Agromorphological Characterization Revealed Three Phenotypic Groups in a Region-Wide Germplasm of Fonio (Digitaria exilis (Kippist) Stapf) from West Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
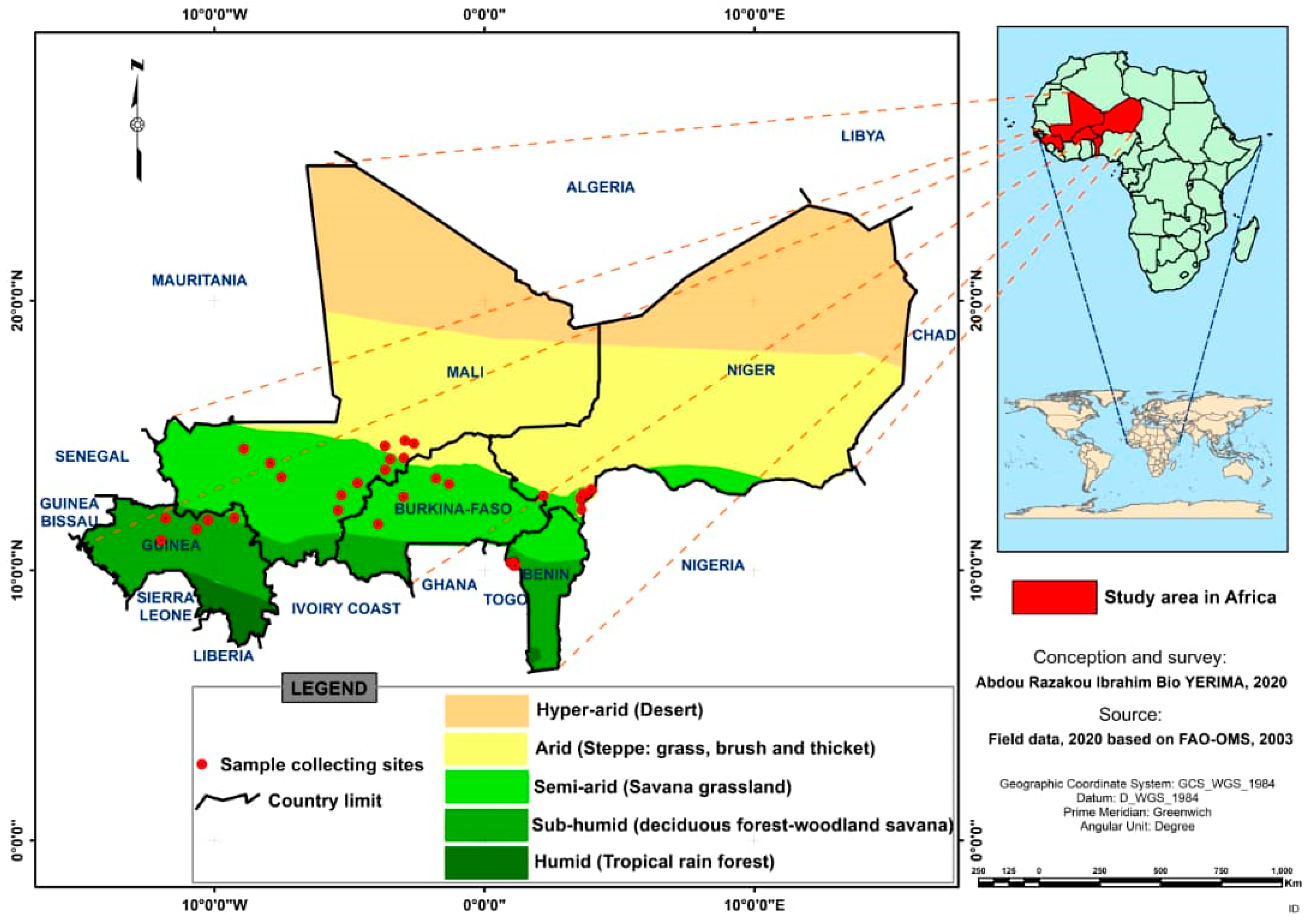
2.2. Description of Site
2.3. Experimental Design and Management
2.4. Data Collection
2.5. Data Analysis
3. Results
3.1. Variation of Qualitative Traits among Fonio Accessions
3.2. Fonio Performance Analysis Based on Country of Provenance
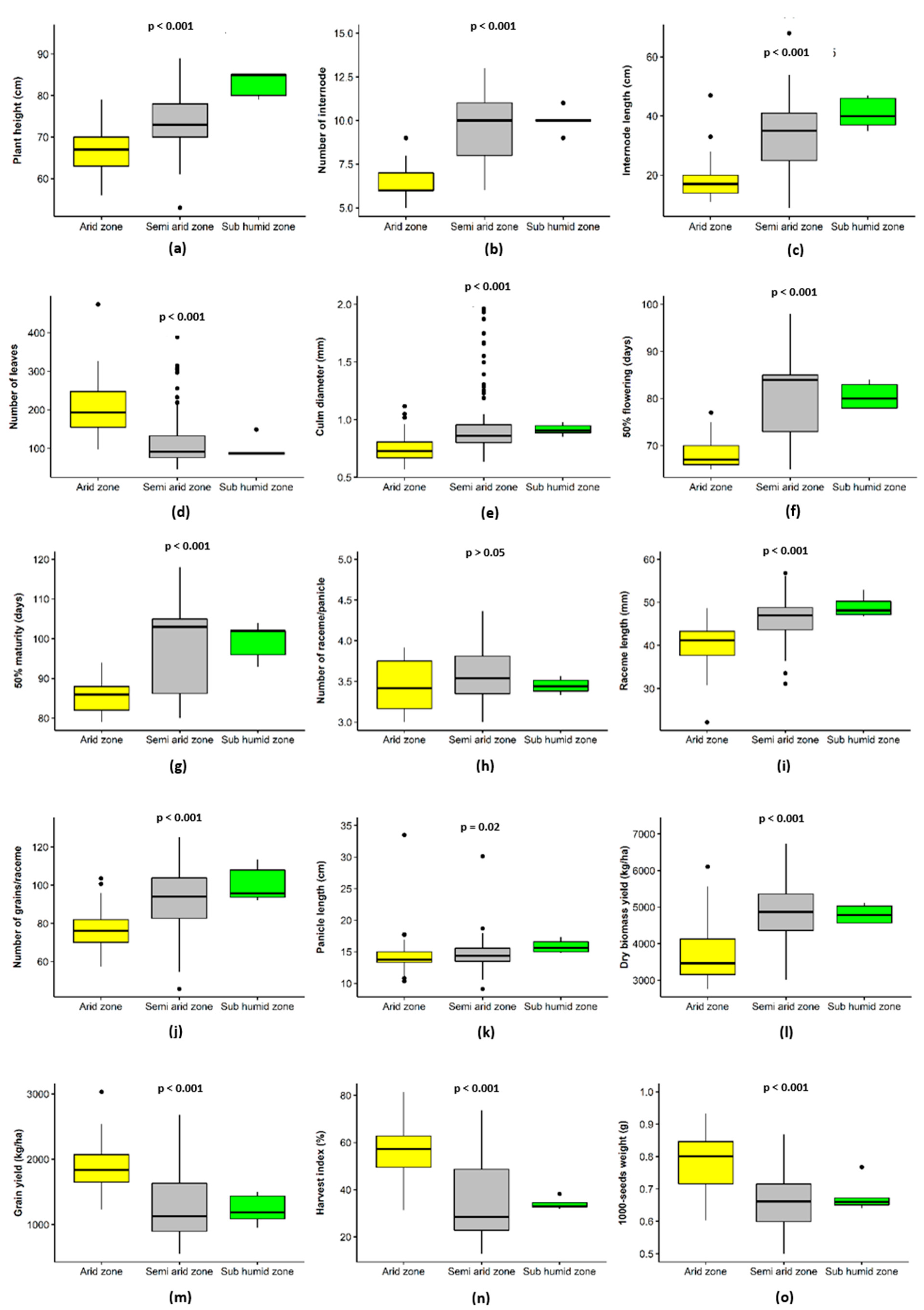
3.3. Fonio Performance Analysis Based on Ecological Zones
3.4. Relationship among Quantitative Traits
3.5. Clustering of Accessions Based on Country of Provenance
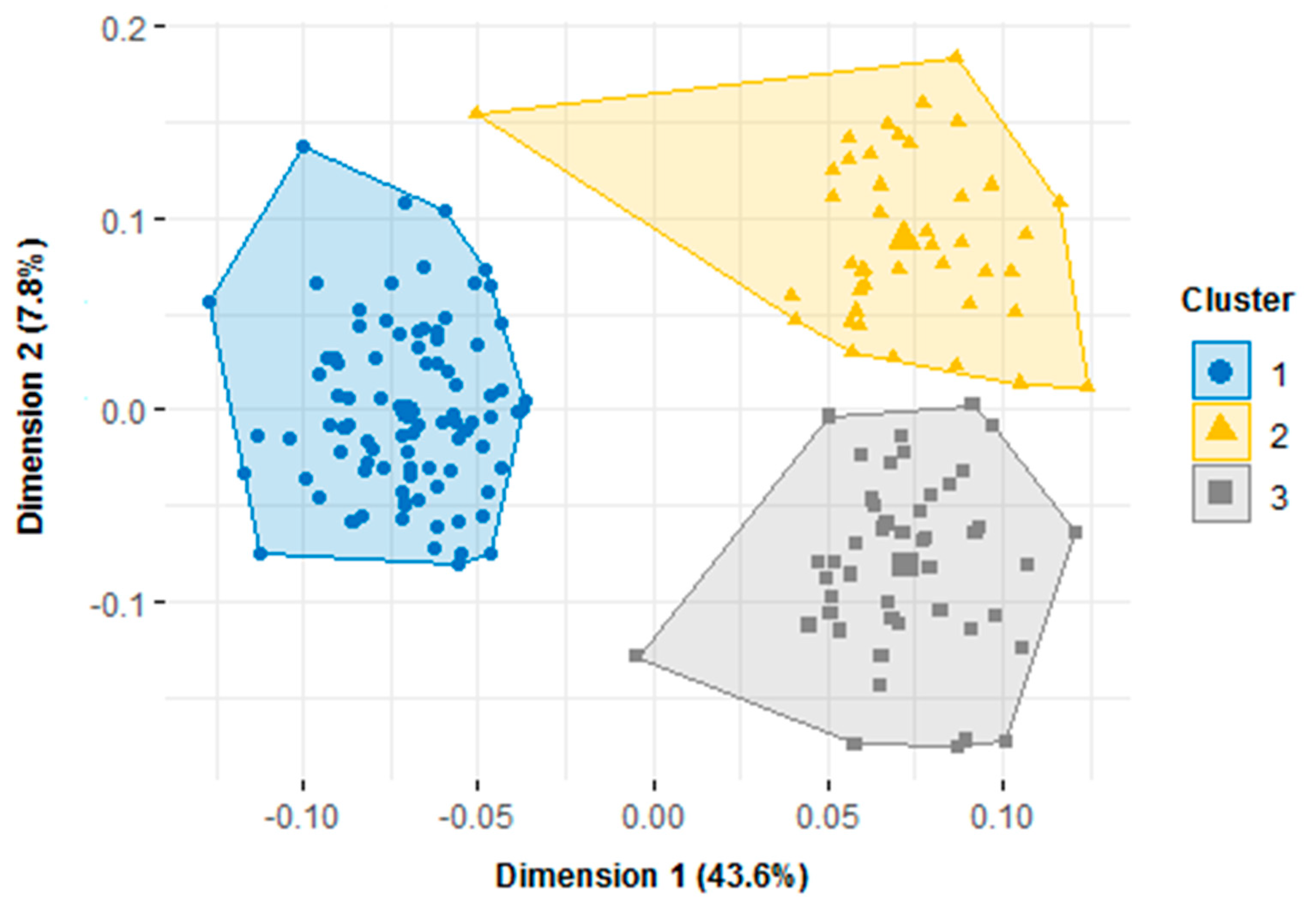
3.6. Partition of Accessions into Phenotypic Groups
4. Discussion
4.1. Qualitative Traits Variation in Fonio
4.2. Performance of Fonio Accessions Based on Provenance
4.3. Quantitative Traits Association and Partition of Phenotypic Groups in Fonio
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garí, J.A. Review of the African millet diversity. In International Workshop on Fonio, Food Security and Livelihood among the Rural Poor in West Africa; IPGRI/IFAD: Bamako, Mali, 2002. [Google Scholar]
- Adoukonou-Sagbadja, H.; Dansi, A.; Vodouhè, R.; Akpagana, K. Indigenous knowledge and traditional conservation of fonio millet (Digitaria exilis, Digitaria iburua) in Togo. Biodivers. Conserv. 2006, 15, 2379–2395. [Google Scholar] [CrossRef]
- Ballogou, V.Y.; Soumanou Mohamed, M.; Toukourou, F.; Hounhouigan Joseph, D. Indigenous knowledge on landraces and fonio-based food in Benin. Ecol. Food Nutr. 2014, 53, 390–409. [Google Scholar] [CrossRef]
- Sartelet, H.; Serghat, S.; Lobstein, A.; Ingenbleek, Y.; Anton, R.; Petitfrere, E.; Aguie-Aguie, G.; Martiny, L.; Haye, B. Flavonoids extracted from fonio millet (Digitaria exilis) reveal potent antithyroid properties. Nutrition 1996, 12, 100–106. [Google Scholar] [CrossRef]
