Agro-Morphological and Biochemical Characterization of Wild Prunus spinosa L. Subsp. dasyphylla (Schur) Domin Genotypes Naturally Grown in Western Black Sea Region of Turkey
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Agro-Morphological Properties of Fruits
2.3. Extraction of Organic Acids
2.4. Extraction of Phenolics
2.5. Analysis of Vitamin C
2.6. Sensory Features of Fruits
2.7. Statistical Analysis
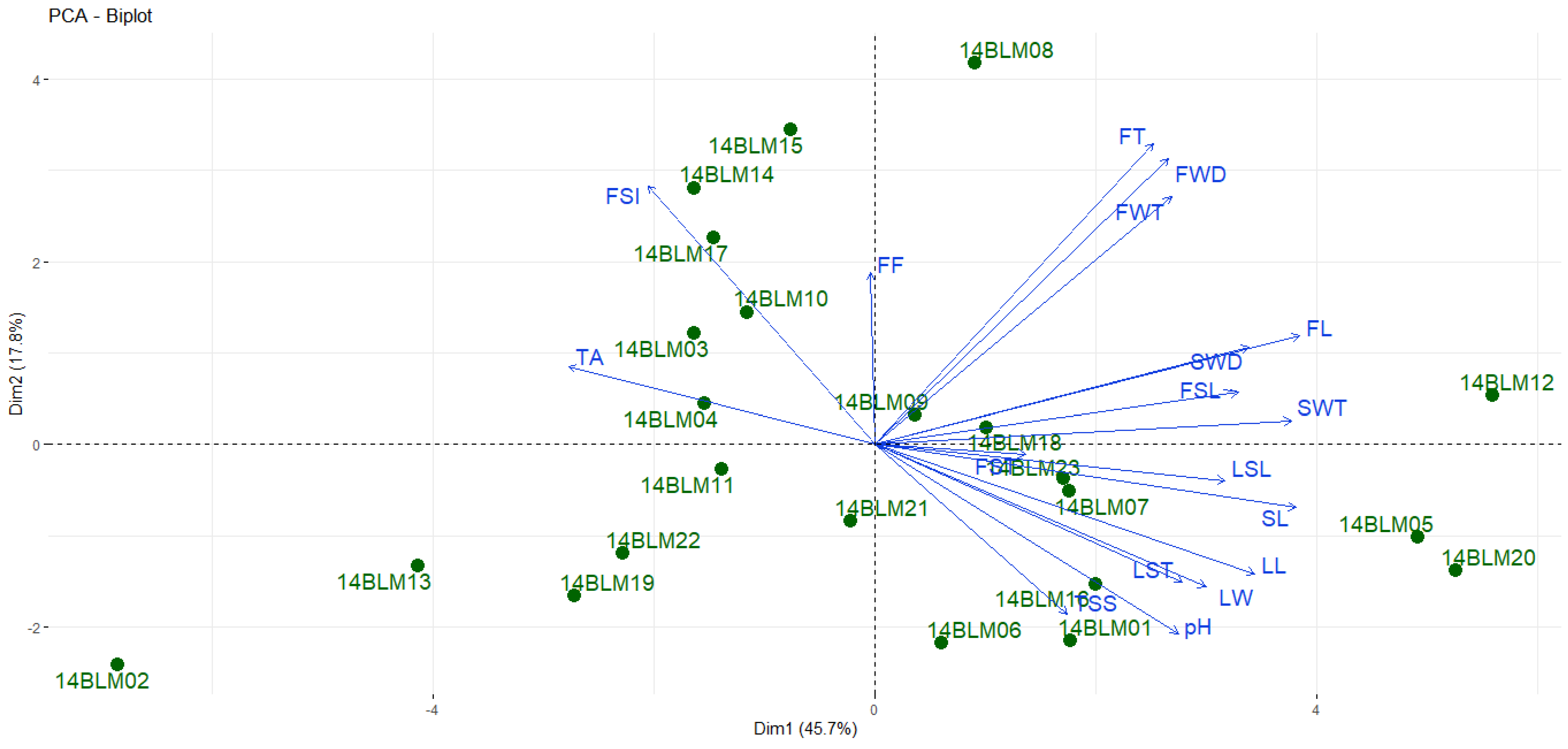
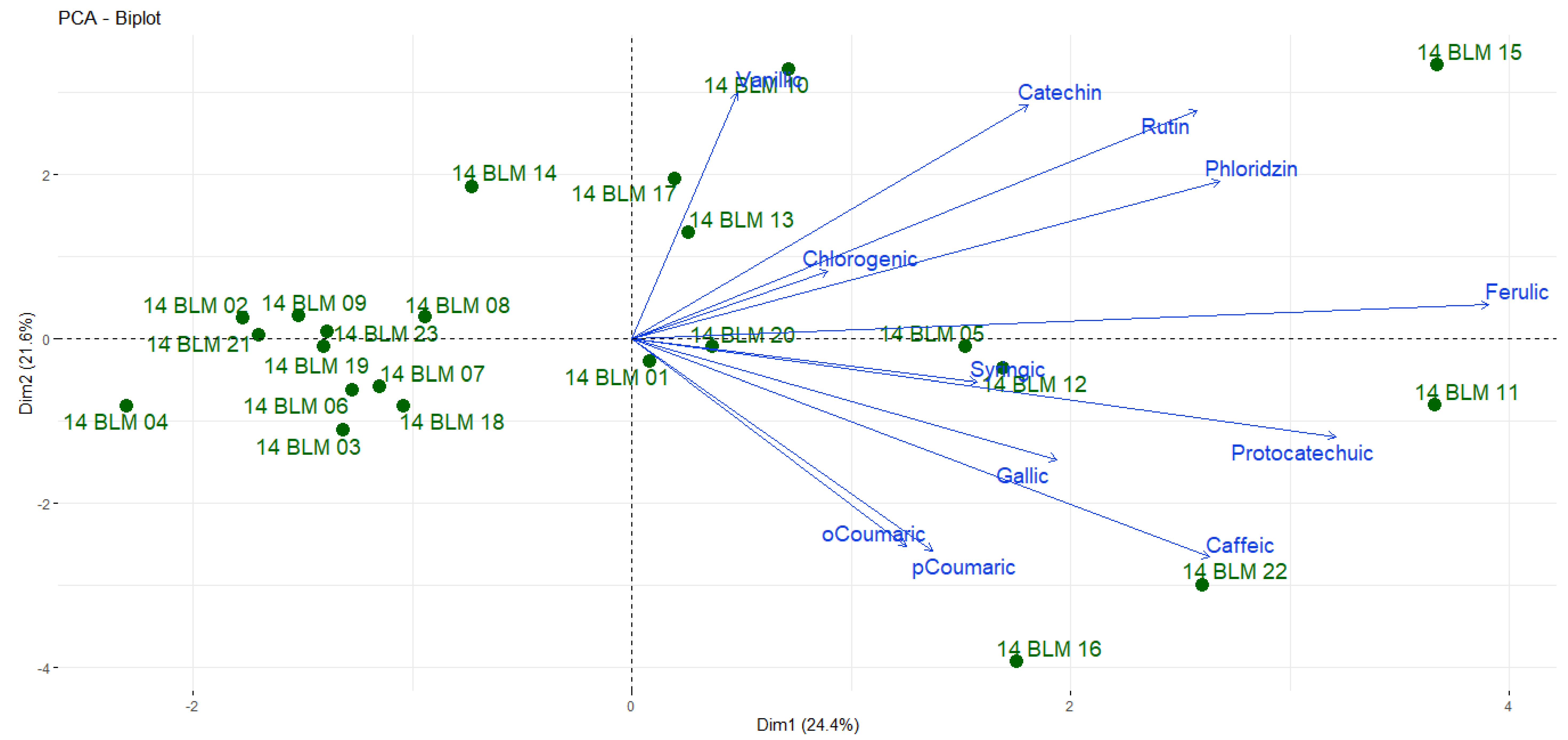
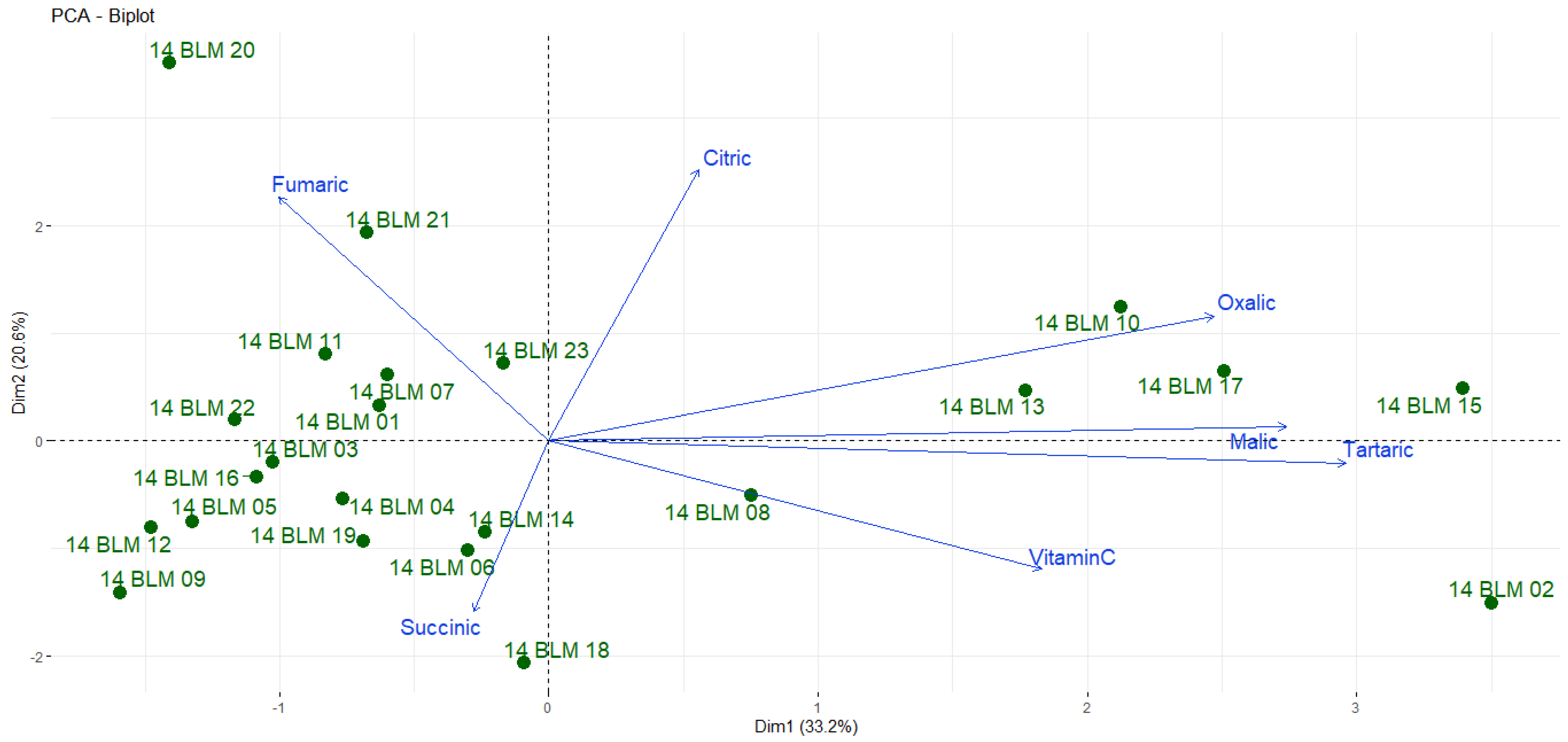
3. Results and Discussion
3.1. Agro-Morphological Properties
3.2. Phenolic Compounds
3.3. Organic Acid and Vitamin C Contents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ercisli, S.; Orhan, E.; Esitken, A.; Yildirim, N.; Agar, G. Relationships among some cornelian cherry genotypes (Cornus mas L.) based on RAPD analysis. Genet. Resour. Crop. Evol. 2008, 55, 613–618. [Google Scholar] [CrossRef]
- Serce, S.; Ozgen, M.; Torun, A.A.; Ercisli, S. Chemical composition, antioxidant activities and total phenolic content of Arbutus andrachne L. (Fam. Ericaceae) (the Greek strawberry tree) fruits from Turkey. J. Food Compos. Anal. 2010, 23, 619–623. [Google Scholar] [CrossRef]
- Ercisli, S.; Ipek, A.; Barut, E. SSR marker-based DNA fingerprinting and cultivar identification of olives (Olea europaea). Biochem. Genet. 2011, 49, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Gecer, M.K.; Kan, T.; Gundogdu, M.; Ercisli, S.; Ilhan, G.; Sagbas, H.I. Physicochemical characteristics of wild and cultivated apricots (Prunus armeniaca L.) from Aras valley in Turkey. Genet. Resour. Crop. Evol. 2020, 67, 935–945. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 October 2020).
- Beyhan, Ö. Some phonological, pomological and morphological characteristics of some standard and local plum cultivars grown in Darende. Bahçe 2005, 34, 47–56. [Google Scholar]
- Rushforth, K. Trees of Britain and Europe; Collins: New York, NY, USA, 1999; ISBN 0-00-220013-9. [Google Scholar]
- Ozcan, T.; Baycu, G. Fatty acid and amino acid profiles in the fruits of Prunus spinosa L. subsp dasyphylla (Schur) Domin from Europe-in-Turkey. Adv. Mol. Biol. 2008, 1, 39–46. [Google Scholar]
- Dönmez, A.A.; Yildirim, S. Taxonomy of the genus Prunus L. (Rosaceae) in Turkey. Turk. J. Bot. 2000, 24, 187–202. [Google Scholar]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Jayasankar, S.; Lay, W. Variation in total phenolic contents and antioxidant capacity among European plum genotypes. Sci. Hortic. 2006, 108, 243–246. [Google Scholar] [CrossRef]
- Rop, O.; Jurikova, T.; Mlcek, J.; Kramarova, D.; Sengee, Z. Antioxidant activity and selected nutritional values of plums (Prunus domestica L.) typical of the white Carpathian Mountains. Sci. Hortic. 2009, 122, 545–549. [Google Scholar] [CrossRef]
- Erturk, Y.; Ercisli, S.; Tosun, M. Physico-chemical characteristics of wild plum fruits (Prunus spinosa L.). Int. J. Plant. Prod. 2009, 3, 89–92. [Google Scholar]
- Celik, F.; Gundogdu, M.; Alp, S.; Muradoglu, F.; Ercişli, S.; Gecer, M.K.; Canan, I. Determination of phenolic compounds, antioxidant capacity and organic acids contents of Prunus domestica L., Prunus cerasifera Ehrh. and Prunus spinosa L. fruits by HPLC. Acta Chromatogr. 2017, 29, 507–510. [Google Scholar] [CrossRef]
- Vlaic, R.; Socaci, S.; Muresan, A.E.; Muresan, C.; Moldovan, O.P.; Muste, S.; Muresan, V. Bioactive compounds and volatile profile dynamics during fruit growth of several plums cultivars. J. Agric. Sci. Technol. 2017, 19, 1565–1576. [Google Scholar]
