Extending Cut Paeonia Lactiflora Pall. Storage Duration Using Sub-Zero Storage Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Exp1—Broad Temperature Range Storage
2.3. Exp2—Near-Freezing Temperature Storage
2.4. Post-Storage Evaluation
2.5. Indices of Physiological Stress and Antioxidant Activity
A = [(Abs532 + TBA) − (Abs600 + TBA)] − (Abs532 − TBA) − (Abs600 − TBA)
B = [(Abs440 + TBA) − (Abs600 + TBA) × 0.0571]
2.6. Experimental Design and Statistics
3. Results and Discussion
3.1. Vase Life (Bud and Open Time)
3.1.1. Exp1—Broad Temperature Range Storage
3.1.2. Exp2—Near-Freezing Temperature Storage
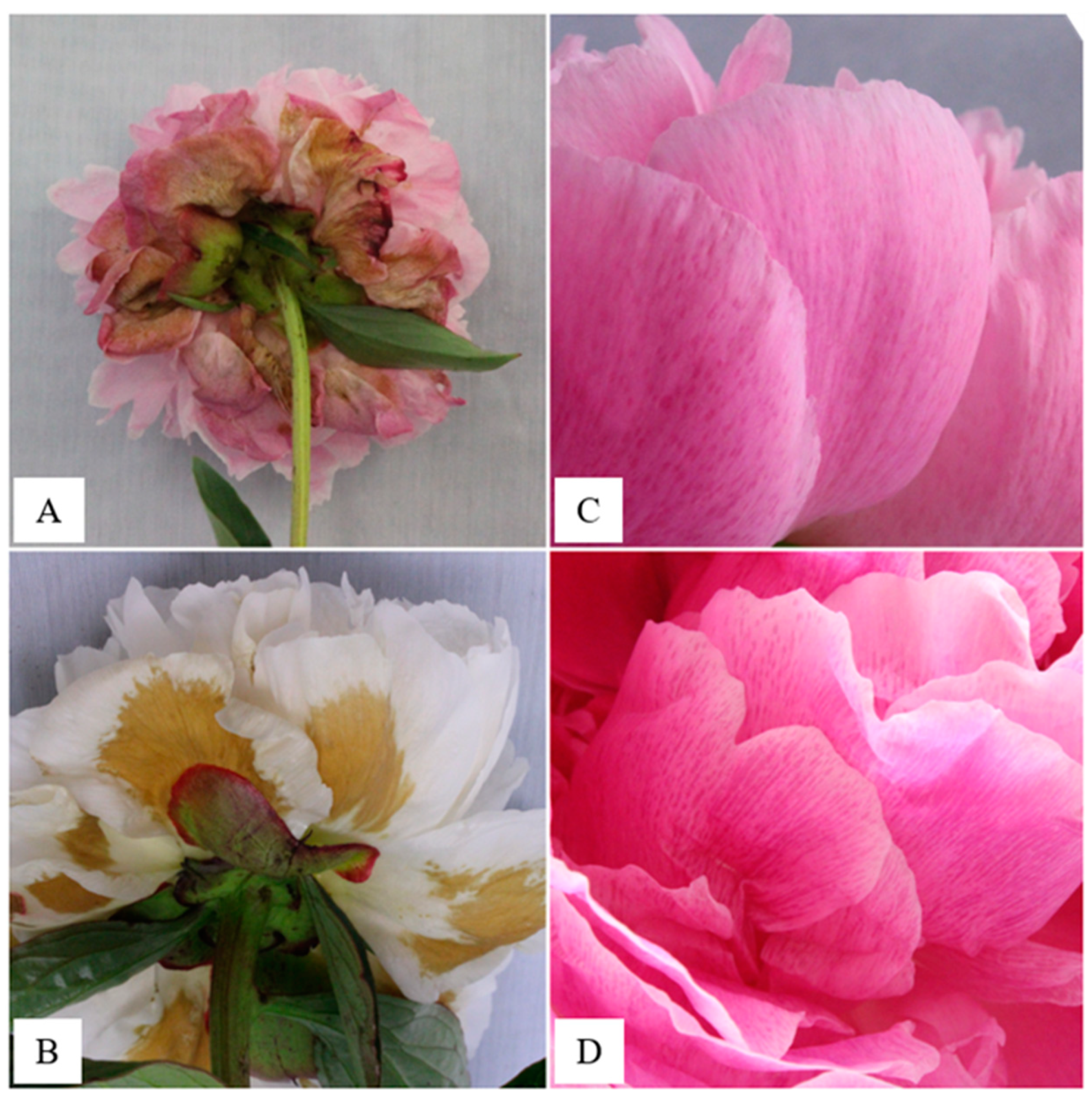
3.2. Flower Quality