- Vodouhè, S.; Achigan Dako, E.G. Digitaria exilis (Kippist) Stapf; Plant Resources of Tropical Africa 1. Cereals and Pulses; Brink, M., Belay, G., Eds.; PROTA Foundations: Wageningen, The Netherlands; Backhuys Publishers: Leiden, The Netherlands, 2006; Volume 1. [Google Scholar]
- Fogny, N.F.; Madode, E.Y.; Laleye, F.F.; Amoussou-Lokossou, Y.; Kayode, A.P. Formulation de farine de fonio enrichie en ressources alimentaires locales pour l’alimentation complémentaire des jeunes enfants au Bénin. Int. J. Biol. Chem. Sci. 2017, 11, 2745–2755. [Google Scholar] [CrossRef][Green Version]
- Cruz, J.; Beavogui, F.; Dramé, D. Fonio, an African Cereal [Le Fonio, une Céréale Africaine]; CTA: Wageningen, The Netherlands, 2011. [Google Scholar]
- Sekloka, E.; Adoukonou-Sagbadja, H.; Paraïso Armand, A.; Yoa Brigitte, K.; Bachabi, F.-X.; Zoumarou-Wallis, N. Evolution de la diversité des cultivars de fonio pratiqués dans la commune de Boukoumbé au Nord-Ouest du Bénin. Int. J. Biol. Chem. Sci. 2015, 9, 2446–2458. [Google Scholar] [CrossRef][Green Version]
- Ayenan, M.A.T.; Sodedji, K.A.F.; Nwankwo, C.I.; Olodo, K.F.; Alladassi, M.E.B. Harnessing genetic resources and progress in plant genomics for fonio (Digitaria spp.) improvement. Genet. Resour. Crop Evol. 2017, 65, 373–386. [Google Scholar] [CrossRef]
- Dansi, A.; Adoukonou-Sagbadja, H.; Vodouhè, R. Diversity, conservation and related wild species of Fonio millet (Digitaria spp.) in the northwest of Benin. Genet. Resour. Crop Evol. 2010, 57, 827–839. [Google Scholar] [CrossRef]
- Bhanu, A.N. Assessment of Genetic Diversity in Crop Plants—An Overview. Adv. Plants Agric. Res. 2017, 7, 279–286. [Google Scholar] [CrossRef]
- Mondini, L.; Noorani, A.; Pagnotta, M. Assessing Plant Genetic Diversity by Molecular Tools. Diversity 2009, 1, 19–35. [Google Scholar] [CrossRef]
- Odjo, T.C.; Dossou-Aminon, I.; Dansi, A.; Bonou-Gbo, Z.; Kombaté, K. Agro-Morphological Characterization and Assessment of Variability within a Germplasm of Benin Rice (Oryza sativa L.) Varieties. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 1–16. [Google Scholar]