- El-Baltagi, H.S.; El-Ansary, A.E.; Mostafa, M.A.; Kamel, T.A.; Safwat, G. Evaluation of the phytochemical, antioxidant, antibacterial and anticancer activity of Prunus domestica. Not. Bot. Horti Agrobot. 2019, 47, 395–404. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Srivastav, M.; Singh, D.; Varghese, E. Edible coatings influence the cold-storage life and quality of Santa Rosa plum (Prunus salicina Lindell). Int. J. Food Sci. Technol. 2018, 55, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ruiz, L.K.; Ruiz-Cruz, S.; Cira-Chàvez, A.L.; Gassos-Ortega, E.L.; Ornelas-Paz, J.; Del-Toro-Sànchez, C.; Marquez-Rios, E.; Lôpez-Mata, A.M.; Rodriguez-Fėlix, F. Evaluation of antioxidant capacity, protective effect on human erythrocytes and phenolic compound identification in two varieties of plum fruit (Spondias spp.) by UPLC-MS. Molecules 2018, 23, 3200. [Google Scholar] [CrossRef]
- Valkom, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Tesler, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Gonzales- Flores, D.; Velardo, B.; Garrido, M.; Gonzalez-Gomez, D.; Lozano, M.; Concepcion Ayuso, M.; Barriga, C.; Paredes, S.; Rodriguez, A. Investigation of Japanese plums (Prunus salicina Lindl. cv. Crimson Globa) increases the urinary 6 -sulfatoxymelatonin and total antioxidant capacity levels in young, middle—Aged and elderly humans: Nutritional and functional characterization of their content. J. Food Nutr. Res. 2011, 50, 229–236. [Google Scholar]
- Williamson, G. The role of poliphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Preedy, V.; Zibadi, S. Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Elsevier Academic Press: Cambridge, MA, USA, 2018; Volume 2. [Google Scholar]
- Tosun, I.; Artik, N. A study on chemical composition of blackberry. Gıda 1988, 23, 403–413. [Google Scholar]
- Cemeroglu, B.; Yemenicioğlu, A.; Özkan, M. Composition of Fruits and Vegetables; 1st Fruit and Vegetable Processing Technology; Cemeroğlu, B., Ed.; Klise Publication: Ankara, Turkey, 2004. [Google Scholar]
- Bevilacqua, A.E.; Califano, A.N. Determination of organic acids in dairy products by high performance liquid chromatography. J. Food Sci. 1989, 54, 1076–1079. [Google Scholar] [CrossRef]
- Rodriguez-Delgado, A.; Malovana, S.; Perez, J.P.; Borges, T.; Garcia-Montelongo, F.J. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Cemeroglu, B. Food Analysis; No. 34; Food Technology Society Publication: Ankara, Turkey, 2007; pp. 168–171. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Crisosto, C.H.; Garner, D.; Crisosto, G.M.; Bowerman, E. Increasing ‘Black Amber’ plum (Prunus salicina Lindll) consumer acceptance. Postharvest Biol. Technol. 2004, 34, 237–244. [Google Scholar] [CrossRef]
- Onal, M.K.; Cinsoy, A.S. Relationships of some pomological traits of Prunus salicina Lindl. and Prunus domestica L. cultivars. Akdeniz Üniv. J. Agric. 2003, 16, 43–50. [Google Scholar]
- Cevik, I.; Bilisli, A. A study on suitability of some plum cultivars to drying. Gıda 2002, 27, 285–290. [Google Scholar]
- Abaci, Z.T.; Sevindik, E.; Selvi, S. Determination of total phenolic content, total anthocyanin and ascorbic acid in local plums (Prunus x domestica L.) grown in Ardahan. Tekirdağ Univ. J. Agric. 2014, 11, 27–32. [Google Scholar]
- Mirheidari, F.; Khadivi, A.; Moradi, Y.; Paryan, S. The selection of superior plum (Prunus domestica L.) accessions based on morphological and pomological characterizations. Euphytica 2020, 216, 87. [Google Scholar] [CrossRef]
- Ionica, M.E.; Nour, V.; Trandafir, I.; Cosmulescu, S.; Botu, M. Physical and chemical properties of some European plum cultivars. Not. Bot. Horti Agrobot. 2013, 41, 499–503. [Google Scholar] [CrossRef]
- Walkowiak- Tomczak, D.; Reguła, J.; Łysiak, G. Physico-chemical properties and antioxidant activity of selected plum cultivars fruit. Acta Sci. Pol. Technol. Aliment. 2008, 7, 15–22. [Google Scholar]
- Lombardi-Boccia, G.; Lucarini, M.; Lanzi, S.; Aguzzi, A.; Cappelloni, M. Nutrients and antioxidant molecules in yellow plums (Prunus domestica L.) from conventional and organic productions: A comparative study. J. Agric. Food Chem. 2004, 52, 90–94. [Google Scholar] [CrossRef]