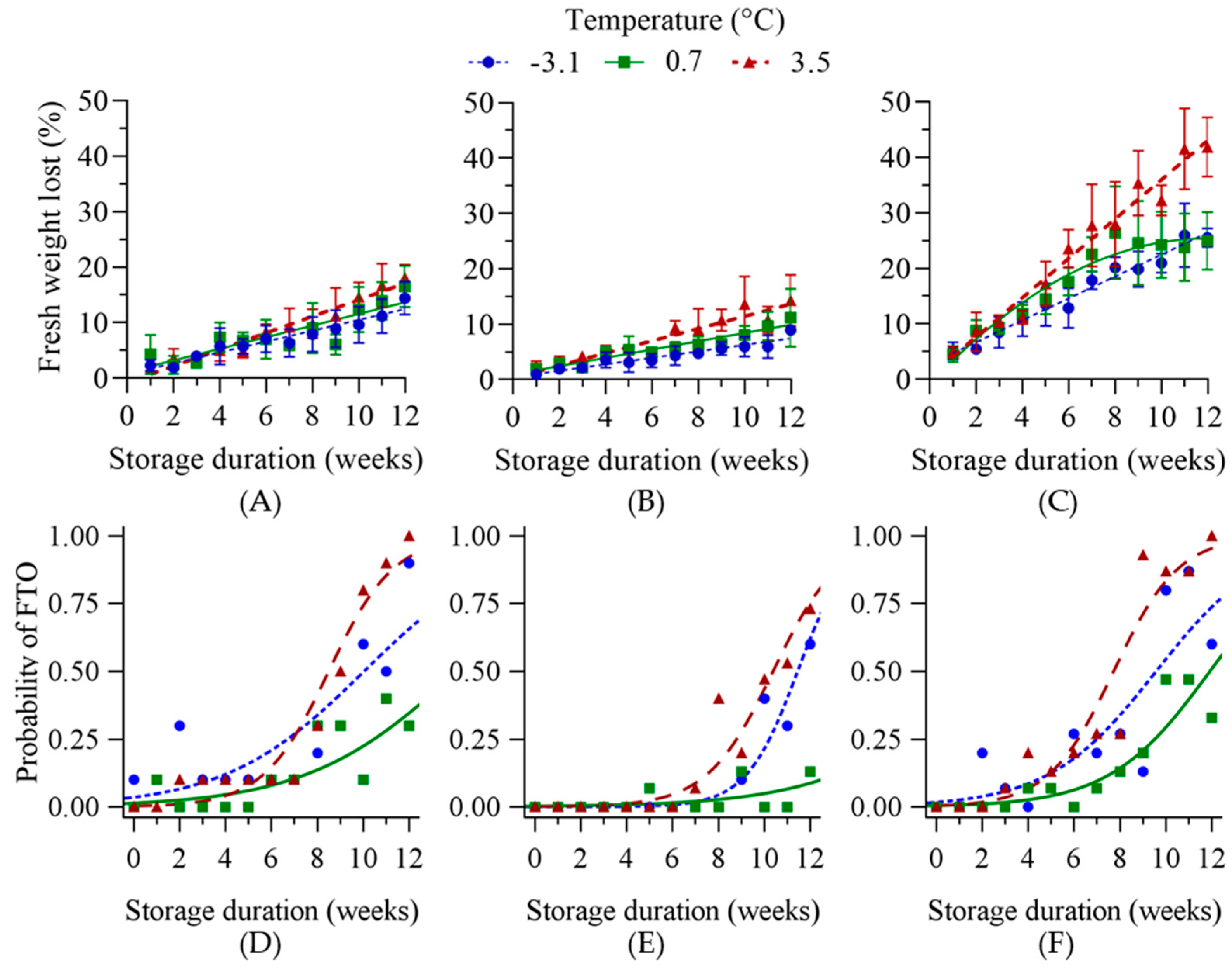
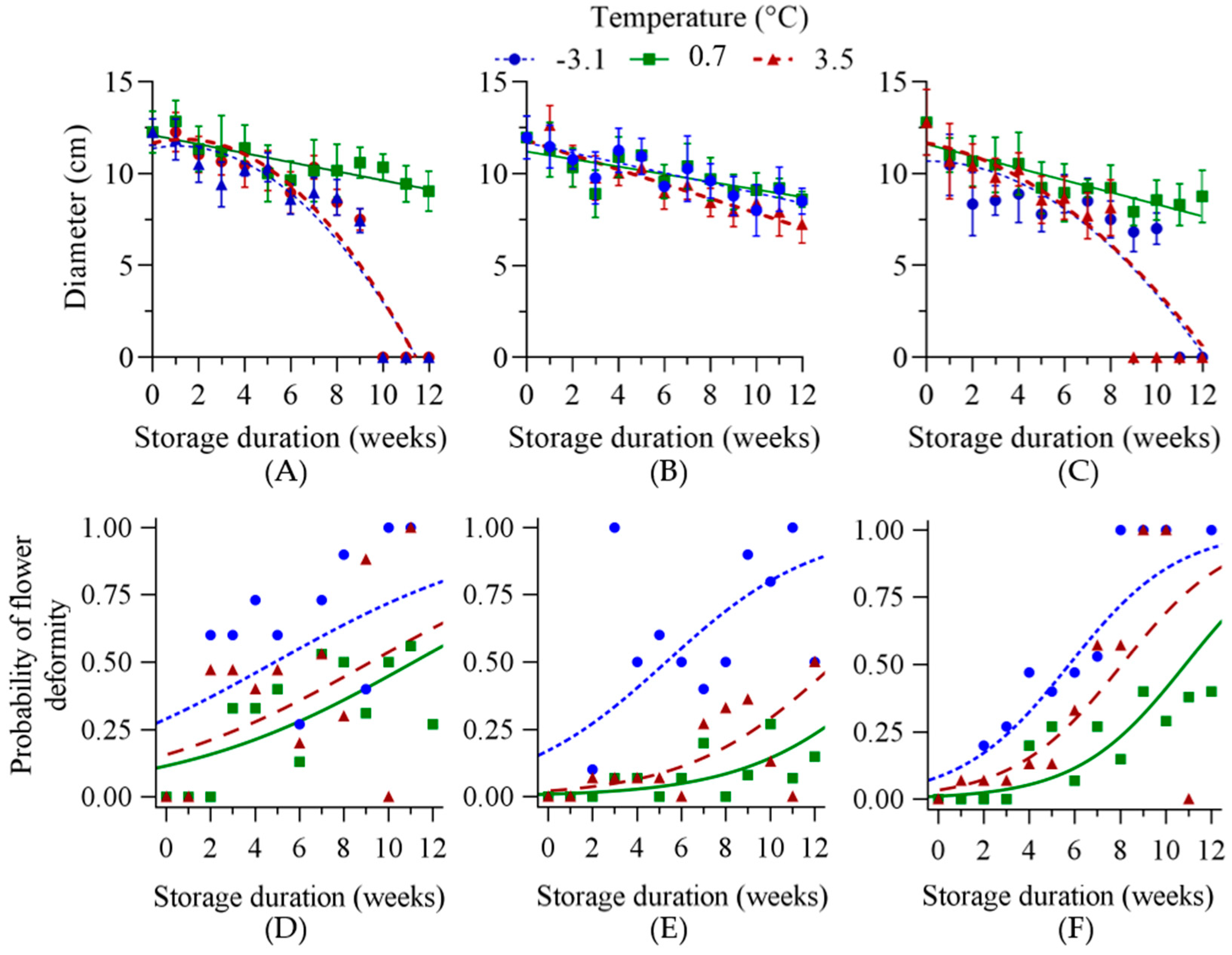
3.2.1. Exp1—Broad Temperature Range Storage
3.2.2. Exp2—Near-Freezing Temperature Storage
3.3. Stress Indices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamenetsky, R.; Dole, J. Herbaceous peony (Paeonia): Genetics, physiology, and cut flower production. Floricult. Ornam. Biotechnol. 2012, 1, 62–77. [Google Scholar]
- Gast, K. Fresh-Cut Peonies. Report of Progress 818; Kansas State University: Manhattan, KS, USA, 1997; Available online: https://www.ksre.k-state.edu/historicpublications/pubs/SRP818.pdf (accessed on 31 October 2020).
- Walton, E.F.; Boldingh, H.L.; McLaren, G.F.; Williams, M.H.; Jackman, R. The dynamics of starch and sugar utilization in cut peony (Paeonia lactiflora Pall.) stems during storage and vase life. Postharvest Biol. Technol. 2010, 58, 142–146. [Google Scholar] [CrossRef]
- Gast, K. Fresh-Cut Peonies. Report of Progress 864; Kansas State University: Manhattan, KS, USA, 1999; Available online: https://www.ksre.k-state.edu/historicpublications/pubs/SRP864.pdf (accessed on 31 October 2020).
- Gast, K. Fresh-Cut Peonies. Report of Progress 866; Kansas State University: Manhattan, KS, USA, 2000; Available online: https://www.ksre.k-state.edu/historicpublications/pubs/SRP866.pdf (accessed on 31 October 2020).