- Babu, B.K.; Sood, S.; Agrawal, P.; Chandrashekara, C.; Kumar, A.; Kumar, A. Molecular and Phenotypic Characterization of 149 Finger Millet Accessions Using Microsatellite and Agro-Morphological Markers. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2016, 87, 1217–1228. [Google Scholar] [CrossRef]
- Sattler, F.; Sanogo, M.; Kassari, I.; Angarawai, I.; Gwadi, K.; Dodo, H.; Haussmann, B. Characterization of West and Central African accessions from a pearl millet reference collection for agro-morphological traits and Striga resistance. Plant Genet. Resour. Charact. Util. 2017, 16, 260–272. [Google Scholar] [CrossRef]
- Naoura, G.; Sawadogo, N.; Atchozou, E.A.; Emendack, Y.; Hassan, M.A.; Reoungal, D.; Amos, D.N.; Djirabaye, N.; Tabo, R.; Laza, H. Assessment of agro-morphological variability of dry-season sorghum cultivars in Chad as novel sources of drought tolerance. Sci. Rep. 2019, 9, 19581. [Google Scholar] [CrossRef]
- Clottey, V.; Agyare, W.; Bayorbor, T.; Abanga, J.; Kombiok, J. Genetic relatedness of fonio (Digitaria spp.) landraces assembled in Ghana. Plant Genet. Resour. Newsl. (Bioversity Int. FAO) 2006, 147, 6–11. [Google Scholar]
- Adoukonou-Sagbadja, H.; Wagner, C.; Dansi, A.; Ahlemeyer, J.; Dainou, O.; Akpagana, K.; Ordon, F.; Friedt, W. Genetic diversity and population differentiation of traditional fonio millet (Digitaria spp.) landraces from different agro-ecological zones of West Africa. Theor. Appl. Genet. 2007, 115, 917–931. [Google Scholar] [CrossRef]
- Sekloka, E.; Kanlindogbe, C.; Biaou Samadori, S.H.; Adoukonou-Sagbadja, H.; Kora, A.; Motouama, F.T.; Seidou, M.; Zinsou Valérien, A.; Afouda, L.; Baba-Moussa, L. Agro-morphological characterization of fonio millet accessions (Digitaria exilis Stapf.) collected from Boukoumb, Northwest of Benin. J. Plant Breed. Crop Sci. 2016, 8, 211–222. [Google Scholar]