- Treutter, D.; Wang, D.; Farag, M.A.; Argueta Baires, G.S.; Rühmann, S.; Neumüller, M. Diversity of phenolic profiles in the fruit skin of Prunus domestica plums and related species. J. Agric. Food Chem. 2012, 60, 12011–12019. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, K.; Saraçoğlu, O. Variation in total phenolic content and antioxidant activity of Prunus cerasifera Ehrh. selections from Mediterranean region of Turkey. Sci. Hortic. 2012, 134, 88–92. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus fruit species as a rich source of bioactive compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Meyer, A.S.; Waterhouse, A.L. Phenolic composition and antioxidant activity of prunes and prune juice (Prunus domestica). J. Agric. Food Chem. 1998, 46, 1247–1252. [Google Scholar] [CrossRef]
- Sahin, U.; Anapali, O.; Ercisli, S. Physico-chemical and physical properties of some substrates used in horticulture. Gartenbauwissenschaft 2002, 67, 55–60. [Google Scholar]
- Ercisli, S.; Esitken, A. Fruit characteristics of native rose hip (Rosa spp.) selections from the Erzurum province of Turkey. New Zeal. J. Crop. Hort. 2004, 32, 51–53. [Google Scholar] [CrossRef]
- Eyduran, S.P.; Akin, M.; Ercisli, S.; Eyduran, E.; Magharadze, D. Sugars, organic acids, and phenolic compounds of ancient grape cultivars (Vitis vinifera L.) from Igdir province of Eastern Turkey. Biol. Res. 2015, 48, 2. [Google Scholar] [CrossRef]
- Eyduran, S.P.; Ercisli, S.; Akin, M.; Beyhan, Ö.; Geçer, M.K. Organic acids, sugars, vitamin c, antioxidant capacity, and phenolic compounds in fruits of white (Morus alba L.) and black (Morus nigra L.) mulberry genotypes. J. Appl. Bot. Food Qual. 2015, 88, 134–138. [Google Scholar]
- Ersoy, N.; Kupe, M.; Gundogdu, M.; Ilhan, G.; Ercisli, S. Phytochemical and antioxidant diversity in fruits of currant (Ribes spp.) cultivars. Not. Bot. Horti Agrobot. 2018, 46, 381–387. [Google Scholar] [CrossRef]
- Ersoy, N.; Kupe, M.; Sagbas, H.I.; Ercisli, S. Phytochemical diversity among barberry (Berberis vulgaris L.). Not. Bot. Horti Agrobot. 2018, 46, 198–204. [Google Scholar]
- Ghafoor, K.; Ahmed, I.A.M.; Doğu, S.; Uslu, N.; Fadimu, G.J.; Al Juhaimi, F.; Babiker, E.E.; Özcan, M.M. The effect of heating temperature on total phenolic. Int. J. Food Eng. 2019, 15, 11–12. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Veberic, R.; Štampar, F. Quality changes during ripening of plums (Prunus domestica L.). Food Chem. 2008, 111, 830–836. [Google Scholar] [CrossRef]
- Poyrazoglu, E.; Gokmen, V.; Artik, N. Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J. Food Compos. Anal. 2002, 15, 567–575. [Google Scholar] [CrossRef]
- Davarynejad, G.H.; Zarei, M.; Nasrabadi, M.E.; Ardakani, E. Effects of salicylic acid and putrescine on storability, quality attributes and antioxidant activity of plum cv. ‘Santa Rosa’. J. Food Sci. Technol. 2015, 52, 2053–2062. [Google Scholar] [CrossRef]
- Satora, P.; Kostrz, M.; Sroka, P.; Tarko, T. Chemical profile of spirits obtained by spontaneous fermentation of different varieties of plum fruits. Eur. Food Res. Technol. 2017, 243, 489–499. [Google Scholar] [CrossRef]
| Genotypes | Coordinates | Altitude (a.s.l) | Fruit Weight (g) | Fruit Width (mm) | Fruit Length (mm) | Fruit Thickness (mm) | Fruit Firmness (kg/cm2) | Fruit Shape Index | Fruit Stalk Length (mm) |
|---|---|---|---|---|---|---|---|---|---|
| 14BLM01 | 40°.47′58.76′’N; 31° 21′48.34′’E | 855 m | 11.58 ± 0.78 fg * | 25.30 ± 0.16 j | 35.22 ± 0.14 c | 25.70 ± 0.20 h | 1.47 ± 0.09 fgh | 0.72 ± 0.00 k | 12.38 ± 0.43 d-h |
| 14BLM02 | 40°.49′37.45′’N; 31°.22′.53.30′’E | 863 m | 1.79 ± 0.05 k | 13.60 ± 0.05 o | 14.69 ± 0.36 m | 13.70 ± 0.13 o | N.D. | 0.93 ± 0.02 f | 4.49 ± 0.33 k |
| 14BLM03 | 40°.50′27.47′’N; 31°.24′36.22′’E | 875 m | 14.92 ± 0.39 de | 29.44 ± 0.45 ef | 27.68 ± 0.31 h | 29.64 ± 0.68 def | 0.87 ± 0.07 h | 1.06 ± 0.00 ab | 9.78 ± 0.78 ij |
