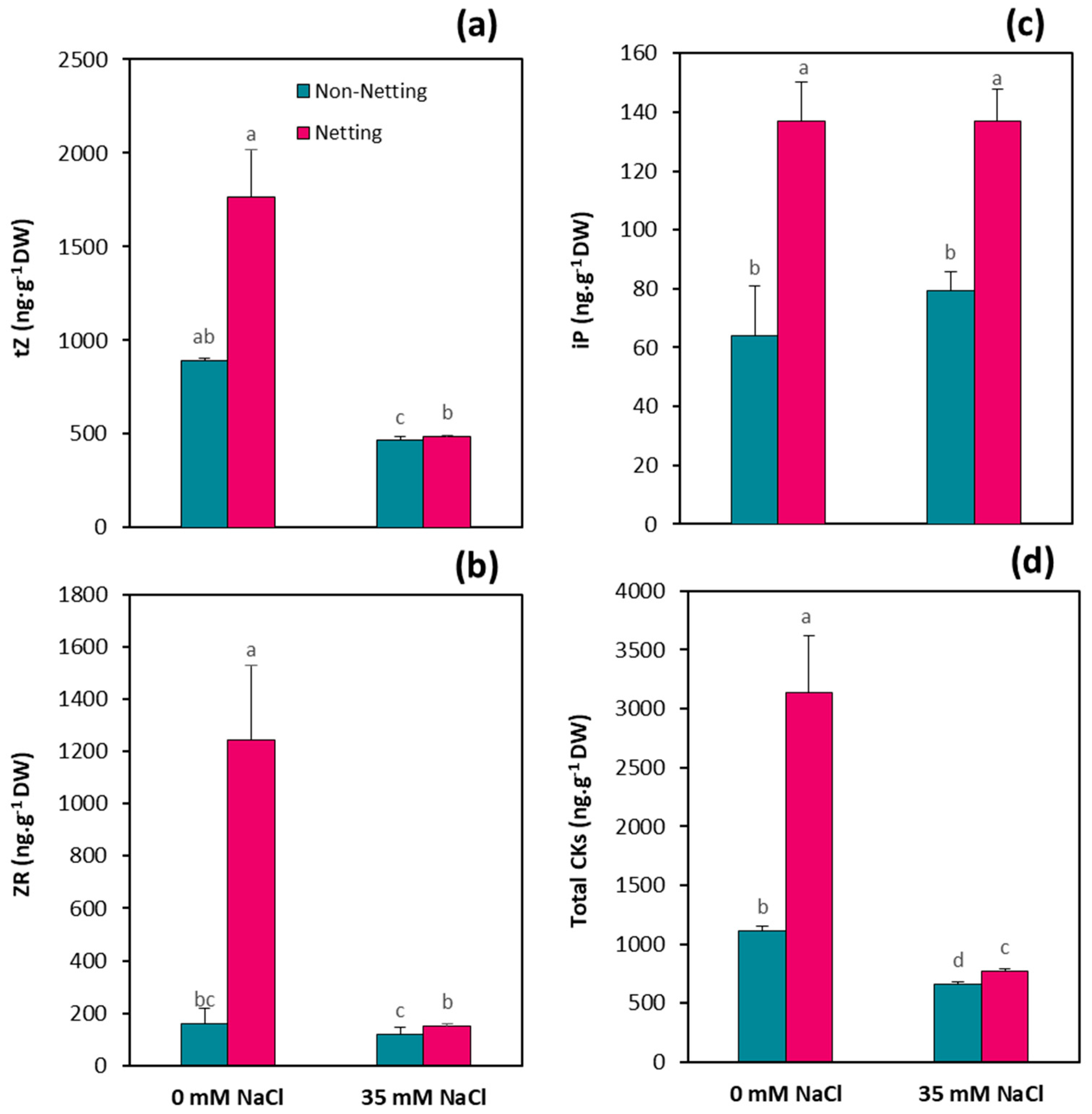
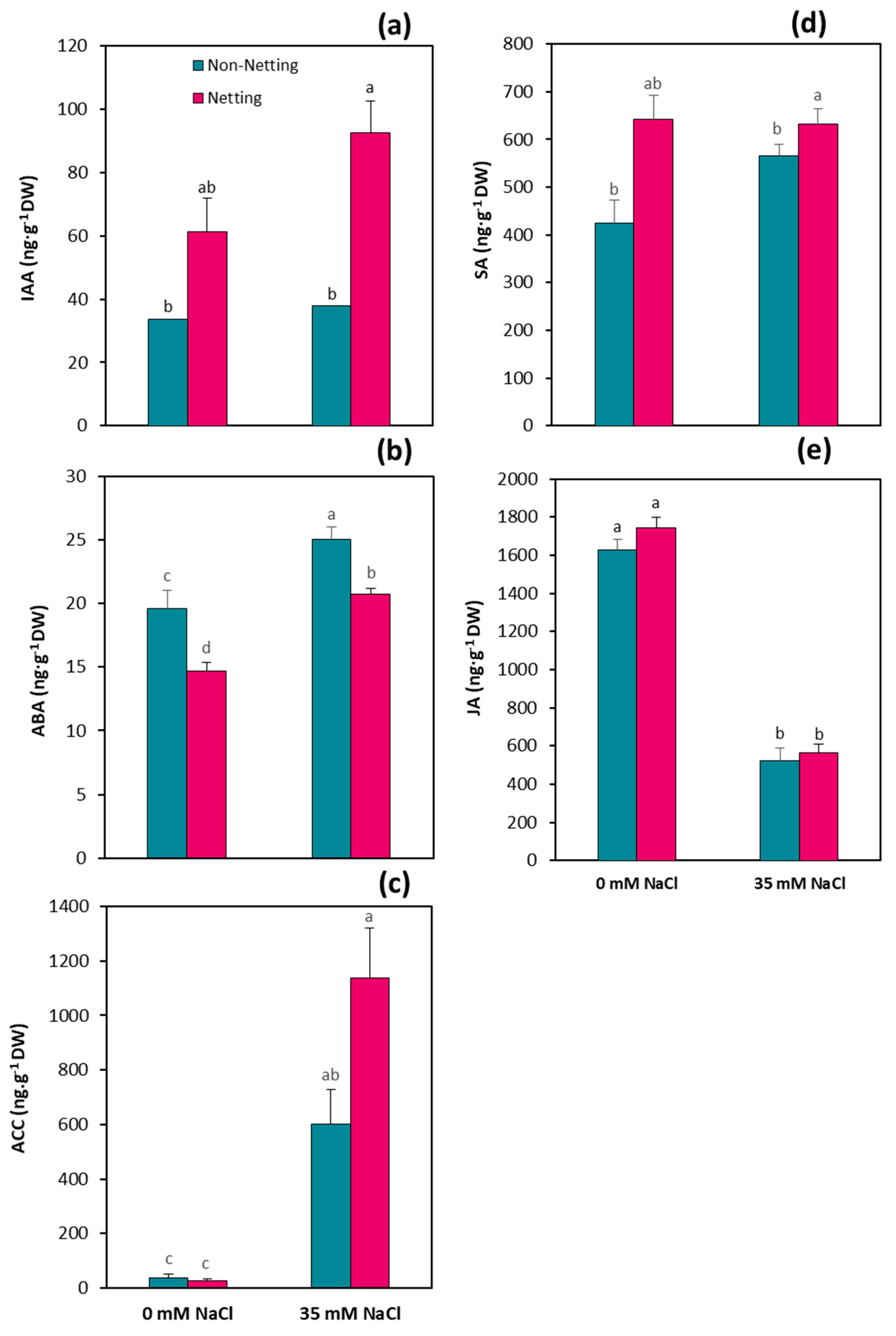
3.7. Hormone Concentrations
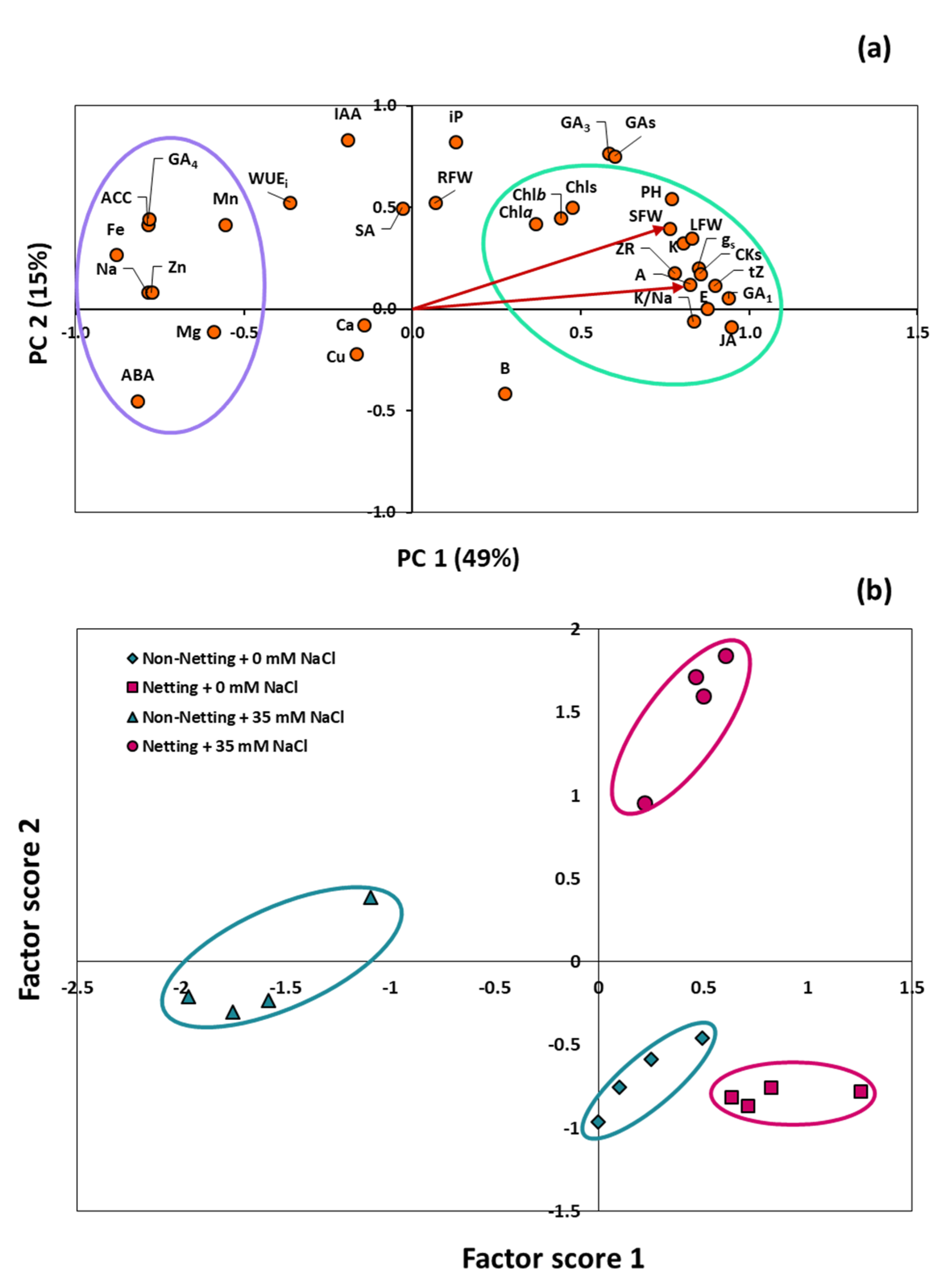
3.8. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, C.R.; Ørum, J.E.; Pedersen, S.M.; Andersen, M.N.; Plauborg, F.; Liu, F.; Jacobsen, S.-E. A Short Overview of Measures for Securing Water Resources for Irrigated Crop Production. J. Agron. Crop Sci. 2014, 200, 333–343. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Piñero, M.C.; Pérez-Jiménez, M.; López-Marín, J.; del Amor, F.M. Changes in the salinity tolerance of sweet pepper plants as affected by nitrogen form and high CO2 concentration. J. Plant Physiol. 2016, 200, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Martínez-Andújar, C.; Pérez-Alfocea, F. Hormonal and metabolic regulation of source–sink relations under salinity and drought: From plant survival to crop yield stability. Biotechnol. Adv. 2014, 32, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Dajic, Z. Salt Stress. In Physiology and Molecular Biology of Stress Tolerance in Plants; Madhava Rao, K.V., Raghavendra, A.S., Janardhan Reddy, K., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 41–99. ISBN 978-1-4020-4225-6. [Google Scholar]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem, M.E.E.; Martinez-Andújar, C.; Albacete, A.; Pospíšilová, H.; Dodd, I.C.C.; Pérez-Alfocea, F.; Lutts, S. Nitrogen Form Alters Hormonal Balance in Salt-treated Tomato (Solanum lycopersicum L.). J. Plant Growth Regul. 2011, 30, 144–157. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Atri, N. Impact of salinity stress in crop plants and mitigation strategies. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 49–63. ISBN 978-981-15-1322-0. [Google Scholar]
- Alarcón, J.J.; Ortuño, M.F.; Nicolás, E.; Navarro, A.; Torrecillas, A. Improving water-use efficiency of young lemon trees by shading with aluminised-plastic nets. Agric. Water Manag. 2006, 82, 387–398. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P. Moderate shade can increase net gas exchange and reduce photoinhibition in citrus leaves. Tree Physiol. 2003, 23, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Nicolás, E.; Barradas, V.L.; Ortuño, M.F.; Navarro, A.; Torrecillas, A.; Alarcón, J.J. Environmental and stomatal control of transpiration, canopy conductance and decoupling coefficient in young lemon trees under shading net. Environ. Exp. Bot. 2008, 63, 200–206. [Google Scholar] [CrossRef]