- Nyam, D.; Kwon-Ndung, E.; Wuyep, A. Genetic affinity and breeding potential of phenologic traits of acha (fonio) in Nigeria. J. Sci. Eng. Res. 2017, 4, 91–101. [Google Scholar]
- Saidou, S.I.; Bakasso, Y.; Inoussa, M.M.; Zaman-Allah, M.; Atta, S.; Barnaud, A.; Billot, C.; Saadou, M. Diversité agro-morphologique des accessions de fonio [Digitaria exilis (Kippist.) Stapf.] au Niger. Int. J. Biol. Chem. Sci. 2014, 8, 1710. [Google Scholar] [CrossRef]
- Peyre, D. Catalogue des Plantes Vasculaires du Niger; Institut d’Elevage et de Médecine Vétérinaire des Pays Tropicaux: Paris, France, 1976; Volume 1, pp. 405–419. [Google Scholar]
- Peyre de Fabregues, B.; Lebrun, J. Catalogue des Plantes Vasculaires du Niger (Etude Botanique n 3.) IEMVT; Laboratoire National d’Elevage: Maisons-Alfort, France; Niamey, Niger, 1976; p. 433. [Google Scholar]
- White, F. The Vegetation of Africa; Natural Resources Research; UNESCO: Paris, France, 1983; Volume 20, p. 356. [Google Scholar]
- Dobignard, A.; Chatelain, C. Éditions des Conservatoire et Jardin Botaniques de la Ville de Genève; Index Synonymique de la Flore d’Afrique du Nord; Geneve, Switzerland, 2010; Volume 1, p. 455. [Google Scholar]
- Prance, G.; Sothers, C. Chrysobalanaceae 1 & 2, Species Plantarum: Flora of the World, Parts 9 &10; Australian Biological Resources Study: Canberra, Australia, 2003. [Google Scholar]
- Bioversity International, I.W. Descriptors for Wild and Cultivated Rice (Oryza spp.); Bioversity International: Rome, Italy, 2007. [Google Scholar]
- Kassambara, A. Practical Guide to Principal Component Methods in R (Multivariate Analysis); CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2017; Volume 2. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package ‘Factoextra’. Extract and Visualize the Results of Multivariate Data Analyses; R pachage. 2017. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 5 August 2020).
- Team, R.C. Version 3.5. 1. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org (accessed on 3 November 2018).