- Loyola-López, N.; Prieto-Labbé, C.; Villouta-Barr, B. Application of calcium, boron and sucrose on cut peony stems (Paeonia lactiflora Pall.) cv. Karl Rosenfield. Agron. Colomb. 2012, 30, 103–110. [Google Scholar]
- Celikel, F.G.; Reid, M.S. Storage temperature affects the quality of cut flowers from the Asteraceae. HortScience 2002, 37, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Heuser, C.W.; Evensen, K.B. Cut flower longevity of peony. J. Am. Soc. Hort. Sci. 1986, 111, 896–899. [Google Scholar]
- Prisa, D.; Burchi, G.; van Doorn, W.G. Effects of low temperature storage and sucrose pulsing on the vase life of Lilium cv. Brindisi inflorescences. Postharvest Biol. Technol. 2013, 79, 39–46. [Google Scholar] [CrossRef]
- Faragher, J.D.; Mayak, S.; Tirosh, T. Physiological response of cut flowers to cold storage. Physiol. Plant. 1986, 67, 205–210. [Google Scholar] [CrossRef]
- Halevy, A.H.; Mayak, S. Senescence and postharvest physiology of cut flowers-Part 2. In Horticulture Reviews; Janick, J., Ed.; Horti AVI Publishing Co. Inc.: Westport, CT, USA, 1981; pp. 59–143. [Google Scholar] [CrossRef]
- Post, K.; Fischer, C.W., Jr. Commercial Storage of Cut Flowers; Cornell Extension Bulletin 853; Cornell University: Ithaca, NY, USA, 1952. [Google Scholar]
- Xue, J.; Tang, Y.; Wang, S.; Xue, Y.; Liu, X.; Zhang, X. Evaluation of dry and wet storage on vase quality of cut peony based on the regulation of starch and sucrose metabolism. Postharvest Bio. Technol. 2019, 155, 11–19. [Google Scholar] [CrossRef]
- Eason, J.; Pickney, T.; Heyes, J.; Brash, D.; Bycroft, B. Effect of storage temperature and harvest bud maturity on bud opening and vase life of Paeonia lactiflora cultivars. N. Z. J. Crop Hort. Sci. 2002, 30, 61–67. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, H.; Liu, B.; Cao, J.; Jiang, W. Improving fresh apricot (Prunus armeniaca L.) quality and antioxidant capacity by storage at near freezing temperature. Sci. Hort. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, W.; Wang, B.; Shen, J.; Zhao, H.; Jiang, W. Near freezing point storage compared with conventional low temperature storage on apricot fruit flavor quality (volatile, sugar, organic acid) promotion during storage and related shelf life. Sci. Hort. 2019, 249, 100–109. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, B.; Cui, K.; Cao, J.; Jiang, W. Improving postharvest quality and antioxidant capacity of sweet cherry fruit by storage at near-freezing temperature. Sci. Hort. 2019, 246, 68–78. [Google Scholar] [CrossRef]
- Zhao, H.; Shu, C.; Fan, X.; Cao, J.; Jiang, W. Near-freezing temperature storage prolongs storage period and improves quality and antioxidant capacity of nectarines. Sci. Hort. 2018, 228, 196–203. [Google Scholar] [CrossRef]
- Nichols, R.; Wallis, L.W. Cool Storage of Cut Narcissus; Agricultural Research Council: Kent, UK, 1972. [Google Scholar]
- Barzilay, A.; Zemah, H.; Kamenetsky, R. Annual life cycle and floral development of ‘Sarah Bernhardt’ peony in Israel. HortScience 2002, 37, 300–303. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Shi, J.; Gao, S.; Wang, Z.; Shi, G. Changes of cold resistance of floral organs during sprout in different tree peony cultivars (In Chinese). China Acad. J. 2013, 21, 52–56. [Google Scholar]
- Walton, E.F.; McLaren, G.F.; Boldingh, H.L. Seasonal patterns of starch and sugar accumulation in herbaceous peony (Paeonia lactiflora Pall.). J. Hort. Sci. Biotechnol. 2007, 82, 365–370. [Google Scholar] [CrossRef]
- Galindo, F.G.; Sjöholm, I.; Rasmusson, A.G.; Widell, S.; Kaack, K. Plant stress physiology: Opportunities and challenges for the food industry. Crit. Rev. Food Sci. Nutr. 2007, 46, 749–763. [Google Scholar] [CrossRef]
- Jones, K.S.; Paroschy, J.; McKersie, B.D.; Bowley, S.R. Carbohydrate composition and freezing tolerance of canes and buds in Vitis Vinifera. J. Plant Physiol. 1999, 155, 101–106. [Google Scholar] [CrossRef]
- Patton, A.J.; Cunningham, S.M.; Volenec, J.J.; Reicher, Z.J. Differences in freeze tolerance of Zoysiagrasses: II. Carbohydrate and proline accumulation. Crop Sci. 2007, 47, 2170–2181. [Google Scholar] [CrossRef]
- Ashworth, E.N.; Stirm, V.E.; Volenec, J.J. Seasonal variations in soluble sugars and starch within woody stems of Cornus sericea L. Tree Physiol. 1993, 13, 379–388. [Google Scholar] [CrossRef]
- Yu, D.J.; Hwang, J.Y.; Chung, S.W.; Ohm, H.D.; Yun, S.K.; Lee, H.J. Changes in cold hardiness and carbohydrate content in peach (Prunus persica) trunk bark and wood tissues during cold acclimation and deacclimation. Sci. Hort. 2017, 219, 45–52. [Google Scholar] [CrossRef]