- Manja, K.; Aoun, M. The use of nets for tree fruit crops and their impact on the production: A review. Sci. Hortic. 2019, 246, 110–122. [Google Scholar] [CrossRef]
- López-Marín, J.; Gálvez, A.; Otálora, G.; del Amor, F.M. Photoselective shade nets for pepper cultivation in southeastern Spain. Acta Hortic. 2019, 1252, 183–189. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Devlin, P.F.; Christie, J.M.; Terry, M.J. Many hands make light work. J. Exp. Bot. 2007, 58, 3071–3077. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Seaton, D.D.; Krahmer, J.; Halliday, K.J. Photoreceptor effects on plant biomass, resource allocation, and metabolic state. Proc. Natl. Acad. Sci. USA 2016, 113, 7667–7672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devlin, P.F. Plants wait for the lights to change to red. Proc. Natl. Acad. Sci. USA 2016, 113, 7301–7303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, J.E.; Sullivan, S.; Hermanowicz, P.; Petersen, J.; Diaz-Ramos, L.A.; Hoey, D.J.; Łabuz, J.; Christie, J.M. Engineering the phototropin photocycle improves photoreceptor performance and plant biomass production. Proc. Natl. Acad. Sci. USA 2019, 116, 12550–12557. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Agrawal, S.B.; Agrawal, M. UVR8 mediated plant protective responses under low UV-B radiation leading to photosynthetic acclimation. J. Photochem. Photobiol. B Biol. 2014, 137, 67–76. [Google Scholar] [CrossRef]
- Tossi, V.E.; Regalado, J.J.; Iannicelli, J.; Laino, L.E.; Burrieza, H.P.; Escandón, A.S.; Pitta-Álvarez, S.I. Beyond Arabidopsis: Differential UV-B response mediated by UVR8 in diverse species. Front. Plant Sci. 2019, 10, 780. [Google Scholar] [CrossRef]
- Rajapakse, N.C.; Shahak, Y. Light-Quality Manipulation by Horticulture Industry. In Light and Plant Development; Whitelam, G.C., Halliday, K.J., Eds.; Blackwell Publishing Ltd: Oxford, UK, 2007; pp. 290–312. ISBN 9780470988893. [Google Scholar]