- Argüelles, M.; Benavides, C.; Fernández, I. A new approach to the identification of regional clusters: Hierarchical clustering on principal components. Appl. Econ. 2014, 46, 2511–2519. [Google Scholar] [CrossRef]
- Rencher, A. Discriminant analysis: Description of group separation. In Methods of Multivariate Analysis, 2nd ed.; John Wiles and Sons: New York, NY, USA, 2002; pp. 270–298. [Google Scholar]
- Pagès, J. Multiple Factor Analysis by Example Using R; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Kanya, J.I.; Hauser, T.P.; Kinyamario, J.I.; Amugune, N.O. Hybridization potential between cultivated rice Oryza sativa and African wild rice Oryza longistaminata. Int. J. Agric. Res. 2012, 7, 291–302. [Google Scholar] [CrossRef]
- Govindaraj, M.; Rao, A.S.; Shivade, H.; Rai, K. Effect of grain colour on iron and zinc density in pearl millet. Indian J. Genet. Plant Breed. 2018, 78, 247. [Google Scholar] [CrossRef]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K.; et al. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Blench, R.M. Vernacular names for African millets and other minor cereals and their significance for agricultural history. Archaeol. Anthropol. Sci. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Lule, D.; Tesfaye, K.; Fetene, M. Qualitative traits diversity and eco-geographical distribution in finger millet (Eleusine coracana) landraces from eastern and south eastern Africa: An implication for germplasm collection and conservation. Afr. J. Plant Sci. 2012, 6, 346–354. [Google Scholar]
- Vigouroux, Y.; Barnaud, A.; Scarcelli, N.; Thuillet, A.-C. Biodiversity, evolution and adaptation of cultivated crops. Comptes Rendus Biol. 2011, 334, 450–457. [Google Scholar] [CrossRef]
- Dehgahi, R.; Joniyas, A.; Latip, S.N.H.B.M. Rainfall Distribution and Temperature Effects on Wheat Yield in Torbate Heydarie. Int. J. Sci. Res. Knowl. ISSN 2014, 2, 2322–4541. [Google Scholar]
- Kyei-Mensah, C.; Kyerematen, R.; Adu-Acheampong, S. Impact of Rainfall Variability on Crop Production within the Worobong Ecological Area of Fanteakwa District, Ghana. Adv. Agric. 2019, 2019. [Google Scholar] [CrossRef]
- Aliero, A.; Morakinyo, J. Photoperiodism in Digitaria exilis (Kipp) Stapf accessions. Afr. J. Biotechnol. 2005, 4, 241–243. [Google Scholar]
- Haussmann, B.I.; Fred Rattunde, H.; Weltzien-Rattunde, E.; Traoré, P.; Vom Brocke, K.; Parzies, H.K. Breeding strategies for adaptation of pearl millet and sorghum to climate variability and change in West Africa. J. Agron. Crop Sci. 2012, 198, 327–339. [Google Scholar] [CrossRef]
- Gueye, M.; Kanfany, G.; Fofana, A.; Noba, K.; Grove, J. Effect of planting date on growth and grain yield of fonio millet (Digitaria exilis Stapf) in the Southeast of Senegal. Int. J. Biol. Chem. Sci. 2015, 9, 581–592. [Google Scholar] [CrossRef]
- Wolabu, T.W.; Tadege, M. Photoperiod response and floral transition in sorghum. Plant Signal. Behave. 2016, 11, e1261232. [Google Scholar] [CrossRef]
- Sultana, H.; Ali, N.; Iqbal, M.M.; Khan, A.M. Vulnerability and adaptability of wheat production in different climatic zones of Pakistan under climate change scenarios. Clim. Chang. 2009, 94, 123–142. [Google Scholar] [CrossRef]