- Heins, R.D.; Howell, G.S.; Wilkins, H.F. The influence of sucrose, ethanol and calcium nitrate on the freezing-point and long-term low-temperature storage of carnation flowers. Sci. Hort. 1981, 14, 269–275. [Google Scholar] [CrossRef]
- Goszczyńska, D.; Rudnicki, R.M. Long-term storage and carnations cut at the green-bud stage. Sci. Hort. 1982, 17, 289–297. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Dole, I.; Celikel, F.G.; Harkema, H. Opening of cut Iris x hollandica flowers as affected by temperature, dry storage, and light. Postharvest Biol. Technol. 2014, 89, 40–43. [Google Scholar] [CrossRef]
- van Meeteren, U.; Arévalo-Galarza, L.; van Doorn, W.G. Inhibition of water uptake after dry storage of cut flowers: Role of aspired air and wound-induced processes in Chrysanthemum. Postharvest Biol. Technol. 2006, 41, 70–77. [Google Scholar] [CrossRef]
- Mayak, S.; Bravdo, B.; Gvilli, A.; Halevy, A.H. Improvement of opening of cut gladioli flowers by pretreatment with high sugar concentrations. Sci. Hort. 1973, 1, 357–365. [Google Scholar] [CrossRef]
- Doi, M.; Reid, M.S. Sucrose improves the postharvest life of cut flowers of a hybrid Limonium. HortScience 1995, 30, 1058–1060. [Google Scholar] [CrossRef]
- Mor, Y.; Reid, M.S.; Kofranek, A.M. Pulse treatments with silver thiosulfate and sucrose improve the vase life of sweet peas. J. Am. Soc. Hort. Sci. 1984, 109, 866–868. [Google Scholar]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Func. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Droillard, M.-J.; Bureau, D.; Paulin, A.; Daussant, J. Identification of different classes of superoxide dismutase in carnation petals. Electrophoresis 1989, 10, 46–48. [Google Scholar] [CrossRef]
- Lee, S.C.; Kwon, Y.S.; Son, K.H.; Heo, M.Y. Antioxidative constituents form Paeonia lactiflora. Arch. Pharm. Res. 2005, 28, 775–783. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Verma, A.K.; Datta, S.K. Oxidative stress and antioxidant activity as the basis of senescence in Hemerocallis (day lily) flowers. J. Hort. For. 2009, 1, 113–119. [Google Scholar]
- Kumar, N.; Srivastava, G.C.; Dixit, K. Rose of superoxide dismutases during petal senescence in rose (Rosa hybrid L.). J. Hort. Sci. Biotechnol. 2007, 82, 673–678. [Google Scholar] [CrossRef]
- Mayak, S.; Legge, R.L.; Thompson, J.E. Superoxide radical production by microsomal membranes from senescing carnation flowers: An effect of membrane fluidity. Phytochemistry 1983, 22, 1375–1380. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Guimet, J.J.; Montaldi, E.; Puntarulo, S. Antioxidant enzymes and lipid peroxidation during aging of Chrysanthemum morifolium RAM petals. Plant Sci. 1995, 104, 161–168. [Google Scholar] [CrossRef]
- Li, W.; Yang, S.; Cui, H.; Hua, Y.; Tao, J.; Zhou, C. Nutritional evaluation of herbaceous peony (Paeonia lactiflora Pall.) petals. Emir. J. Food Agri. 2017, 29, 518–531. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, T.; Yu, X.; Teixeira da Silva, J.A.; Byrne, D.H. Physiological and biochemical responses of six herbaceous peony cultivars to cold stress. S. Afr. J. Bot. 2014, 94, 140–148. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, F.; Zhang, W.; Liu, C.; Zhao, X. Antioxidant mechanism and lipid peroxidation patterns in leaves and petals of marigold in response to drought stress. Hort. Environ. Biotechnol. 2012, 53, 183–192. [Google Scholar] [CrossRef]
- Fan, J.; Wenxue, Z.; Kang, H.; Ma, H.; Tao, G. Flavonoid constituents and antioxidant capacity in flowers of different Zhongyuan tree peony cultivars. J. Funct. Foods 2012, 4, 147–157. [Google Scholar] [CrossRef]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Wu, S.-H.; Wu, D.-G.; Chen, Y.-W. Chemical constituents and bioactivities of plants from the genus Paeonia. Chem. Biodivers. 2010, 7, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Singleton, V.L.; Otherofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Miller, W.B.; Langhans, R.W. Low temperature alters carbohydrate metabolism in Easter lily bulbs. HortScience 1990, 25, 463–465. [Google Scholar] [CrossRef]
- Rehman, S.I.; Qadir, Z.A.; Sheikh, M.Q.; Ahmed, Z. Effect of pulsing with sucrose and silver thiosulphate complex on keeping quality of cut peony (Paeonia lactiflora Pall.) cv. Sarah Bernhardt. Afr. J. Agri. Res. 2013, 8, 2360–2365. [Google Scholar] [CrossRef][Green Version]
- Hall, A.J.; Catley, J.L.; Walton, E.F. The effect of forcing temperature on peony shoot and flower development. Sci. Hort. 2007, 133, 188–195. [Google Scholar] [CrossRef]
- CRONOS. State Climate Office of North Carolina; NC State University: Raleigh, NC, USA, 2019; Available online: http://climate.ncsu.edu/cronos/ (accessed on 22 November 2019).