- Kong, Y.; Avraham, L.; Ratner, K.; Shahak, Y. Response of photosynthetic parameters of sweet pepper leaves to light quality manipulation by photoselective shade nets. Acta Hortic. 2012, 956, 501–506. [Google Scholar] [CrossRef]
- Shahak, Y.; Gussakovsky, E.E.; Cohen, Y.; Lurie, S.; Stern, R.; Kfir, S.; Naor, A.; Atzmon, I.; Doron, I.; Greenblat-Avron, Y. Colornets: A new approach for light manipulation in fruit trees. Acta Hortic. 2004, 636, 609–616. [Google Scholar] [CrossRef]
- Rajapakse, N.C.; Shahak, Y. Light-quality manipulation by horticulture industry. In Annual Plant Reviews Online; Roberts, J.A., Ed.; Wiley Online Library: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Dovjek, I.; Nemera, D.B.; Wachsmann, Y.; Shlizerman, L.; Ratner, K.; Kamara, I.; Morozov, M.; Charuvi, D.; Shahak, Y.; Cohen, S.; et al. Top netting as a practical tool to mitigate the effect of climate change and induce productivity in citrus: Summary of experiments using photo-selective nets. Acta Hortic. 2020, 1268, 265–270. [Google Scholar] [CrossRef]
- Shahak, Y.; Yehezkel, H.; Matan, E. Colored shade nets improve production in bell peppers. Gan Sade Vameshek 2006, 4, 37–40. [Google Scholar]
- Santana, J.Q.; Balbino, M.A.; Tavares, T.R.; Bezerra, R.S.; Farias, J.G.; Ferreira, R.C. Effect of photoselective screens in the development and productivity of red and yellow sweet peper. Acta Hortic. 2012, 956, 493–500. [Google Scholar] [CrossRef]
- Santos, T.M.; Ferreira, R.C.; Seleguini, A. Growing tomatoes under a red photoselective screenhouse with different management strategies. Acta Hortic. 2017, 1170, 381–388. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Stanojević, L.; Cvetković, D.; Fallik, E. Effects of the modification of light intensity by color shade nets on yield and quality of tomato fruits. Sci. Hortic. 2012, 139, 90–95. [Google Scholar] [CrossRef]
- Mashabela, N.M.; Sivakumar, D.; Soundy, P. Variation in fruit quality in HTSP-5 green bell pepper grown under different photo-selective nets. Acta Hortic. 2016, 1123, 61–68. [Google Scholar] [CrossRef]
- Ghanem, M.E.M.E.E.; Albacete, A.; Martínez-Andújar, C.; Acosta, M.; Romero-Aranda, R.; Dodd, I.C.I.C.; Lutts, S.; Pérez-Alfocea, F. Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J. Exp. Bot. 2008, 59, 3039–3050. [Google Scholar] [CrossRef] [Green Version]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef]