- Jyothsna, S.; Patro, T.; Ashok, S.; Rani, Y.S.; Neeraja, B. Studies on genetic parameters, character association and path analysis of yield and its components in finger millet (Eluesine coracana L. Gaertn). Int. J. Theor. Appl. Sci. 2016, 8, 25. [Google Scholar]
- Assefa, E. Correlation and Path Coefficient Studies of Yield and Yield Associated Traits in Bread Wheat (Triticum aestivum L.) Genotypes. Adv. Plants Agric. Res. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Chavan, B.; Jawale, L.N.; Shinde, A.V. Correlation and path analysis studies in finger millet for yield and yield contributing traits (Eleusine coracana L. Gaertn). Int. J. Chem. Stud. 2020, 8, 2911–2914. [Google Scholar]
- Jifar, H.; Dagne, K.; Tesfaye, K.; Assefa, K.; Tadele, Z. Agro-Morphological Traits Diversity in Tef [Eragrostis Tef (Zucc.) Trotter] Genotypes from Various Sources. Ethiop. J. Agric. Sci. 2018, 28, 131–148. [Google Scholar]
- Sidibé, A.; Meldrum, G.; Coulibaly, H.; Padulosi, S.; Traore, I.; Diawara, G.; Sangaré, A.R.; Mbosso, C. Revitalizing cultivation and strengthening the seed systems of fonio and Bambara groundnut in Mali through a community biodiversity management approach. Plant Genet. Resour. 2020, 19, 31–48. [Google Scholar]


| Qualitative | ||
|---|---|---|
| Descriptors | Code | Description |
| Vigour at seedling | 1 = good establishment | Recorded three weeks after planting |
| 2 = moderate establishment | ||
| 3 = poor establishment | ||
| Panicle exertion | 1 = well exerted | Recorded near maturity |
| 3 = moderately exerted | ||
| 5 = lightly exerted | ||
| 7 = partially exerted | ||
| Panicle type | 1 = compact | Recorded near maturity |
| 3 = intermediate | ||
| 5 = open | ||
| Panicle leaf attitude | 1 = erect | Recorded after heading |
| 3 = intermediate | ||
| 5 = horizontal | ||
| 7 = descending | ||
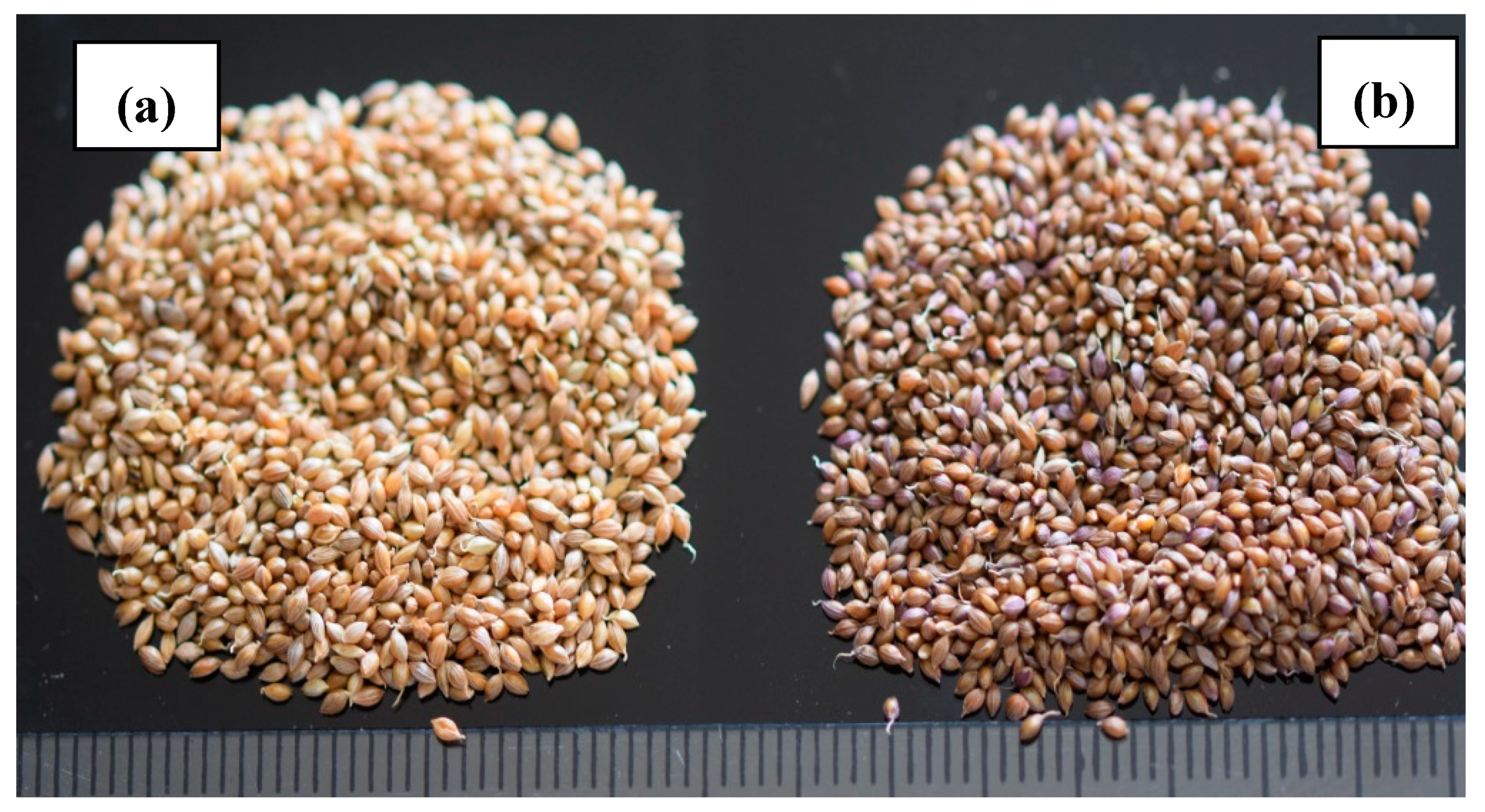
| Phenotypic grain colour | 1 = brown | Recorded after harvesting |
| 2 = greyed orange | ||
| Quantitative | ||
| Descriptors | Code | Description |
| Plant height (cm) | PHT | Measured from the soil level to the tip of longest panicle |
| Number internode | NIN | Mean number of internodes on the main stem from 10 plants |
| Internode length (cm) | INL | Measured from the 2nd internode to last internode below the panicle |
| Number of leaves/plant | NFP | Mean number of leaves from 10 plants |
| Culm diameter (mm) | CD | Measured at the mid portion of the culm. |
| Days to 50% flowering (days) | FLO | Date on which 50% of the plants are flowering |
| Days to 50% maturity (days) | MAT | Date on which 50% of the plants are maturing |
| Number of racemes/panicle | NRP | Mean number of raceme from 10 panicles |
| Raceme length (cm) | RLT | Length of main axis measured from the raceme base to the tip |
| Number of grains/raceme | NGR | Mean number of grain from 10 racemes |
| Panicle length (cm) | PLT | Measured from panicle leaf insertion to the tip of longest raceme |
| Dry biomass yield (kg.ha−1) | DMY | Ratio of shoot dry weight (kg) to the plot area (ha) |
| Grain yield (kg.ha−1) | GRY | Ratio of grain weight (kg) to the plot area (ha) |
| Harvest index (%) | HI | Ration of grain yield to total dry biomass × 100 |
