Is It Possible to Replace Part of the Mineral Nitrogen Dose in Maize for Grain by Using Growth Activators and Plant Growth-Promoting Rhizobacteria?
Abstract
1. Introduction
2. Materials and Methods
- Ammonium sulfate 32 (16% N in ammonium form and 16% N in nitrate form)
- Korn-Kali: potassium chloride with added magnesium salt (33.2% K, 3.6% Mg, 3% Na and 5% S)
- Mocznik (46% N in amide form)
- Polidap: ammonium phosphate (18% N in ammonium form, 20.1% P as mono- and diammonium phosphate, 2.8% S as sulfate)
- Polifoska 6 fertilizer (6% N in ammonium form, 8.7% P as mono- and diammonium phosphate, 24.9% K as potassium chloride, 2.8% S as sulfate)
- Potassium chloride (49.8% K as potassium chloride)
- Saletrzak Standard 27: ammonium nitrate with added dolomite flour containing calcium and magnesium (13.5% N in ammonium form and 13.5% N in nitrate form, 1.4% Ca, 2.4% Mg)
- RSM 28: urea-ammonium nitrate solution (7% N in nitrate form, 7% N in ammonium form, 14% N in amide form)
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO Home Page. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 28 September 2020).
- Regulation of the Polish Council of Ministers on the Adoption of the Action Program to Reduce Water Pollution with Nitrates from Agricultural Sources and to Prevent Further Pollution. J. Laws Repub. Pol. 2020, 243. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20200000243/O/D20200243.pdf (accessed on 26 October 2020).
- Lucy, M.; Reed, E.; Glick, B.R. Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 2004, 86, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Arshad, M.; Shaharoona, B.; Mahmood, T. Plant growth promoting rhizobacteria and sustainable agriculture. In Microbial Strategies for Crop Improvement; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 133–160. [Google Scholar] [CrossRef]
- Mrkovački, N.; Bjelić, D. Rizobakterije koje promovišu biljni rast (PGPR) i njihov efekat na kukuruz/Plant growth promoting rhizobacteria (PGPR) and their effect on maize. Ratar. Povrt./Field Veg. Crop Res. 2011, 48, 305–312. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Zaeim, A.N.; Torkaman, M.; Ghasemeeyan, H. Effects of biofertilizer application on growth and yield of corn (Zea mays L.): A review. Int. J. Sci. Res. Sci. Technol. 2017, 3, 245–251. [Google Scholar]
- Mrkovački, N.; Jarak, M.; Đalović, I.; Jocković, Đ. Značaj i efekat primene PGPR na mikrobiološku aktivnost u rizosferi kukuruza/Importance of PGPR application and its effect on microbial activity in maize rhizosphere. Ratar. Povrt. 2012, 49, 335–344. [Google Scholar] [CrossRef]