- Albacete, A.; Martínez-Andújar, C.; Ghanem, M.E.; Acosta, M.; Sánchez-Bravo, J.; Asins, M.j.; Cuartero, J.; Lutts, S.; Doss, I.c.; Pérez-Alfocea, F. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato. Plant Cell Environ. 2009, 32, 928–938. [Google Scholar] [CrossRef]
- Ghanem, M.E.E.; Albacete, A.; Smigocki, A.C.C.; Frébort, I.; Pospisilová, H.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Lutts, S.; Dodd, I.C.C.; et al. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2011, 62, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Cantero-Navarro, E.; Balibrea, M.E.M.E.; Großkinsky, D.K.D.K.; de la Cruz González, M.; Martínez-Andújar, C.; Smigocki, A.C.A.C.; Roitsch, T.; Pérez-Alfocea, F. Hormonal and metabolic regulation of tomato fruit sink activity and yield under salinity. J. Exp. Bot. 2014, 65, 6081–6095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Carlos, D.O.; Bárbara, H.; Vicent, A.; Aurelio, G.-C. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef]
- Muñoz-Espinoza, V.A.; López-Climent, M.F.; Casaretto, J.A.; Gómez-Cadenas, A. Water stress responses of tomato mutants impaired in hormone biosynthesis reveal abscisic acid, jasmonic acid and salicylic acid Interactions. Front. Plant Sci. 2015, 6, 997. [Google Scholar] [CrossRef] [Green Version]
- Priel, A. Coloured nets can replace chemical growth regulators. FlowerTECH 2001, 4, 12–13. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. FAO Irrigation and Drainage Paper 29 Rev. 1; Food and Agricultural Organization: Rome, Italy, 1985. [Google Scholar]
- Machado, R.M.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Wungrampha, S.; Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Photosynthesis and salinity: Are these mutually exclusive? Photosynthetica 2018, 56, 366–381. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- López-Marín, J.; González, A.; Pérez-Alfocea, F.; Egea-Gilabert, C.; Fernández, J.A. Grafting is an efficient alternative to shading screens to alleviate thermal stress in greenhouse-grown sweet pepper. Sci. Hortic. 2013, 149, 39–46. [Google Scholar] [CrossRef]