| Thousand seeds weight (g) | TSW | Counting and weighting of 1000 seeds |
| Descriptors | Modalities | Number of Accessions | Percentage (%) |
|---|---|---|---|
| Vigour at seedling | Good | 54 | 30 |
| Moderate | 114 | 63 | |
| Poor | 12 | 7 | |
| Panicle leaf attitude | Erect | 86 | 48 |
| Intermediate | 82 | 45 | |
| Horizontal | 12 | 7 | |
| Panicle type | Compact | 90 | 50 |
| Intermediate | 86 | 48 | |
| Open | 4 | 2 | |
| Panicle exertion | Well exerted | 117 | 65 |
| Moderately exerted | 48 | 27 | |
| Lightly exerted | 15 | 8 | |
| Phenotypic grain color | Brown | 152 | 84 |
| Greyed orange | 28 | 16 |
| Quantitative Traits | Countries | |||||||
|---|---|---|---|---|---|---|---|---|
| Benin | Burkina Faso | Guinea | Mali | Niger | Mean | SD | Pr (>F) | |
| Plant height (cm) | 72 ± 4.62b | 71 ± 12.29bc | 81 ± 4.92a | 68 ± 6.27c | 70 ± 4.98bc | 72 | 5.03 | *** |
| Number of internodes | 10 ± 0.85ab | 10 ± 1.76b | 11 ± 2.04a | 6 ± 0.78c | 7 ± 0.62c | 9 | 2.17 | *** |
| Internode length (cm) | 21.42 ± 6.1ab | 22.62 ± 9.6ab | 24.24 ± 11.6a | 19.44 ± 9.3b | 18.44 ± 3.9b | 20.73 | 2.34 | ** |
| Number of leaves/plant | 92 ± 10.8c | 81 ± 10.7c | 79 ± 8.1c | 212 ± 70.2a | 186 ± 64.9b | 144 | 63.85 | *** |
| Culm diameter (mm) | 1.48 ± 0.55a | 1.40 ± 0.13ab | 1.36 ± 0.20b | 1.21 ± 0.11c | 1.29 ± 0.09b | 1.33 | 0.10 | ** |
| Days to flowering | 81 ± 3.24b | 80 ± 2.30b | 85 ± 7.95a | 69 ± 1.06d | 74 ± 2.20c | 77 | 6.30 | *** |
| Days to 50% Maturity | 105 ± 3.75a | 98 ± 3.87b | 105 ± 4.57a | 85 ± 5.07c | 84 ± 1.94c | 95 | 10.36 | *** |
| Number of racemes/panicle | 4 ± 0.21a | 4 ± 0.11a | 3 ± 0.14b | 3 ± 0.32b | 3 ± 0.25b | 3 | 0.55 | *** |
| Raceme length (cm) | 5.10 ± 1.70b | 4.80 ± 1.16bc | 5.52 ± 0.59a | 4.41 ± 0.93c | 4.50 ± 0.60c | 4.82 | 0.46 | *** |
| Number of grains/raceme | 105 ± 12.03a | 86 ± 22.3bc | 94 ± 11.9b | 76 ± 11.18c | 79 ± 9.18c | 88 | 11.77 | *** |
| Panicle length (cm) | 15.1 ± 2.56c | 15.9 ± 1.73bc | 15.8 ± 1.07c | 17.2 ± 3.2ab | 17.4 ± 1.40a | 16.4 | 0.98 | ** |
| Dry biomass yield (kg.ha−1) | 5095 ± 145.3a | 4603 ± 159.3ab | 5075 ± 96.47a | 3398 ± 98.27c | 4536 ± 125.8b | 4490 | 689.64 | *** |
| Grain yield (kg.ha−1) | 1057 ± 45.99b | 1063 ± 70.53b | 924 ± 45.15b | 1910 ± 68.75a | 1936 ± 62.25a | 1474 | 500.69 | *** |
| Harvest index (%) | 26 ± 4.90c | 27 ± 8.98c | 25 ± 6.23c | 59 ± 9.48a | 54 ± 7.18b | 41 | 16.81 | *** |
| Thousand seed weight (g) | 0.59 ± 0.05d | 0.63 ± 0.10c | 0.68 ± 0.04b | 0.82 ± 0.04a | 0.69 ± 0.03b | 0.70 | 0.09 | *** |
| PHT | NIN | INL | NLP | CD | MAT | FLO | PLT | RLT | NRP | NGR | DBY | GRY | HI | TSW | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHT | 1 | ||||||||||||||
| NIN | 0.53 | 1 | |||||||||||||
| INL | 0.55 | 0.77 | 1 | ||||||||||||
| NLP | −0.38 | −0.75 * | −0.70 | 1 | |||||||||||
| CD | 0.35 | 0.53 | 0.51 | −0.51 | 1 | ||||||||||
| MAT | 0.36 | 0.77 ** | 0.70 | −0.71 | 0.51 | 1 | |||||||||
| FLO | 0.35 | 0.77 | 0.64 | −0.71 | 0.56 | 0.80 ** | 1 | ||||||||
| PLT | 0.46 | 0.25 | 0.34 | −0.19 | 0.02 | 0.24 | 0.05 | 1 | |||||||
| RLT | 0.57 | 0.74 | 0.73 | −0.64 | 0.42 | 0.81 ** | 0.66 | 0.49 | 1 | ||||||
| NRP | −0.02 | 0.21 | 0.16 | −0.27 | 0.31 | 0.27 | 0.23 | −0.21 | 0.13 | 1 | |||||
| NGR | 0.43 | 0.69 | 0.64 | −0.60 | 0.50 | 0.64 | 0.67 | 0.15 | 0.61 | 0.30 | 1 | ||||