| 14BLM04 | 40°.49′19.77′’N; 31°.30′55.38′’E | 860 m | 9.14 ± 0.30 hi | 26.19 ± 0.30 j | 24.46 ± 0.26 j | 26.20 ± 0.25 h | 3.23 ± 0.32 bc | 1.07 ± 0.02 a | 15.13 ± 0.57 bc |
| 14BLM05 | 40°.73′27.36′’N; 31°.58′45.13′’E | 910 m | 19.02 ± 0.48 c | 29.17 ± 0.08 efg | 35.76 ± 0.63 c | 29.86 ± 1.08 de | 3.33 ± 0.29 bc | 0.82 ± 0.02 hi | 18.66 ± 1.24 a |
| 14BLM06 | 40°.49′55.89′’N; 31°.22′17.68′’E | 863 m | 8.82 ± 0.07 hi | 22.60 ± 0.23 l | 27.37 ± 0.31 h | 22.75 ± 0.35 k | 2.93 ± 0.18 cd | 0.83 ± 0.01 hi | 12.68 ± 0.27 d–g |
| 14BLM07 | 40°.48′19.55′’N; 31°.30′62.84′’E | 878 m | 15.06 ± 0.89 de | 27.33 ± 0.20 i | 29.50 ± 0.19 g | 28.48 ± 0.10 fg | 1.07 ± 0.18 h | 0.93 ± 0.01 ef | 14.07 ± 0.95 cd |
| 14BLM08 | 40°.38′60.81′’N; 31°.20′49.73′’E | 1040 m | 38.42 ± 1.28 a | 37.51 ± 0.11 a | 37.44 ± 0.26 b | 40.13 ± 0.14 a | 1.73 ± 0.29 e-h | 1.00 ± 0.01 cd | 12.97 ± 0.75 def |
| 14BLM09 | 40°.39′38.51′’N; 31°.09′80.58′’E | 1008 m | 13.37 ± 0.99 ef | 27.96 ± 0.14 hi | 26.34 ± 0.55 hi | 28.91 ± 0.22 d–g | 3.97 ± 0.26 b | 1.06 ± 0.02 ab | 13.83 ± 0.23 cde |
| 14BLM10 | 40°.56′61.55′’N; 31°.32′22.46′’E | 880 m | 16.14 ± 0.43 d | 28.66 ± 0.35 fgh | 29.28 ± 0.23 g | 29.97 ± 0.25 d | 2.30 ± 0.17 def | 0.98 ± 0.01 cde | 11.66 ± 0.17 fgh |
| 14BLM11 | 40°.54′78.13′’N; 31°.23′50.67′’E | 903 m | 9.34 ± 0.28 hi | 24.16 ± 0.26 k | 27.05 ± 0.45 hi | 24.32 ± 0.35 ij | 2.20 ± 0.12 d–g | 0.90 ± 0.02 fg | 12.66 ± 0.69 d–g |
| 14BLM12 | 40°.50′0918′’N; 31°.18′39.56′’E | 920 m | 26.41 ± 0.53 b | 31.80 ± 0.10 c | 39.87 ± 0.44 a | 32.91 ± 0.32 c | 0.97 ± 0.12 h | 0.80 ± 0.01 ij | 20.14 ± 0.99 a |
| 14BLM13 | 40°.38′45.49′’N; 31°.33′60.45′’E | 1020 m | 4.65 ± 0.10 j | 17.92 ± 0.44 n | 20.74 ± 0.31 l | 18.17 ± 0.64 n | 2.73 ± 0.15 cd | 0.86 ± 0.01 gh | 8.71 ± 0.39 j |
| 14BLM14 | 40°.38′07.34′’N; 31°.21′18.35′’E | 1007 m | 13.24 ± 0.44 ef | 27.52 ± 0.21 i | 27.01 ± 0.28 hi | 29.07 ± 0.11 d–g | 9.07 ± 0.87 a | 1.02 ± 0.02 bc | 10.51 ± 0.38 hij |
| 14BLM15 | 40°.45′09.76′’N; 31°.29′72.09′’E | 845 m | 19.41 ± 0.89 c | 33.18 ± 0.18 b | 31.14 ± 0.82 f | 34.48 ± 0.20 b | 3.97 ± 0.15 b | 1.06 ± 0.02 ab | 12.59 ± 0.34 d–g |
| 14BLM16 | 40°.45′51.62′’N; 31°.19′63.68′’E | 861 m | 13.42 ± 0.44 ef | 25.42 ± 0.33 j | 33.61 ± 0.35 d | 25.26 ± 0.37 hi | 2.33 ± 0.18 def | 0.76 ± 0.00 jk | 10.96 ± 0.17 ghi |
| 14BLM17 | 40°.45′09.41′’N; 31°.20′19.41′’E | 857 m | 12.96 ± 0.57 f | 27.79 ± 0.35 hi | 26.15 ± 0.13 hi | 27.94 ± 0.33 g | 3.87 ± 0.29 b | 1.06 ± 0.01 ab | 13.10 ± 0.32 def |
| 14BLM18 | 40°.53′30.70′’N; 31°.35′36.37′’E | 1105 m | 15.09 ± 0.61 de | 30.58 ± 0.25 d | 32.84 ± 0.52 de | 28.59 ± 0.35 efg | 2.33 ± 0.24 def | 0.93 ± 0.01 ef | 9.12 ± 0.67 ij |
| 14BLM19 | 40°.52′03.16′’N; 31°.20′86.23′’E | 853 m | 5.38 ± 0.31 j | 19.50 ± 0.31 m | 21.39 ± 0.27 kl | 19.66 ± 0.17 m | 2.07 ± 0.18 d–g | 0.91 ± 0.00 f | 9.36 ± 0.42 ij |
| 14BLM20 | 40°.50′45.23′’N; 31°.10′19.12′’E | 850 m | 19.01 ± 1.11 c | 28.32 ± 0.20 ghi | 35.76 ± 1.09 c | 28.27 ± 0.53 g | 2.50 ± 0.21 cde | 0.80 ± 0.03 ij | 16.58 ± 0.48 b |
| 14BLM21 | 40°.52′34.23′’N; 31°.23′60.15′’E | 865 m | 10.13 ± 0.05 gh | 23.69 ± 0.33 k | 25.62 ± 0.64 ij | 23.79 ± 0.41 jk | 1.20 ± 0.23 h | 0.93 ± 0.03 f | 14.15 ± 0.32 cd |
| 14BLM22 | 40°.45′08.03′’N; 31°.25′56.34′’E | 835 m | 7.49 ± 0.43 i | 22.54 ± 0.65 l | 22.67 ± 1.22 k | 21.18 ± 0.30 l | 1.33 ± 0.18 gh | 1.00 ± 0.02 cd | 11.98 ± 1.01 e–h |
| 14BLM23 | 40°.51′70.91′’N; 31°.17′56.19′’E | 840 m | 16.74 ± 0.59 d | 29.77 ± 0.76 de | 31.49 ± 0.34 ef | 28.49 ± 0.56 fg | 2.20 ± 0.15 d–g | 0.95 ± 0.02 def | 9.07 ± 0.34 ij |
| Genotypes | Stone Weight (g) | Stone Length (mm) | Stone Width (mm) | Leaf Width (cm) | Leaf Length (cm) | Leaf Stalk Length (mm) | Leaf Stalk Thickness (mm) |
|---|---|---|---|---|---|---|---|
| 14 BLM 01 | 0.77 ± 0.06 fgh * | 17.44 ± 0.03 de | 9.96 ± 0.12 ef | 4.03 ± 0.24 c | 6.65 ± 0.25 d | 16.55 ± 0.59 c–f | 1.12 ± 0.06 bcd |
| 14 BLM 02 | 0.28 ± 0.00 l | 9.14 ± 0.20 l | 7.35 ± 0.39 h | 1.27 ± 0.07 h | 3.20 ± 0.10 k | 5.75 ± 0.33 l | 0.60 ± 0.03 k |