- Jarak, M.; Jeličić, Z.; Kuzevski, J.; Mrkovački, N.; Đurić, S. The use of Azotobacter in maize production: The effect on microbiological activity of soil, early plant growth and grain yield. Contemp. Agric. 2011, 60, 80–85. [Google Scholar]
- Almaghrabi, O.A.; Abdelmoneim, T.S.; Hassan, M.A.; Moussa, T.A.A. Enhancement of maize growth using some plant growth promoting rhizobacteria (PGPR) under laboratory conditions. Life Sci. J. 2014, 11, 764–772. [Google Scholar]
- Mrkovački, N.; Đalović, I.; Jošić, D.; Bjelić, D.; Jokanović, D.B. The effect of PGPR strains on microbial abundance in maize rhizosphere in field conditions. Ratar. Povrt. 2016, 53, 15–19. [Google Scholar] [CrossRef]
- Mrkovački, N.; Bjelić, D.; Jošić, D.; Đalović, I. Yield response of five maize hybrids to inoculation with rhizobacteria. J. Agric. Food Environ. Sci. 2016, 70, 94–97. [Google Scholar]
- Weber, N.F.; Herrmann, I.; Hochholdinger, F.; Ludewig, U.; Neumann, G. PGPR-induced growth stimulation and nutrient acquisition in maize: Do root hairs matter? Sci. Agric. Bohem. 2018, 49, 164–172. [Google Scholar] [CrossRef]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B. Effects of selected functional bacteria on maize growth and nutrient use efficiency. Microorganisms 2020, 8, 854. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Kadziuliene, Z.; Feiziene, D.; Leistrumaite, A.; Semaskiene, R. Peculiarities of some legumes and cereals under organic farming system. NJF-Seminar 369. Organic farming for a new millennium—Status and future challenges. Published by Nordic Association of Agricultural Scientists (NJF), Section I: Soil, Water and Environment Swedish University of Agricultural Sciences Alnarp, Sweden June 15–17 2005. NJF Rep. 2005, 1, 103–106. [Google Scholar]
- Jakiene, E.; Venskutonis, V.; Mickevicius, V. The effect of additional fertilization with liquid complex fertilizers and growth regulators on potato productivity. Sci. Works Lith. Inst. Hortic. Lith. Univ. Agric. Sodinink. ir Daržinink. 2008, 27, 259–267. [Google Scholar]
- Jakiene, E.; Venskutonis, V.; Liakas, V. Fertilization of sugar beet root with ecological fertilizers. Agron. Res. 2009, 7, 269–276. [Google Scholar]
- Jankauskienė, J.; Survilienė, E. Influence of growth regulators on seed germination energy and biometrical parameters of vegetables. Sci. Works Lith. Inst. Hortic. Lith. Univ. Agric. Sodinink. Daržinink. 2009, 28, 69–77. [Google Scholar]