- van Doorn, W.G.; Perik, R.R.J.; Abadie, P.; Harkema, H. A treatment to improve the vase life of cut tulips: Effects on tepal senescence, tepal abscission, leaf yellowing and stem elongation. Postharvest Bio. Technol. 2011, 61, 56–63. [Google Scholar] [CrossRef]
- Bravdo, B.; Mayak, S.; Gravrieli, Y. Sucrose and water uptake from concentrated sucrose solutions by gladiolus shoots and the effect of these treatments on floret life. Can. J. Plant Sci. 1974, 52, 1271–1281. [Google Scholar] [CrossRef]
- Xue, J.; Tang, Y.; Wang, S.; Yang, R.; Xue, Y.; Wu, C.; Zang, X. Assessment of vase quality and transcriptional regulation of sucrose transporter and invertase genes in cut peony (Paeonia lactiflora ‘Yang Fei Chu Yu’) treated by exogenous sucrose. Postharvest Bio. Technol. 2018, 143, 92–101. [Google Scholar] [CrossRef]
- Mayak, S.; Halevy, A.H. Water stress as the cause for failure of flower bud opening in iris. J. Am. Soc. Hort. Sci. 1971, 96, 482–483. [Google Scholar]
- Cevallos, J.-C.; Reid, M.S. Effect of dry and wet storage at different temperatures on the vase life of cut flowers. HortTechnology 2001, 11, 199–202. [Google Scholar] [CrossRef]
- Pearce, R.S. Plant freezing and damage. Annu. Bot. 2007, 87, 417–424. [Google Scholar] [CrossRef]
- Min, K.; Chen, K.; Arora, R. Effect of short-term versus prolonged freezing on freeze-thaw injury and post-thaw recovery in spinach: Importance in laboratory freeze-thaw protocols. Environ. Exp. Bot. 2014, 106, 124–131. [Google Scholar] [CrossRef]
- Wright, R.C. The Freezing Temperatures of Some Fruits, Vegetables, and Florists’ Stocks; United States Department of Agriculture: Washington, DC, USA, 1942; p. 447. [CrossRef]
- Jahnke, N.J.; Dole, J.M.; Livingston, D.P., III; Bergmann, B.A. Impacts of carbohydrate pulses and short-term sub-zero temperatures on vase life and quality of cut Paeonia lactiflora Pall. hybrids. Postharvest Biol. Technol. 2020, 161. [Google Scholar] [CrossRef]
- Dole, J.; Stamps, B.; Carlson, A.; Ahmad, I.; Greer, L.; Laushman, J. Postharvest Handling of Cut Flowers and Greens; ASCFG Press: Oberlin, OH, USA, 2017. [Google Scholar]
- Dole, J.M. Storage and simulated shipping of cut ‘Renaissance Red’ poinsettias. Acta Hort. 2005, 683, 103–110. [Google Scholar] [CrossRef]
- Kitamura, Y.; Ueno, S. Inhibition of transpiration from the inflorescence extends the vase life of cut hydrangea flowers. Hort. J. 2015, 84, 156–160. [Google Scholar] [CrossRef]
- Han, S.S. Role of sugar in the vase solution on postharvest flower and leaf quality of Oriental lily ‘Stargazer’. HortScience 2003, 38, 412–416. [Google Scholar] [CrossRef]
- Shin, Y.; Liu, R.H.; Nock, J.F.; Holliday, D.; Watkins, C.B. Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biol. Technol. 2007, 45, 349–357. [Google Scholar] [CrossRef]