| 14 BLM 03 | 0.67 ± 0.02 g–j | 12.46 ± 0.41 ijk | 10.74 ± 0.13 de | 2.67 ± 0.09 fg | 5.08 ± 0.12 f–i | 10.71 ± 0.98 jk | 0.88 ± 0.02 fgh |
| 14 BLM 04 | 0.59 ± 0.03 h–k | 12.38 ± 0.30 ijk | 10.02 ± 0.04 ef | 3.20 ± 0.25 ef | 4.63 ± 0.04 hij | 14.77 ± 1.17 e–h | 0.72 ± 0.07 h–k |
| 14 BLM 05 | 1.28 ± 0.09 b | 23.96 ± 0.32 b | 12.86 ± 0.33 b | 4.85 ± 0.34 a | 9.10 ± 0.28 a | 17.84 ± 1.04 bcd | 1.23 ± 0.11 bc |
| 14 BLM 06 | 0.80 ± 0.03 efg | 18.04 ± 0.26 de | 10.43 ± 0.07 de | 3.90 ± 0.15 c | 5.73 ± 0.11 ef | 16.79 ± 0.58 b–f | 1.06 ± 0.02 cde |
| 14 BLM 07 | 1.15 ± 0.03 bc | 18.79 ± 0.33 d | 12.18 ± 0.22 b | 4.30 ± 0.25 bc | 7.47 ± 0.35 bc | 13.20 ± 1.29 g–j | 0.83 ± 0.09 f–j |
| 14 BLM 08 | 0.80 ± 0.06 efg | 14.97 ± 0.99 fg | 10.27 ± 0.75 de | 2.52 ± 0.19 g | 4.73 ± 0.36 g–j | 12.58 ± 0.73 h–k | 0.65 ± 0.04 jk |
| 14 BLM 09 | 0.63 ± 0.09 g–j | 12.12 ± 0.25 jk | 10.64 ± 0.55 de | 3.77 ± 0.09 cd | 6.83 ± 0.37 cd | 17.66 ± 1.04 b–f | 1.24 ± 0.03 b |
| 14 BLM 10 | 0.73 ± 0.09 f–j | 13.56 ± 0.53 g–j | 11.20 ± 0.16 cd | 2.58 ± 0.09 g | 4.75 ± 0.09 g–j | 12.76 ± 0.86 h–k | 0.83 ± 0.02 f–j |
| 14 BLM 11 | 0.54 ± 0.04 jk | 15.33 ± 0.61 f | 10.87 ± 0.26 de | 2.85 ± 0.09 efg | 4.63 ± 0.09 hij | 16.18 ± 1.46 def | 0.65 ± 0.02 jk |
| 14 BLM 12 | 1.70 ± 0.02 a | 25.86 ± 0.44 a | 14.80 ± 0.33 a | 3.33 ± 0.16 de | 6.20 ± 0.38 de | 17.85 ± 0.28 bcd | 0.87 ± 0.03 f–i |
| 14 BLM 13 | 0.44 ± 0.03 kl | 13.18 ± 0.21 h–k | 9.05 ± 0.11 fg | 2.58 ± 0.21 g | 4.22 ± 0.25 j | 9.82 ± 0.88 k | 0.69 ± 0.05 ijk |
| 14 BLM 14 | 0.74 ± 0.02 f–i | 14.88 ± 0.12 fg | 11.07 ± 0.47 d | 2.42 ± 0.25 g | 4.13 ± 0.29 j | 13.92 ± 0.87 f–i | 0.69 ± 0.05 ijk |
| 14 BLM 15 | 0.69 ± 0.02 f–j | 14.35 ± 0.02 fgh | 12.43 ± 0.21 b | 2.58 ± 0.11 g | 4.18 ± 0.06 j | 12.29 ± 0.41 h–k | 0.80 ± 0.02 g–j |
| 14 BLM 16 | 1.03 ± 0.09 cd | 21.78 ± 0.31 c | 11.19 ± 0.20 cd | 4.03 ± 0.04 c | 6.08 ± 0.34 de | 21.10 ± 0.36 a | 0.78 ± 0.01 g–k |
| 14 BLM 17 | 0.97 ± 0.15 cde | 16.79 ± 0.34 e | 12.45 ± 0.10 b | 2.97 ± 0.16 efg | 4.47 ± 0.23 ij | 17.58 ± 1.43 b–f | 0.65 ± 0.01 jk |
| 14 BLM 18 | 0.88 ± 0.02 def | 17.49 ± 0.43 de | 12.12 ± 0.15 bc | 2.87 ± 0.12 efg | 4.83 ± 0.27 g–j | 18.25 ± 1.00 a–d | 0.96 ± 0.02 d–g |
| 14 BLM 19 | 0.56 ± 0.02 ijk | 13.69 ± 0.49 ghi | 9.97 ± 0.07 ef | 2.98 ± 0.15 efg | 5.42 ± 0.18 e–h | 12.08 ± 0.64 h–k | 0.74 ± 0.04 h–k |
| 14 BLM 20 | 1.32 ± 0.05 b | 25.30 ± 0.22 a | 14.03 ± 0.31 a | 4.63 ± 0.12 ab | 7.97 ± 0.45 b | 19.83 ± 1.05 ab | 1.52 ± 0.09 a |
| 14 BLM 21 | 1.01 ± 0.02 cd | 18.88 ± 0.30 d | 12.91 ± 0.15 b | 2.88 ± 0.12 efg | 5.18 ± 0.06 f–i | 11.72 ± 0.62 ijk | 0.93 ± 0.06 efg |
| 14 BLM 22 | 0.31 ± 0.00 l | 11.95 ± 0.33 k | 8.45 ± 0.21 g | 4.20 ± 0.26 bc | 4.90 ± 0.31 g–j | 15.97 ± 1.49 d–g | 0.99 ± 0.08 def |
| 14 BLM 23 | 0.89 ± 0.10 def | 17.21 ± 1.22 e | 12.35 ± 0.60 b | 2.77 ± 0.20 efg | 5.53 ± 0.09 efg | 19.40 ± 0.50 abc | 1.10 ± 0.11 b–f |
| TSS (%) | pH | Total Acidity (%) | Taste | Aroma | Stone Separation from Flesh | |
|---|---|---|---|---|---|---|
| 14 BLM 01 | 17.47 ± 0.35 def * | 3.93 ± 0.03 bc | 1.11 ± 0.06 jk | 5.7± 0.2 c | 7.1± 0.4 b | Very difficult |
| 14 BLM 02 | 19.00 ± 1.10 bcd | 3.47 ± 0.07 gh | 3.01 ± 0.37 b | 3.3± 0.2 d | 5.2± 0.3 bc | Difficult |
| 14 BLM 03 | 16.40 ± 0.31 fgh | 3.47 ± 0.03 gh | 2.06 ± 0.04 efg | 3.5± 0.3 d | 8.9± 0.5 a | Easy |
| 14 BLM 04 | 11.40 ± 0.60 j | 4.13 ± 0.07 a | 1.90 ± 0.04 fgh | 3.2± 0.1 d | 8.8± 0.5 a | Difficult |
| 14 BLM 05 | 19.13 ± 1.16 bcd | 4.07 ± 0.03 ab | 1.77 ± 0.03 f–i | 5.1± 0.4 c | 6.9± 0.3 bc | Easy |
| 14 BLM 06 | 18.00 ± 0.23 def | 4.07 ± 0.07 ab | 1.69 ± 0.09 ghi | 5.0± 0.3 c | 6.9± 0.3 bc | Easy |
| 14 BLM 07 | 18.53 ± 0.29 cde | 3.67 ± 0.13 ef | 1.08 ± 0.04 jk | 5.9± 0.2 c | 8.8± 0.6 a | Easy |
| 14 BLM 08 | 15.60 ± 0.31 gh | 3.43 ± 0.03 gh | 1.02 ± 0.04 jk | 5.6± 0.1 c | 5.2± 0.4 bc | Difficult |