- Mupambi, G.; Musacchi, S.; Serra, S.; Kalcsits, L.A.; Layne, D.R.; Schmidt, T. Protective netting improves leaf-level photosynthetic light use efficiency in ‘honeycrisp’ apple under heat stress. HortScience 2018, 53, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Shahak, Y. Photoselective netting: An overview of the concept, research and development and practical implementation in agriculture. Acta Hortic. 2014, 1015, 155–162. [Google Scholar] [CrossRef]
- Tinyane, P.P.; Sivakumar, D.; Soundy, P. Influence of photo-selective netting on fruit quality parameters and bioactive compounds in selected tomato cultivars. Sci. Hortic. 2013, 161, 340–349. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C. Bell pepper (Capsicum annum L.) crop as affected by shade level: Microenvironment, plant growth, leaf gas exchange, and leaf mineral nutrient concentration. HortScience 2013, 48, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Pérez, J.C.; John, K.S. Bell Pepper (Capsicum annum L.) under colored shade nets: Plant Growth and physiological responses. HortScience 2019, 54, 1795–1801. [Google Scholar] [CrossRef] [Green Version]
- Kosma, C.; Triantafyllidis, V.; Papasavvas, A.; Salahas, G.; Patakas, A. Yield and nutritional quality of greenhouse lettuce as affected by shading and cultivation season. Emir. J. Food Agric. 2017, 25, 974–979. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.; Yu, J.; Liu, H.; Cao, Z.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. Var. youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Beneragama, C.; Goto, K. Chlorophyll a:b Ratio Increases Under Low-light in “Shade-tolerant” Euglena gracilis. Trop. Agric. Res. 2011, 22, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, T.; Endo, R.; Kitaya, Y.; Hayashi, S. Growth analysis and photosynthesis measurements of cucumber seedlings grown under light with different red to far-red ratios. HortScience 2016, 51, 843–846. [Google Scholar] [CrossRef] [Green Version]
- Nicolás, E.; Torrecillas, A.S.; Amico, J.D.; Alarcón, J.J. Sap flow, gas exchange, and hydraulic conductance of young apricot trees growing under a shading net and different water supplies. J. Plant Physiol. 2005, 162, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H., Jr. Shade-cloth microclimate of soybeans. Agron. J. 1975, 67, 175–181. [Google Scholar] [CrossRef]