- Kim, M.J.; Perkins-Veazie, P.; Ma, G.; Fernandez, G. Shelf life and changes in phenolic compounds of organically grown blackberries during refrigerated storage. Postharvest Biol. Technol. 2015, 110, 257–263. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. A thorough study of reactivity of various compound classes towards the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Liu, M.; Zhang, Q.; Wang, T.; Sun, X. Factors affecting the color of herbaceous peony. J. Am. Soc. Hort. Sci. 2020, 145, 257–266. [Google Scholar] [CrossRef]
- Jia, N.; Shu, Q.-Y.; Wang, D.-H.; Wang, L.-S.; Liu, Z.-A.; Ren, H.-X.; Xu, Y.-J.; Tian, D.-K.; Tilt, K.M. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-mass spectrometry in herbaceous peony species. J. Am. Hort. Sci. 2008, 133, 418–426. [Google Scholar] [CrossRef]
- Pennycooke, J.C.; Cox, S.; Stushnoff, C. Relationship of cold acclimation, total phenolic content and antioxidant capacity with chilling tolerance in petunia (Petunia × hybrida). Environ. Exp. Bot. 2005, 53, 225–232. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, 1064–1070. [Google Scholar] [CrossRef]


| Cultivar | FM | MJE | SB | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Open Time (d) | Open Time (d) | Open Time (d) | ||||||||
| ST (°C) | −3.1 | 0.7 | 3.5 | −3.1 | 0.7 | 3.5 | −3.1 | 0.7 | 3.5 | |
| Storage duration (weeks) | 1 | 2.9 * ns | 2.6 * ns | 3.3 * ns | 5.5 * bs | 6.4 * as | 5.3 * bs | 4.5 * ns | 5.5 * ns | 5.0 * ns |
| 2 | 2.0 * ns | 2.6 * ns | 2.3 * ns | 4.0 * ns | 4.3 * ns | 4.0 * ns | 3.5 * bs | 5.1 * as | 4.3 * ab | |
| 3 | 2.0 * ns | 2.9 * ns | 2.5 * ns | 4.1 * ns | 3.9 * ns | 4.4 * ns | 2.8 * bs | 4.4 * as | 3.3 * bs | |
| 4 | 1.5 * b1 | 2.6 * as | 2.6 * as | 3.9 * ns | 4.5 * ns | 4.2 * ns | 3.0 * bs | 4.8 * as | 3.9 * ab | |
| 5 | 2.0 * b1 | 3.4 * as | 3.4 * as | 4.0 * bs | 4.5 * bs | 5.1 s as | 2.6 * bs | 3.9 * as | 3.7 * ab | |
| 6 | 2.0 * b1 | 3.0 * as | 3.0 * as | 3.4 * ns | 4.2 * ns | 4.0 * ns | 2.8 * ns | 3.1 * ns | 3.4 * ns | |
| 7 | 0.9 * b1 | 2.2 * as | 2.2 * as | 2.9 * bs | 4.0 * as | 4.0 * as | 2.7 * bs | 4.0 * as | 3.3 * ab | |
| 8 | 1.2 * b1 | 3.3 * as | 3.8 * as | 3.9 * ns | 4.6 * ns | 3.9 * ns | 2.0 * bs | 3.9 * as | 3.3 * ab | |
| 9 | 0.0 2.3 b* | 3.0 * ss | 1.8 * ss | 2.4 * bs | 4.0 * as | 3.7 * as | 0.0 * . s | 3.3 * ss | 3.0 * ss | |
| 10 | 0.0 2.3 b* | 2.5 * ss | 1.0 * ss | 2.0 * bs | 4.3 * as | 3.8 * as | 0.0 * . s | 3.0 * ss | 3.0 * ss | |
| 11 | 0.0 2.3 b* | 2.4 * ss | 1.0 * ss | 1.9 * bs | 4.3 * as | 3.0 * bs | 0.0 * . s | 3.4 * ss | 3.5 * ss | |
| 12 | 0.0 2.3 b* | 3.0 * ss | 0.0 * ss | 1.0 * . s | 3.6 * ss | 2.3 * ss | 0.0 * . s | 3.4 * ss | 3.0 * ss | |
| Non-stored | 4.6 | 5.5 | 7.0 | |||||||
| Bud Time (d) | Open Time (d) | FTO (%) | Deformation (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ST (°C) | 0.7 | −0.6 | 0.7 | −0.6 | 0.7 | −0.6 | 0.7 | −0.6 | |
| Storage duration (weeks) | 2 | 1.1 | 1.0 | 3.3 * a1 | 3.3 * a1 | 00 | 0 | 00 | 00 |
| 4 | 1.0 | 1.0 | 3.9 * a1 | 4.2 * a | 00 | 0 | 00 | 00 | |
| 6 | 1.0 | 1.0 | 3.2 * b1 | 3.9 ** a | 00 | 0 | 00 | 00 | |
| 8 | 1.0 | 1.1 | 3.5 * b1 | 4.1 * a | 00 | 0 | 07 | 00 | |
| 10 | 1.1 | 1.1 | 3.8 ** b1 | 4.4 ** a | 00 | 0 | 07 | 03 | |
| 12 | 1.1 | 1.0 | 4.0 ** b1 | 5.4 * a | 00 | 0 | 10 | 00 | |
| 14 | 1.0 | 1.1 | 3.2 * b1 | 4.5 ** a | 10 | 0 | 43 | 10 | |