| 14 BLM 09 | 13.27 ± 0.41 i | 3.77 ± 0.03 de | 1.10 ± 0.05 jk | 5.4± 0.4 c | 5.3± 0.2 bc | Very easy |
| 14 BLM 10 | 16.33 ± 0.24 fgh | 3.37 ± 0.03 h | 2.17 ± 0.08 def | 3.5± 0.3 d | 8.7± 0.5 a | Easy |
| 14 BLM 11 | 15.47 ± 0.29 h | 3.57 ± 0.03 fg | 1.57 ± 0.09 hi | 5.0± 0.2 c | 8.8± 0.3 a | Very easy |
| 14 BLM 12 | 19.93 ± 0.58 bc | 4.13 ± 0.07 a | 0.87 ± 0.04 k | 9.0± 0.7 a | 5.4± 0.2 bc | Easy |
| 14 BLM 13 | 13.47 ± 0.29 i | 3.40 ± 0.00 gh | 2.47 ± 0.18 cde | 3.2± 0.2 d | 5.0± 0.2 c | Easy |
| 14 BLM 14 | 9.33 ± 0.18 k | 3.33 ± 0.03 h | 2.06 ± 0.08 efg | 3.1± 0.1 d | 9.0± 0.6 a | Easy |
| 14 BLM 15 | 13.07 ± 0.18 i | 3.33 ± 0.03 h | 2.88 ± 0.09 bc | 3.1± 0.1 d | 5.3± 0.2 bc | Middle |
| 14 BLM 16 | 18.40 ± 0.31 cde | 3.83 ± 0.03 cd | 0.89 ± 0.09 k | 8.8± 0.2 a | 8.7± 0.4 a | Very easy |
| 14 BLM 17 | 13.67 ± 0.24 i | 3.17 ± 0.03 i | 4.26 ± 0.47 a | 3.0± 0.2 d | 5.5± 0.1 bc | Easy |
| 14 BLM 18 | 20.33 ± 0.48 b | 3.87 ± 0.03 cd | 1.44 ± 0.03 ij | 6.9± 0.4 b | 8.8± 0.3 a | Very easy |
| 14 BLM 19 | 17.20 ± 0.46 efg | 3.57 ± 0.09 fg | 2.10 ± 0.06 efg | 3.7± 0.2 d | 5.2± 0.2 bc | Difficult |
| 14 BLM 20 | 18.40 ± 0.31 cde | 3.93 ± 0.03 bc | 0.86 ± 0.03 k | 8.9± 0.6 a | 7.7± 0.3 b | Easy |
| 14 BLM 21 | 23.33 ± 0.29 a | 3.53 ± 0.03 fg | 2.57 ± 0.11 cd | 5.0± 0.2 c | 7.3± 0.3 b | Easy |
| 14 BLM 22 | 12.47 ± 0.29 ij | 3.57 ± 0.03 fg | 2.05 ± 0.07 efg | 3.3± 0.1 d | 7.4± 0.3 b | Easy |
| 14 BLM 23 | 24.33 ± 1.35 a | 3.93 ± 0.03 bc | 1.09 ± 0.04 jk | 8.9± 0.5 a | 5.3± 0.2 bc | Easy |
| Genotypes | Gallic Acid | Protocatechuic Acid | Catechin | Chlorogenic Acid | Rutin | Phloridzin |
|---|---|---|---|---|---|---|
| 14 BLM 01 | 1.63 ± 0.06 e * | 0.59 ± 0.01 j | 1.97 ± 0.05 h | 4.18 ± 0.08 j | 0.69 ± 0.09 ijk | 0.73 ± 0.03 fgh |
| 14 BLM 02 | 0.61 ± 0.03 jk | 0.48 ± 0.04 j | 0.95 ± 0.03 l | 7.69 ± 0.03 e | 0.95 ± 0.03 g | 0.74 ± 0.03 fgh |
| 14 BLM 03 | 0.65 ± 0.04 ij | 1.45 ± 0.02 fg | 2.52 ± 0.06 f | 2.21 ± 0.06 k | 0.44 ± 0.03 m | 0.59 ± 0.04 jkl |
| 14 BLM 04 | 0.82 ± 0.08 h | 0.65 ± 0.04 ij | 1.11 ± 0.08 k | 2.07 ± 0.04 kl | 0.45 ± 0.04 m | 0.47 ± 0.03 mn |
| 14 BLM 05 | 2.67 ± 0.06 b | 0.95 ± 0.01 hi | 1.12 ± 0.04 k | 9.91 ± 0.03 b | 1.66 ± 0.03 cd | 1.20 ± 0.05 cd |
| 14 BLM 06 | 0.77 ± 0.04 hi | 1.24 ± 0.08 gh | 2.21 ± 0.05 g | 1.62 ± 0.07 mn | 0.74 ± 0.04 ij | 0.36 ± 0.03 o |
| 14 BLM 07 | 1.46 ± 0.02 f | 1.32 ± 0.03 g | 0.71 ± 0.03 mn | 8.27 ± 0.18 d | 0.55 ± 0.01 klm | 0.76 ± 0.03 efg |
| 14 BLM 08 | 1.02 ± 0.08 g | 0.75 ± 0.04 ij | 2.57 ± 0.10 f | 4.32 ± 0.04 j | 1.75 ± 0.01 c | 0.38 ± 0.03 no |
| 14 BLM 09 | 0.49 ± 0.04 k | 0.72 ± 0.03 ij | 1.93 ± 0.04 h | 6.22 ± 0.04 h | 0.82 ± 0.06 hi | 0.68 ± 0.02 g–j |
| 14 BLM 10 | 0.81 ± 0.03 h | 0.63 ± 0.02 ij | 5.45 ± 0.06 b | 5.32 ± 0.06 i | 2.65 ± 0.04 a | 0.81 ± 0.02 ef |
| 14 BLM 11 | 1.92 ± 0.02 d | 3.95 ± 0.05 c | 0.57 ± 0.02 n | 6.84 ± 0.01 g | 1.65 ± 0.04 cd | 1.52 ± 0.05 a |
| 14 BLM 12 | 3.10 ± 0.04 a | 0.45 ± 0.01 j | 3.51 ± 0.08 d | 1.48 ± 0.07 no | 1.45 ± 0.03 e | 0.84 ± 0.05 e |
| 14 BLM 13 | 2.75 ± 0.07 b | 0.73 ± 0.01 ij | 3.68 ± 0.03 c | 9.78 ± 0.27 b | 1.49 ± 0.01 e | 0.53 ± 0.02 klm |
| 14 BLM 14 | 1.05 ± 0.04 g | 0.56 ± 0.05 j | 0.66 ± 0.04 n | 1.84 ± 0.06 lm | 1.53 ± 0.05 de | 1.24 ± 0.01 c |
| 14 BLM 15 | 0.52 ± 0.02 jk | 4.46 ± 0.06 b | 6.78 ± 0.10 a | 7.22 ± 0.04 f | 2.12 ± 0.04 b | 1.42 ± 0.04 b |
| 14 BLM 16 | 2.29 ± 0.01 c | 3.47 ± 0.43 d | 1.41 ± 0.05 j | 1.18 ± 0.08 o | 1.17 ± 0.03 f | 0.53 ± 0.02 klm |
| 14 BLM 17 | 1.65 ± 0.04 e | 2.09 ± 0.08 e | 3.23 ± 0.04 e | 4.06 ± 0.05 j | 1.29 ± 0.03 f | 1.12 ± 0.03 d |
| 14 BLM 18 | 1.13 ± 0.03 g | 0.70 ± 0.03 ij | 0.85 ± 0.01 lm | 8.76 ± 0.06 c | 0.88 ± 0.02 gh | 0.70 ± 0.04 ghi |
| 14 BLM 19 | 1.93 ± 0.04 d | 1.65 ± 0.05 f | 0.64 ± 0.03 n | 6.61 ± 0.04 g | 0.81 ± 0.06 hi | 0.64 ± 0.03 hij |
| 14 BLM 20 | 1.76 ± 0.05 e | 4.20 ± 0.09 bc | 1.64 ± 0.04 i | 11.45 ± 0.31 a | 1.16 ± 0.06 f | 0.45 ± 0.03 mno |