- Ketehouli, T.; Carther, K.F.I.; Noman, M.; Wang, F.-W.; Li, X.-W.; Li, H.-Y. Adaptation of plants to salt stress: Characterization of Na+ and K+ transporters and role of Cbl gene family in regulating salt stress response. Agronomy 2019, 9, 687. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Shabala, L.; Azzarello, E.; Huang, Y.; Pandolfi, C.; Su, N.; Wu, Q.; Cai, S.; Bazihizina, N.; Wang, L.; et al. Na + extrusion from the cytosol and tissue-specific Na+ sequestration in roots confer differential salt stress tolerance between durum and bread wheat. J. Exp. Bot. 2018, 69, 3987–4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, C.; Higgs, D.; Ince, F.; Amador, B.M.; Cakir, A.; Sakar, E. Ameliorative Effects of Potassium Phosphate on Salt-Stressed Pepper and Cucumber. J. Plant Nutr. 2003, 26, 807–820. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D. Supplementary Potassium Nitrate Improves Salt Tolerance in Bell Pepper Plants. J. Plant Nutr. 2003, 26, 1367–1382. [Google Scholar] [CrossRef]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef]
- Voisin, A.-S.; Reidy, B.; Parent, B.; Rolland, G.; Redondo, E.; Gerentes, D.; Tardieu, F.; Muller, B. Are ABA, ethylene or their interaction involved in the response of leaf growth to soil water deficit? An analysis using naturally occurring variation or genetic transformation of ABA production in maize. Plant Cell Environ. 2006, 29, 1829–1840. [Google Scholar] [CrossRef]
- Rosado, A.; Amaya, I.; Valpuesta, V.; Cuartero, J.; Botella, M.A.; Borsani, O. ABA- and ethylene-mediated responses in osmotically stressed tomato are regulated by the TSS2 and TOS1 loci. J. Exp. Bot. 2006, 57, 3327–3335. [Google Scholar] [CrossRef] [Green Version]
- Davies, W.J.; Wilkinson, S.; Loveys, B. Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytol. 2002, 153, 449–460. [Google Scholar] [CrossRef] [Green Version]


| Cover 1 | Salt Treatment 1 | Ψw (Mpa) | Ψs (Mpa) | RWC (%) |
|---|---|---|---|---|
| Non netting | 0 mM | −0.74 ab | −1.76 b | 88.80 a |
| 35 mM | −0.94 b | −2.04 a | 87.98 ab | |
| Netting | 0 mM | −0.53 a | −1.64 b | 89.33 a |