- Pekarskas, J.; Vilkenyte, L.; Sileikiene, D.; Cesoniene, L.; Makarenko, N. Effect of organic nitrogen fertilizers Provita and fermentator Penergetic–K winter wheat and on soil quality. In Proceedings of the 8th International Conference, Environmental Engineering, Vilnius, Lithuania, 19–20 May 2011; pp. 248–254. [Google Scholar]
- Brito, O.R.; Dequech, F.K.; Brito, R.M. Use of Penergetic products P and K in the snap bean production. Annu. Rep. 2012, 55, 279–280. [Google Scholar]
- Nascente, A.S.; Cobucci, T. Phosphate fertilization in the soil and Penergetic application in the grain yield of common bean. Soils Embrace Life and Universe. In Proceedings of the 20th World Congress of Soil Science, Jeju, Korea, 8–13 June 2014. [Google Scholar]
- De Souza, A.A.; de Almeida, F.Z.; Alberton, O. Growth and yield of soybean with Penergetic application. Sci. Agrar. 2017, 18, 95–98. [Google Scholar]
- Franco Junior, K.S.; Terra, A.B.C.; Teruel, T.R.; Mantovani, J.R.; Florentino, L.A. Effect of cover crops and bioactivators in coffee and chemical properties of soil. Coffee Sci. Lavras 2018, 13, 559–567. [Google Scholar]
- Artyszak, A.; Gozdowski, D. The effect of growth activators and Plant Growth-Promoting Rhizobacteria (PGPR) on the soil properties, root yield, and technological quality of sugar beet. Agronomy 2020, 10, 1262. [Google Scholar] [CrossRef]
- Chukwuneme, C.F.; Babalola, O.O.; Kutu, F.R.; Ojuederie, O.B. Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J. Plant Interact. 2020, 15, 93–105. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015; World Soil Resources Report 106; FAO: Rome, Italy, 2015. [Google Scholar]
- PKN. PN-ISO 10390:1997. Soil Quality—pH Determination; Polish Committee for Standardization: Warsaw, Poland, 1997. [Google Scholar]
- Regional Agrochemical Station. Research Procedure of the Regional Agrochemical Station in Warsaw; No. PB 01 ed.; Regional Agrochemical Station: Warsaw, Poland, 2009. [Google Scholar]
- Regional Agrochemical Station. Research Procedure of the Regional Agrochemical Station in Warsaw; No. PB 46 ed.; Regional Agrochemical Station: Warsaw, Poland, 2017. [Google Scholar]
- PKN. Polish Standard PN-R-04023:1996. Agro-Chemical Analysis of Soil—Determination of Available Phosphorus. Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- PKN. Polish Standard PN-R-04022:1996/Az1:2002. Agro-Chemical Analysis of Soil—Determination of Available Potassium Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- PKN. Polish Standard PN-R-04020:1994/Az1:2004. Agro-Chemical Analysis of Soil—Determination of Available Magnesium Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1994. [Google Scholar]