| 14 BLM 21 | 1.99 ± 0.08 d | 0.53 ± 0.01 j | 1.15 ± 0.05 k | 1.21 ± 0.06 o | 0.53 ± 0.03 lm | 0.72 ± 0.01 fgh |
| 14 BLM 22 | 2.16 ± 0.03 c | 5.27 ± 0.06 a | 1.19 ± 0.09 k | 6.23 ± 0.10 h | 0.60 ± 0.10 jkl | 0.61 ± 0.02 ijk |
| 14 BLM 23 | 1.02 ± 0.07 g | 0.78 ± 0.06 ij | 0.86 ± 0.04 lm | 5.37 ± 0.04 i | 0.66 ± 0.03 jkl | 0.50 ± 0.05 lm |
| Genotypes | Ferulic Acid | O-Coumaric Acid | Vanillic Acid | Caffeic Acid | Syringic Acid | p-Coumaric Acid |
| 14 BLM 01 | 1.38 ± 0.05 d * | 0.39 ± 0.01 c | 0.32 ± 0.02 ghi | 0.74 ± 0.04 k | 3.68 ± 0.07 a | 0.92 ± 0.04 j |
| 14 BLM 02 | 0.21 ± 0.02 n | 0.07 ± 0.00 j–m | 0.23 ± 0.01 ijk | 1.39 ± 0.05 f–i | 0.69 ± 0.03 efg | 1.25 ± 0.02 f |
| 14 BLM 03 | 0.29 ± 0.02 mn | 0.32 ± 0.02 def | 0.15 ± 0.02 kl | 2.51 ± 0.10 d | 0.80 ± 0.05 efg | 1.23 ± 0.06 f |
| 14 BLM 04 | 0.25 ± 0.04 mn | 0.19 ± 0.01 gh | 0.19 ± 0.04 jkl | 1.25 ± 0.06 g–j | 0.89 ± 0.05 ef | 1.21 ± 0.04 fg |
| 14 BLM 05 | 1.04 ± 0.05 e | 0.35 ± 0.02 cd | 0.13 ± 0.01 l | 1.49 ± 0.04 fg | 3.61 ± 0.06 ab | 0.98 ± 0.06 ij |
| 14 BLM 06 | 0.76 ± 0.04 fg | 0.45 ± 0.03 b | 0.35 ± 0.02 fgh | 1.13 ± 0.04 g–j | 0.90 ± 0.03 ef | 1.13 ± 0.03 fgh |
| 14 BLM 07 | 0.45 ± 0.03 ijk | 0.28 ± 0.03 f | 0.17 ± 0.01 jkl | 1.05 ± 0.02 ijk | 1.48 ± 0.07 d | 0.86 ± 0.02 jk |
| 14 BLM 08 | 0.54 ± 0.03 hi | 0.30 ± 0.03 ef | 0.18 ± 0.01 jkl | 1.18 ± 0.05 g–j | 0.96 ± 0.03 ef | 1.06 ± 0.05 hi |
| 14 BLM 09 | 0.34 ± 0.04 j–n | 0.05 ± 0.00 lm | 0.27 ± 0.03 hij | 1.08 ± 0.06 ijk | 1.60 ± 0.04 d | 1.53 ± 0.04 e |
| 14 BLM 10 | 0.84 ± 0.03 f | 0.11 ± 0.01 ijk | 0.71 ± 0.06 b | 1.31 ± 0.04 g–j | 1.00 ± 0.03 e | 1.17 ± 0.04 fgh |
| 14 BLM 11 | 1.95 ± 0.07 b | 0.44 ± 0.01 b | 0.20 ± 0.02 jkl | 3.14 ± 0.07 c | 3.47 ± 0.06 ab | 1.20 ± 0.06 fg |
| 14 BLM 12 | 1.70 ± 0.03 c | 0.33 ± 0.03 de | 0.20 ± 0.01 jkl | 1.05 ± 0.11 ijk | 3.46 ± 0.30 ab | 1.66 ± 0.03 d |
| 14 BLM 13 | 0.64 ± 0.05 gh | 0.07 ± 0.00 j–m | 0.61 ± 0.04 c | 1.46 ± 0.04 fgh | 1.94 ± 0.03 c | 1.09 ± 0.05 ghi |
| 14 BLM 14 | 0.34 ± 0.04 j–n | 0.03 ± 0.00 m | 0.92 ± 0.05 a | 0.95 ± 0.04 jk | 0.74 ± 0.04 efg | 1.59 ± 0.04 de |
| 14 BLM 15 | 2.25 ± 0.12 a | 0.12 ± 0.01 ij | 0.62 ± 0.04 c | 2.24 ± 0.12 de | 0.69 ± 0.05 efg | 0.94 ± 0.03 j |
| 14 BLM 16 | 0.64 ± 0.02 gh | 0.74 ± 0.02 a | 0.24 ± 0.03 ijk | 4.80 ± 0.03 b | 0.63 ± 0.03 fg | 2.76 ± 0.04 a |
| 14 BLM 17 | 0.36 ± 0.02 j–m | 0.15 ± 0.01 hi | 0.93 ± 0.06 a | 1.70 ± 0.01 f | 0.55 ± 0.04 g | 0.94 ± 0.03 j |
| 14 BLM 18 | 0.33 ± 0.01 k–n | 0.06 ± 0.00 klm | 0.16 ± 0.01 kl | 1.50 ± 0.08 fg | 1.29 ± 0.18 d | 2.33 ± 0.01 c |
| 14 BLM 19 | 0.29 ± 0.02 lmn | 0.08 ± 0.00 jkl | 0.26 ± 0.02 hij | 1.50 ± 0.09 fg | 0.53 ± 0.01 g | 0.57 ± 0.02 l |
| 14 BLM 20 | 0.43 ± 0.04 i–l | 0.09 ± 0.01 jkl | 0.44 ± 0.04 def | 2.08 ± 0.10 e | 2.07 ± 0.07 c | 1.08 ± 0.05 hi |
| 14 BLM 21 | 0.45 ± 0.05 ijk | 0.19 ± 0.02 gh | 0.45 ± 0.03 de | 1.16 ± 0.07 g–j | 0.53 ± 0.03 g | 0.57 ± 0.02 l |
| 14 BLM 22 | 1.35 ± 0.06 d | 0.10 ± 0.01 jk | 0.36 ± 0.03 efg | 6.01 ± 0.45 a | 1.39 ± 0.06 d | 2.60 ± 0.04 b |
| 14 BLM 23 | 0.47 ± 0.03 ij | 0.21 ± 0.02 g | 0.53 ± 0.03 cd | 1.08 ± 0.03 h–k | 3.33 ± 0.26 b | 0.77 ± 0.01 k |
| Genotypes | Citric Acid | Tartaric Acid | Malic Acid | Oxalic Acid | Succinic acid | Fumaric Acid | Vitamin C |
|---|---|---|---|---|---|---|---|
| 14 BLM 01 | 74.62 ± 1.40 c * | 10.07 ± 0.31 gh | 686.09 ± 9.14 i | 3.75 ± 0.00 ijk | 59.47 ± 0.96 b | 3.08 ± 0.04 h | 32.96 ± 0.29 f |
| 14 BLM 02 | 22.74 ± 0.21 k | 30.09 ± 0.08 a | 1071.62 ± 7.87 d | 10.84 ± 0.34 d | 52.79 ± 0.57 cd | 0.88 ± 0.02 op | 47.40 ± 0.49 b |
| 14 BLM 03 | 11.32 ± 0.72 m | 6.41 ± 0.07 l | 867.33 ± 7.05 g | 3.50 ± 0.25 ijk | 20.80 ± 0.07 k | 3.34 ± 0.05 g | 29.39 ± 0.33 g |
| 14 BLM 04 | 23.57 ± 0.36 k | 12.59 ± 1.01 e | 610.50 ± 6.29 j | 3.38 ± 0.13 ijk | 31.40 ± 1.59 i | 2.09 ± 0.01 j | 28.14 ± 1.03 gh |
| 14 BLM 05 | 21.08 ± 0.42 k | 10.50 ± 0.07 fg | 455.42 ± 4.81 n | 2.51 ± 0.04 k | 54.96 ± 0.12 c | 4.84 ± 0.04 c | 39.12 ± 0.19 d |