| 35 mM | −0.68 ab | −1.77 b | 88.17 b | |
| ANOVA 2 | ||||
| Cover | * | * | * | |
| Salinity | ** | ** | * | |
| C × S | * | * | ns |
| Macronutrients (mg·g−1 DW) | Micronutrients (mg·g−1 DW) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cover 1 | Salt Treatment 1 | K+ | P5+ | Ca2+ | Mg2+ | Fe2+ | B3+ | Cu2+ | Mn2+ | Zn2+ | Na+ | K+/Na+ |
| Non netting | 0 mM | 42.370 a | 6.903 a | 42.935 a | 17.020 a | 0.439 a | 0.111 a | 0.002 a | 0.076 b | 0.016 b | 0.363 c | 142.540 b |
| 35 mM | 24.617 c | 3.403 b | 42.849 a | 14.522 c | 0.321 b | 0.096 a | 0.002 a | 0.079 b | 0.033 a | 7.365 a | 3.441 a | |
| Netting | 0 mM | 42.938 a | 6.957 a | 44.876 a | 17.168 a | 0.469 a | 0.094 a | 0.001 a | 0.070 b | 0.017 b | 0.309 c | 165.687 a |
| 35 mM | 34.728 b | 3.464 b | 40.578 a | 12.978 bc | 0.316 b | 0.089 a | 0.001 a | 0.101 a | 0.030 a | 5.762 b | 9.582 b | |
| ANOVA 2 | ||||||||||||
| Cover | * | ns | ns | ns | ns | ns | ns | ns | ns | * | * | |
| Salinity | *** | *** | ns | * | ns | ns | ns | * | ** | ** | *** | |
| C × S | * | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gálvez, A.; Albacete, A.; del Amor, F.M.; López-Marín, J. The Use of Red Shade Nets Improves Growth in Salinized Pepper (Capsicum annuum L.) Plants by Regulating Their Ion Homeostasis and Hormone Balance. Agronomy 2020, 10, 1766. https://doi.org/10.3390/agronomy10111766
Gálvez A, Albacete A, del Amor FM, López-Marín J. The Use of Red Shade Nets Improves Growth in Salinized Pepper (Capsicum annuum L.) Plants by Regulating Their Ion Homeostasis and Hormone Balance. Agronomy. 2020; 10(11):1766. https://doi.org/10.3390/agronomy10111766
Chicago/Turabian StyleGálvez, Amparo, Alfonso Albacete, Francisco M. del Amor, and Josefa López-Marín. 2020. "The Use of Red Shade Nets Improves Growth in Salinized Pepper (Capsicum annuum L.) Plants by Regulating Their Ion Homeostasis and Hormone Balance" Agronomy 10, no. 11: 1766. https://doi.org/10.3390/agronomy10111766
APA StyleGálvez, A., Albacete, A., del Amor, F. M., & López-Marín, J. (2020). The Use of Red Shade Nets Improves Growth in Salinized Pepper (Capsicum annuum L.) Plants by Regulating Their Ion Homeostasis and Hormone Balance. Agronomy, 10(11), 1766. https://doi.org/10.3390/agronomy10111766