- PKN. Polish Standard PN-93/R-04018. Agro-Chemical Analysis of Soil. Determination of Available Boron; Polish Committee for Standardization: Warsaw, Poland, 1993. [Google Scholar]
- PKN. Polish Standard PN-92/R-04017. Agro-Chemical Analysis of Soil. Determination of Available Copper; Polish Committee for Standardization: Warsaw, Poland, 1992. [Google Scholar]
- PKN. Polish Standard PN-R-04021: 1994. Agro-Chemical Analysis of Soil. Determination of Available Iron; Polish Committee for Standardization: Warsaw, Poland, 1994. [Google Scholar]
- PKN. Polish Standard PN-93/R-04019. Agro-Chemical Analysis of Soil. Determination of Available Manganese; Polish Committee for Standardization: Warsaw, Poland, 1993. [Google Scholar]
- PKN. Polish Standard PN-92/R-04016. Agro-Chemical Analysis of Soil. Determination of Available Zinc; Polish Committee for Standardization: Warsaw, Poland, 1992. [Google Scholar]
- Żarski, J.; Dudek, S.; Kuśmierek-Tomaszewska, R.; Januszewska-Klapa, K. Needs and effects of irrigation in corn cultivated for grain in the Kujawsko-pomorski region. Infrastruct. Ecol. Rural Areas 2013, 3/IV, 77–90. [Google Scholar]
- PKN. Polish Standard PN-R-74017. Cereal Grains and Edible Pulses. Determination of 1000 Grain Weight; Polish Committee for Standardization: Warsaw, Poland, 1968. [Google Scholar]
- Thonar, C.; Lekfeldt, J.D.S.; Cozzolino, V.; Kundel, D.; Kulhanek, M.; Mosimann, C.; Neumann, G.; Piccolo, A.; Rex, M.; Symanczik, S.; et al. Potential of three microbial bio-effectors to promote maize growth and nutrient acquisition from alternative phosphorous fertilizers in contrasting soils. Chem. Biol. Technol. Agric. 2017, 4, 7. [Google Scholar] [CrossRef]
- Nagaraju, Y.; Triveni, S.; Gopal, A.V.; Thirumal, G.; Kumar, B.P.; Jhansi, P. In vitro screening of Zn solubilizing and potassium releasing isolates for plant growth promoting (PGP) characters. Bull. Environ. Pharmacol. Life Sci. 2017, 6, 590–597. [Google Scholar]
- Goteti, P.K.; Emmanuel, L.D.A.; Desai, S.; Shaik, M.H.A. Prospective Zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013, 2013, 869697. [Google Scholar] [CrossRef] [PubMed]