| 14 BLM 06 | 15.91 ± 0.49 l | 7.72 ± 0.17 i–l | 928.48 ± 5.39 f | 3.38 ± 0.13 ijk | 21.53 ± 0.08 k | 0.80 ± 0.04 pr | 35.86 ± 0.14 e |
| 14 BLM 07 | 85.45 ± 0.89 a | 7.84 ± 0.10 ijk | 562.01 ± 7.15 l | 4.38 ± 0.13 hi | 54.45 ± 0.68 cd | 3.60 ± 0.07 f | 43.65 ± 1.49 c |
| 14 BLM 08 | 22.78 ± 1.67 k | 11.08 ± 0.52 efg | 1149.29 ± 2.66 b | 7.63 ± 0.63 e | 27.99 ± 1.17 j | 0.72 ± 0.03 r | 29.30 ± 0.26 g |
| 14 BLM 09 | 13.94 ± 0.04 klm | 6.65 ± 0.14 kl | 440.68 ± 6.50 no | 5.75 ± 0.75 fgh | 85.79 ± 1.61 a | 4.10 ± 0.06 e | 33.43 ± 0.24 f |
| 14 BLM 10 | 79.89 ± 1.58 b | 16.56 ± 0.11 d | 1294.64 ± 0.89 a | 6.25 ± 0.50 efg | 27.48 ± 0.53 j | 4.71 ± 0.03 d | 53.74 ± 1.36 a |
| 14 BLM 11 | 57.54 ± 1.86 g | 8.93 ± 0.49 hi | 546.40 ± 3.02 lm | 5.75 ± 0.25 fgh | 34.74 ± 0.26 h | 3.68 ± 0.06 f | 31.92 ±1.26 f |
| 14 BLM 12 | 15.33 ± 0.19 kl | 8.37 ± 0.24 ij | 386.68 ± 5.73 p | 6.17 ± 0.06 efg | 46.17 ± 0.13 f | 1.72 ± 0.05 k | 22.73 ± 0.03 j |
| 14 BLM 13 | 61.07 ± 1.65 f | 11.77 ± 0.21 def | 1132.28 ± 6.72 c | 12.27 ± 0.66 c | 49.03 ± 0.15 ef | 2.57 ± 0.07 i | 42.57 ± 0.41 c |
| 14 BLM 14 | 12.28 ± 0.27 lm | 16.22 ± 0.65 d | 613.77 ± 4.73 j | 2.50 ± 0.25 k | 21.35 ± 0.23 k | 2.48 ± 0.01 i | 37.08 ± 0.10 de |
| 14 BLM 15 | 52.83 ± 1.25 h | 22.33 ± 1.04 b | 1008.32 ± 8.36 e | 16.09 ± 0.73 a | 32.93 ± 1.96 hi | 2.10 ± 0.03 j | 48.86 ± 1.27 b |
| 14 BLM 16 | 42.57 ± 1.19 j | 7.70 ± 0.44 i–l | 606.99 ± 4.75 j | 4.13 ± 0.13 ij | 39.92 ± 0.10 g | 1.25 ± 0.01 m | 26.69 ± 0.88 hi |
| 14 BLM 17 | 54.58 ± 1.15 gh | 19.02 ± 0.57 c | 1279.28 ± 7.22 a | 13.72 ± 0.72 b | 39.13 ± 0.74 g | 1.05 ± 0.04 n | 25.84 ± 0.74 i |
| 14 BLM 18 | 14.14 ± 0.17 klm | 11.84 ± 0.06 de | 428.17 ± 4.03 o | 4.88 ± 0.13 ghi | 51.66 ± 1.40 de | 1.59 ± 0.04 l | 54.42 ± 0.75 a |
| 14 BLM 19 | 46.97 ± 1.21 i | 7.19 ± 0.07 jkl | 531.73 ± 7.37 m | 6.25 ± 0.50 efg | 61.89 ± 0.01 b | 0.95 ± 0.02 no | 35.99 ± 0.57 e |
| 14 BLM 20 | 70.64 ± 1.73 d | 9.92 ± 0.10 gh | 269.65 ± 2.07 r | 10.38 ± 0.38 d | 23.83 ± 2.48 k | 9.13 ± 0.01 a | 22.62 ± 0.52 j |
| 14 BLM 21 | 53.93 ± 0.97 h | 10.31 ± 0.20 g | 791.95 ± 2.14 h | 6.50 ± 0.75 ef | 16.80 ± 0.67 l | 5.11 ± 0.05 b | 20.32 ± 0.66 k |
| 14 BLM 22 | 64.66 ± 1.70 e | 7.14 ± 0.01 jkl | 586.85 ± 4.30 k | 2.75 ± 0.25 jk | 31.67 ± 0.82 hi | 0.86 ± 0.01 op | 27.26 ± 0.19 ghi |
| 14 BLM 23 | 48.39 ± 0.94 i | 12.08 ± 0.37 de | 431.56 ± 3.76 o | 10.67 ± 0.82 d | 21.81 ± 1.19 k | 1.02 ± 0.00 n | 22.37 ± 0.19 jk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuru Berk, S.; Tas, A.; Orman, E.; Gundogdu, M.; Necas, T.; Ondrasek, I.; Karatas, N.; Ercisli, S. Agro-Morphological and Biochemical Characterization of Wild Prunus spinosa L. Subsp. dasyphylla (Schur) Domin Genotypes Naturally Grown in Western Black Sea Region of Turkey. Agronomy 2020, 10, 1748. https://doi.org/10.3390/agronomy10111748
Kuru Berk S, Tas A, Orman E, Gundogdu M, Necas T, Ondrasek I, Karatas N, Ercisli S. Agro-Morphological and Biochemical Characterization of Wild Prunus spinosa L. Subsp. dasyphylla (Schur) Domin Genotypes Naturally Grown in Western Black Sea Region of Turkey. Agronomy. 2020; 10(11):1748. https://doi.org/10.3390/agronomy10111748
Chicago/Turabian StyleKuru Berk, Selma, Akgul Tas, Erdal Orman, Muttalip Gundogdu, Tomas Necas, Ivo Ondrasek, Neva Karatas, and Sezai Ercisli. 2020. "Agro-Morphological and Biochemical Characterization of Wild Prunus spinosa L. Subsp. dasyphylla (Schur) Domin Genotypes Naturally Grown in Western Black Sea Region of Turkey" Agronomy 10, no. 11: 1748. https://doi.org/10.3390/agronomy10111748
APA StyleKuru Berk, S., Tas, A., Orman, E., Gundogdu, M., Necas, T., Ondrasek, I., Karatas, N., & Ercisli, S. (2020). Agro-Morphological and Biochemical Characterization of Wild Prunus spinosa L. Subsp. dasyphylla (Schur) Domin Genotypes Naturally Grown in Western Black Sea Region of Turkey. Agronomy, 10(11), 1748. https://doi.org/10.3390/agronomy10111748