| 16 | 1.0 | 1.0 | 3.6 * b1 | 4.3 * a | 20 | 0 | 33 | 27 | |
| Non-stored | 1.2 | 1.2 | 4.1 b1 | 4.1 a | 00 | 0 | 00 | 00 | |
| Pre-Storage Pulse Treatment 1 | ||||
|---|---|---|---|---|
| Non-Pulsed | Hydrator | Sucrose | ||
| Open Time (d) | ||||
| Storage duration (weeks) | 2 | 2.8 * a2 | 3.1 * a | 2.8 * a |
| 4 | 3.5 * a1 | 3.2 * a | 3.4 a | |
| 6 | 3.0 * a1 | 2.8 * a | 2.9 a | |
| 8 | 3.0 * a1 | 3.0 * a | 2.7 * a | |
| 10 | 3.2 * ab | 3.3 * a | 2.6 * b | |
| 12 | 3.1 b1 | 4.4 * a | 3.0 b | |
| 14 | 3.0 * a1 | 2.8 * a | 2.8 * a | |
| 16 | 2.9 * a1 | 2.9 * a | 2.9 a | |
| Non-stored | 3.7 * a1 | 3.3 a | 3.6 a | |
| Storage Temperature (°C) | |||
|---|---|---|---|
| 0.7 | −0.6 | ||
| Diameter (cm) | |||
| Storage duration (weeks) | 2 | 12.1 a1 | 12.1 a |
| 4 | 19.8 * a1 | 19.6 * a | |
| 6 | 10.3 * a1 | 10.6 * a | |
| 8 | 19.2 * a1 | 19.7 * a | |
| 10 | 18.6 * b1 | 10.1 * a | |
| 12 | 19.4 * a1 | 19.6 * a | |
| 14 | 18.8 * b1 | 10.0 * a | |
| 16 | 18.6 * a1 | 19.5 * a | |
| Non-stored | 13.3 a1 | 12.8 a | |
| FM | MJE | SB | ||
|---|---|---|---|---|
| TPC (μg·kg−1 FW) | ||||
| Storage duration (weeks) | 2 | 31.08 edd | 38.83 e1d | 38.89 cde |
| 4 | 34.06 ded | 42.42 de1 | 39.61 cdd | |
| 6 | 38.12 abc | 42.91 de1 | 42.07 abc | |
| 8 | 37.17 bcd | 46.30 cd1 | 41.04 bcd | |
| 10 | 36.33 bcd | 47.50 cd1 | 41.39 bcd | |
| 12 | 35.09 cdd | 58.13 ad1 | 35.96 ded | |
| 14 | 39.82 abd | 59.90 ad1 | 47.35 add | |
| 16 | 41.344 add | 53.63 bd1 | 45.91 abd | |
| Non-stored | 34.81 cddg | 38.53 e1d | 33.92 edd | |
| p-value | <0.0001 | <0.0001 | <0.0001 | |
| FM | MJE | SB | ||
|---|---|---|---|---|
| MDA (nmol·g−1 FW) | ||||
| Storage duration (weeks) | 2 | 0.40 | 6.20 bc | 3.60 |
| 4 | 0.40 | 6.88 bc | 3.64 | |
| 6 | 0.40 | 7.64 ab | 3.98 | |
| 8 | 0.40 | 7.64 ab | 4.12 | |
| 10 | 0.40 | 9.43 ad | 3.73 | |
| 12 | 0.40 | 7.95 ab | 3.30 | |
| 14 | 0.40 | 4.79 cd | 3.43 | |
| 16 | 0.40 | 7.06 bd | 4.95 | |
| Non-stored | 0.40 | 4.78 cd | 3.09 | |
| p-value | NS | <0.0001 | NS | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahnke, N.J.; Dole, J.M.; Bergmann, B.A.; Ma, G.; Perkins-Veazie, P. Extending Cut Paeonia Lactiflora Pall. Storage Duration Using Sub-Zero Storage Temperatures. Agronomy 2020, 10, 1694. https://doi.org/10.3390/agronomy10111694
Jahnke NJ, Dole JM, Bergmann BA, Ma G, Perkins-Veazie P. Extending Cut Paeonia Lactiflora Pall. Storage Duration Using Sub-Zero Storage Temperatures. Agronomy. 2020; 10(11):1694. https://doi.org/10.3390/agronomy10111694
Chicago/Turabian StyleJahnke, Nathan J., John M. Dole, Ben A. Bergmann, Guoying Ma, and Penelope Perkins-Veazie. 2020. "Extending Cut Paeonia Lactiflora Pall. Storage Duration Using Sub-Zero Storage Temperatures" Agronomy 10, no. 11: 1694. https://doi.org/10.3390/agronomy10111694
APA StyleJahnke, N. J., Dole, J. M., Bergmann, B. A., Ma, G., & Perkins-Veazie, P. (2020). Extending Cut Paeonia Lactiflora Pall. Storage Duration Using Sub-Zero Storage Temperatures. Agronomy, 10(11), 1694. https://doi.org/10.3390/agronomy10111694