- Amogou, O.; Dagbenonbakin, G.; Agbodjato, N.A.; Noumavo, P.A.; Salako, K.V.; Adoko, M.Y.; Kakai, R.G.; Adjanohoun, A.; Baba-Moussa, L. Applying rhizobacteria on maize cultivation in Northern Benin: Effect on growth and yield. Agric. Sci. 2019, 10, 763–782. [Google Scholar] [CrossRef][Green Version]
- Yazdani, M.; Pirdashti, H. Efficiency of co-inoculation phosphate solubilizer microorganisms (psm) and plant growth promoting rhizobacteria (PGPR) on micronutrients uptake in corn (Zea mays L.). Int. Res. J. Appl. Basic Sci. 2011, 2, 28–34. [Google Scholar]
- Nkebiwe, P.M.; Weinmann, M.; Mueller, T. Improving fertilizer-depot exploitation and maize growth by inoculation with plant growth-promoting bacteria: From lab to field. Chem. Biol. Technol. Agric. 2016, 3, 15. [Google Scholar] [CrossRef]
- de Lima, B.C.; Moro, A.L.; Santos, A.C.P.; Bonifacio, A.; Araujo, A.S.F.; de Araujo, F.F. Bacillus subtilis ameliorates water stress tolerance in maize and common bean. J. Plant Interact. 2019, 14, 432–439. [Google Scholar] [CrossRef]
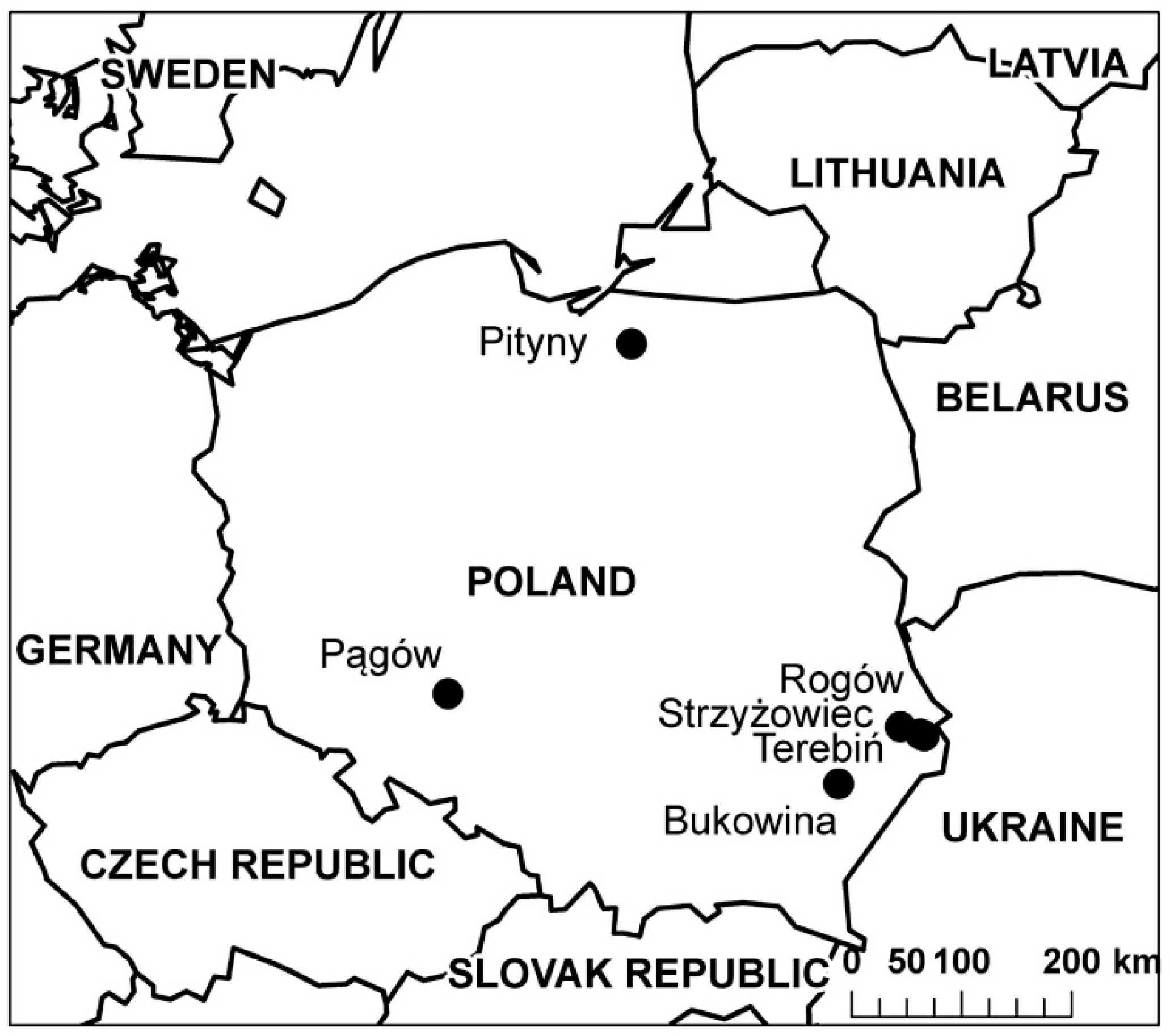
| Location | Forecrop | Side Yield of Forecrop, t ha−1 | Cultivar of Maize | Mineral Fertilization, kg ha−1 | Sowing Date | Harvest Date | Length of Vegetation Period (Days) |
|---|---|---|---|---|---|---|---|
| 2017 | |||||||
| Pągów | Winter wheat | 6.00 (straw) | LG 30.215 (FAO 230) | N–126 (variant No 0) and 88 (variants No 1 and No 2); P–34; K–173; Mg - 14.4, Na- 12, S–20 | 26.04 | 19.10 | 176 |
| Pityny | Potato | (haulm) | Ambrosini (FAO 220) | N–128 (variant No 0) and 90 (variants No 1 and No 2); P–17; K–50; S–6 | 12.05 | 25.10 | 166 |
| Rogów | Winter wheat | 6.00 (straw) | SY Talisman (FAO 220–230) | N–150 (variant No 0) and 90 (variants No 1 and No 2); P–72 K–72 | 20.04 | 06.11 | 200 |
| Strzyżowiec | Sugar beet | 40 (leaves) | SY Talisman (FAO 220–230) | N–56 (variant No 0) and 39 (variants No 1 and No 2); P–30 K–30 | 24.04 | 16.10 | 175 |
| 2018 | |||||||
| Rogów | Winter wheat | 6 (straw) | SY Talisman (FAO 220–230) | N–150 (variant No 0) and 90 (variants No 1 and No 2); P–72; K–72 | 18.04 | 28.09 | 163 |
| Terebiń | Sugar beet | 40 (leaves) | SY Talisman (FAO 220–230) | N–56 (variant No 0) and 39 (variants No 1 and No 2); P- 30; K–30 | 20.04 | 09.10 | 172 |
| 2019 | |||||||
| Bukowina | Winter wheat | 4.00 (straw) | Farmagic (FAO 240) | N–184 (variant No 0) and 129 (variants No 1 and No 2); P - 86; K–90 | 25.04 | 09.10 | 167 |
| Rogów | Maize for grain | 30 (straw) | SY Talisman (FAO 220–230) | N–150 (variant No 0) and 90 (variants No 1 and No 2); P–72; K–72 | 16.04 | 15.10 | 182 |
| Treatment | p-Value Based on ANOVA | |||||