| DBY | 0.48 | 0.65 | 0.52 | −0.50 | 0.43 | 0.51 | 0.66 | 0.17 | 0.53 | 0.11 | 0.59 | 1 | |||
| GRY | −0.35 ** | −0.76 ** | −0.70 | 0.68 * | −0.42 | −0.77 * | −0.79 ** | −0.18 | −0.69 | −0.17 | −0.56 | −0.43 | 1 | ||
| HI | −0.37 | −0.76 | −0.74 | 0.69 | −0.48 | −0.79 ** | −0.84 ** | −0.21 | −0.69 | −0.16 | −0.61 | −0.62 | 0.89 ** | 1 | |
| TSW | −0.24 | −0.63 | −0.45 | 0.61 | −0.53 | −0.64 | −0.79 | 0.08 | −0.49 | −0.34 | −0.62 | −0.60 | 0.60 ** | 0.66 | 1 |
| Variables | Cluster 1, N = 91 ML = 48 and NG = 43 | Cluster 2, N = 43 BN = 6, BF = 2, GN = 33, ML = 2 | Cluster 3, N = 46 BN = 43 and BF = 3 | Pr (>F) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Plant height (cm) | 69 ± 5.76 | 79 ± 5.42 | 72 ± 5.33 | *** |
| Number of internodes/plant | 7 ± 0.77 | 11 ± 1.20 | 10 ± 1.02 | *** |
| Internode length (cm) | 19 ± 7.69 | 39 ± 6.16 | 37 ± 6.68 | ** |
| Number of leaves/plant | 202 ± 69.74 | 90 ± 23.84 | 83 ± 17.75 | *** |
| Culm diameter (mm) | 0.77 ± 0.11 | 0.87 ± 0.09 | 1.14 ± 0.38 | *** |
| Flowering (days) | 69 ± 3.06 | 84 ± 5.21 | 86 ± 3.38 | *** |
| Maturity (days) | 86 ± 3.99 | 104 ± 5.50 | 105 ± 3.38 | ** |
| Panicle exertion | 1 ± 0.68 | 3 ± 1.42 | 3 ± 1.48 | *** |
| Raceme length (cm) | 8.1 ± 0.79 | 9.90 ± 0.61 | 9.4 ± 0.58 | *** |
| Panicle length (cm) | 14 ± 1.63 | 17 ± 3.54 | 14 ± 1.05 | *** |
| Number of racemes/panicle | 3 ± 0.29 | 3 ± 0.23 | 4 ± 0.21 | *** |
| Number of grains/raceme | 77 ± 10.39 | 96 ± 13.22 | 104 ± 14.27 | ** |
| Grain yield (kg/ha) | 3840 ± 660.10 | 2019 ± 550.80 | 2048 ± 450.40 | ** |
| Harvest index (%) | 57 ± 8.78 | 26 ± 7.68 | 26 ± 5.43 | ** |
| Thousand seeds Weight (g) | 0.76 ± 0.07 | 0.68 ± 0.06 | 0.59 ± 0.04 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim Bio Yerima, A.R.; Achigan-Dako, E.G.; Aissata, M.; Sekloka, E.; Billot, C.; Adje, C.O.A.; Barnaud, A.; Bakasso, Y. Agromorphological Characterization Revealed Three Phenotypic Groups in a Region-Wide Germplasm of Fonio (Digitaria exilis (Kippist) Stapf) from West Africa. Agronomy 2020, 10, 1653. https://doi.org/10.3390/agronomy10111653
Ibrahim Bio Yerima AR, Achigan-Dako EG, Aissata M, Sekloka E, Billot C, Adje COA, Barnaud A, Bakasso Y. Agromorphological Characterization Revealed Three Phenotypic Groups in a Region-Wide Germplasm of Fonio (Digitaria exilis (Kippist) Stapf) from West Africa. Agronomy. 2020; 10(11):1653. https://doi.org/10.3390/agronomy10111653
Chicago/Turabian StyleIbrahim Bio Yerima, Abdou R., Enoch G. Achigan-Dako, Mamadou Aissata, Emmanuel Sekloka, Claire Billot, Charlotte O. A. Adje, Adeline Barnaud, and Yacoubou Bakasso. 2020. "Agromorphological Characterization Revealed Three Phenotypic Groups in a Region-Wide Germplasm of Fonio (Digitaria exilis (Kippist) Stapf) from West Africa" Agronomy 10, no. 11: 1653. https://doi.org/10.3390/agronomy10111653
APA StyleIbrahim Bio Yerima, A. R., Achigan-Dako, E. G., Aissata, M., Sekloka, E., Billot, C., Adje, C. O. A., Barnaud, A., & Bakasso, Y. (2020). Agromorphological Characterization Revealed Three Phenotypic Groups in a Region-Wide Germplasm of Fonio (Digitaria exilis (Kippist) Stapf) from West Africa. Agronomy, 10(11), 1653. https://doi.org/10.3390/agronomy10111653