|---|---|---|---|---|---|---|
| Trait | 0 | 1 | 2 | Treatment (T) | Environment (E: Year x Location) | Inter- Action: TxE |
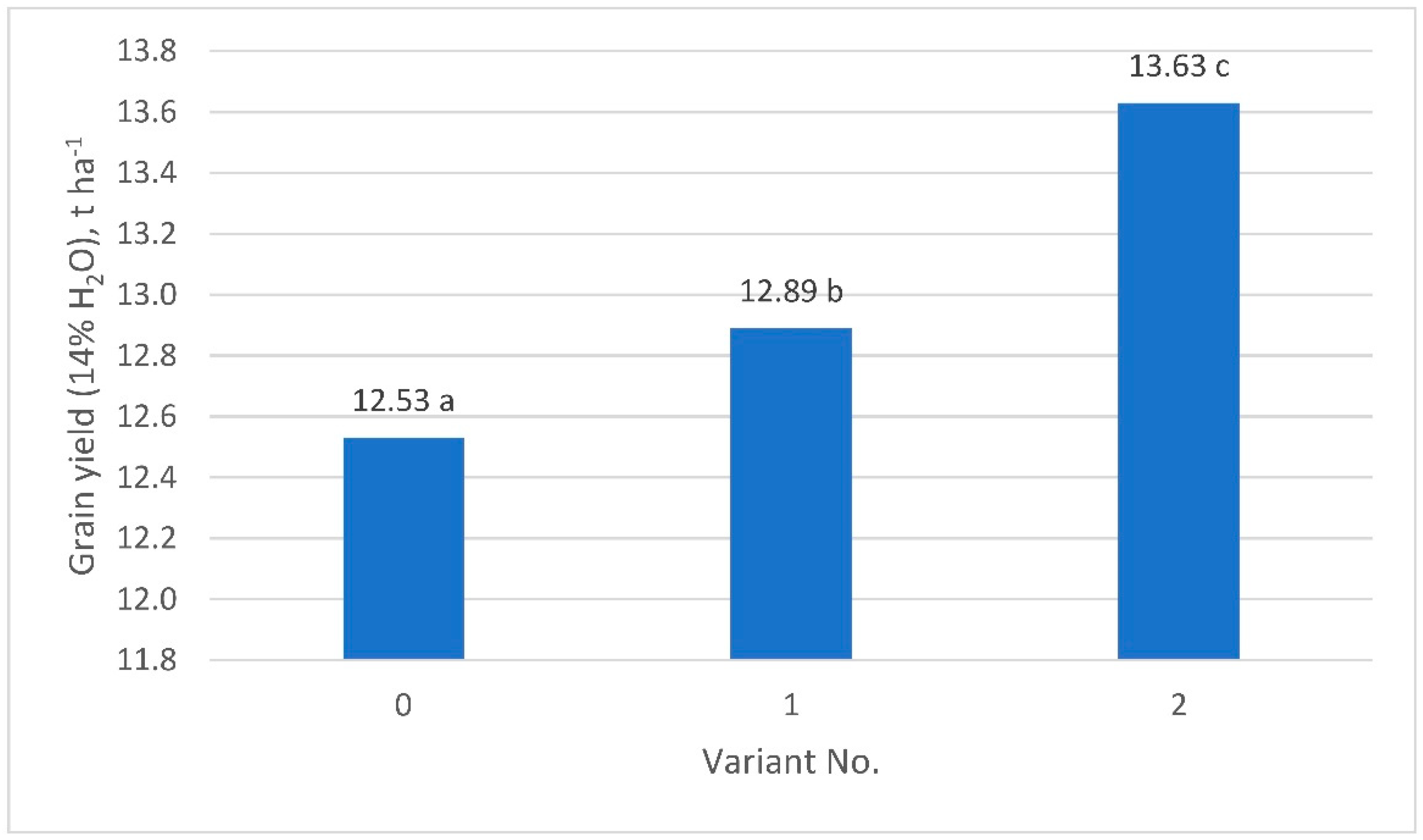
| Grain yield (14% H2O), t ha−1 | 12.53 a* | 12.89 b | 13.63 c | <0.001 | <0.001 | 0.205 |
| Grain moisture, % | 28.65 c | 26.91 a | 27.99 b | <0.001 | <0.001 | <0.001 |
| Yield of straw, t ha−1 | 31.91 b | 29.05 a | 28.18 a | <0.001 | <0.001 | <0.001 |
| Height of plants, cm | 260.20 a | 267.50 b | 271.75 c | <0.001 | <0.001 | <0.001 |
| Grain yield per plant (14% H2O), g | 170.05 a | 174.66 b | 183.57 c | <0.001 | <0.001 | 0.088 |
| Weight of 1000 grains (14% H2O), g | 431.88 a | 444.25 b | 443.50 b | <0.001 | <0.001 | <0.001 |
| Number of grains per cob, pcs. | 391.1 a | 395.2 a | 416.7 b | <0.001 | <0.001 | 0.017 |
| Trait | Mean | Minimum | Maximum | Standard Deviation (SD) | Coefficient of Variation (CV), % |
|---|---|---|---|---|---|
| Grain yield (14% H2O), t ha−1 | 13.02 | 5.90 | 19.76 | 3.45 | 26.49 |
| Grain moisture, % | 27.85 | 20.80 | 45.20 | 5.55 | 19.91 |
| Yield of straw, t ha−1 | 29.72 | 17.00 | 51.50 | 8.53 | 28.72 |
| Height of plants, cm | 266.48 | 190.00 | 319.00 | 24.58 | 9.22 |
| Grain yield per plant (14% H2O), g | 176.09 | 76.82 | 250.14 | 49.13 | 27.90 |
| Weight of 1000 grains (14% H2O), g | 439.88 | 400.00 | 476.00 | 19.29 | 4.38 |
| Number of grain s per cob, pcs. | 401.0 | 174.6 | 618.4 | 114.1 | 28.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artyszak, A.; Gozdowski, D. Is It Possible to Replace Part of the Mineral Nitrogen Dose in Maize for Grain by Using Growth Activators and Plant Growth-Promoting Rhizobacteria? Agronomy 2020, 10, 1647. https://doi.org/10.3390/agronomy10111647
Artyszak A, Gozdowski D. Is It Possible to Replace Part of the Mineral Nitrogen Dose in Maize for Grain by Using Growth Activators and Plant Growth-Promoting Rhizobacteria? Agronomy. 2020; 10(11):1647. https://doi.org/10.3390/agronomy10111647
Chicago/Turabian StyleArtyszak, Arkadiusz, and Dariusz Gozdowski. 2020. "Is It Possible to Replace Part of the Mineral Nitrogen Dose in Maize for Grain by Using Growth Activators and Plant Growth-Promoting Rhizobacteria?" Agronomy 10, no. 11: 1647. https://doi.org/10.3390/agronomy10111647
APA StyleArtyszak, A., & Gozdowski, D. (2020). Is It Possible to Replace Part of the Mineral Nitrogen Dose in Maize for Grain by Using Growth Activators and Plant Growth-Promoting Rhizobacteria? Agronomy, 10(11), 1647. https://doi.org/10.3390/agronomy10111647






