Chemical and Biological Properties of Sandy Loam Soil in Response to Long-Term Organic–Mineral Fertilisation in a Warm-Summer Humid Continental Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Location and Layout
2.2. Soil Samples
2.3. Chemical Analysis and Microbiological Evaluation
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, B.-Q.; Li, X.-Y.; Li, X.-P.; Shi, X.-J.; Huang, S.-M.; Wang, B.-R.; Zhu, P.; Yang, X.-Y.; Liu, H.; Chen, Y.; et al. Long-term fertilizer experiment network in China: Crop yields and soil nutrient trends. Agron. J. 2010, 102, 216–230. [Google Scholar] [CrossRef]
- Hmielowski, T. The value of long-term data in agricultural systems. CSA News 2017, 62, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Johnston, A.E.; Poulton, P.R. The importance of long-term experiments in agriculture: Their management to ensure continued crop production and soil fertility; the Rothamsted experience. Eur. J. Soil Sci. 2018, 69, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Wu, L.; Clark, I.M.; Xue, K.; Yang, Y.; Van Nostrand, J.D.; Deng, Y.; He, Z.; McGrath, S.; Storkey, J.; et al. Over 150 years of long-term fertilization alters spatial scaling of microbial biodiversity. mBio 2015, 6, e00240-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidd, J.; Manning, P.; Simkin, J.; Peacock, S.; Stockdale, E. Impacts of 120 years of fertilizer addition on a temperate grassland ecosystem. PLoS ONE 2017, 12, e0174632. [Google Scholar] [CrossRef] [PubMed]
- Suwara, I.; Pawlak-Zaręba, K.; Gozdowski, D.; Perzanowska, A. Physical properties of soil after 54 years of long-term fertilization and crop rotation. Plant Soil Environ. 2016, 62, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Šimanský, V.; Jonczak, J. Formation of soil structure in long-term fertilised sandy soil: Role of the manganese oxides. Bull. Geogr. Phys. Geogr. Ser. 2019, 17, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Mazur, Z.; Mazur, T. The influence of long-term fertilization with slurry, manure and NPK on the soil content of trace elements. J. Elem. 2016, 21, 131–139. [Google Scholar] [CrossRef]
- Ahmed, W.; Jing, H.; Kaillou, L.; Qaswar, M.; Khan, M.N.; Jin, C. Changes in phosphorus fractions associated with soil chemical properties under long-term organic and inorganic fertilization in paddy soils of southern China. PLoS ONE 2019, 14, e0216881. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Han, X.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H. Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Eo, J.; Park, K.C. Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agric. Ecosyst. Environ. 2016, 231, 176–182. [Google Scholar] [CrossRef]
- Li, C.; Ma, S.; Shao, Y.; Zhang, L. Effects of long-term organic fertilization on soil microbiologic characteristics, yield and sustainable production of winter wheat. J. Integr. Agric. 2018, 17, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Czarnecki, S.; Düring, R.A. Influence of long-term mineral fertilization on metal contents and properties of soil samples taken from different locations in Hesse, Germany. Soil 2015, 1, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Jaskulska, I.; Jaskulski, D.; Kobierski, M. Effect of liming on the change of some agrochemical soil properties in a long-term fertilization experiment. Plant Soil Environ. 2014, 60, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Černý, J.; Balík, J.; Kulhánek, M.; Časová, K.; Nedvěd, V. Mineral and organic fertilization efficiency in long-term stationary experiments. Plant Soil Environ. 2010, 56, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C. Influence of long-term chemical fertilizers and organic manures on soil fertility—A Review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar] [CrossRef]
- Triberti, L.; Nastri, A.; Baldoni, G. Long-term effects of crop rotation, manure and mineral fertilisation on carbon sequestration and soil fertility. Eur. J. Agron. 2016, 74, 47–55. [Google Scholar] [CrossRef]
- Lemanowicz, J. Mineral fertilisation as a factor determining selected sorption properties of soil against the activity of phosphatases. Plant Soil Environ. 2013, 59, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Jaskulska, I.; Jaskulski, D.; Piekarczyk, M.; Kotwica, K.; Gałęzewski, L.; Wasilewski, P. Magnesium content in the leaves of winter wheat in a long-term fertilization experiment. Plant Soil Environ. 2015, 61, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Yousaf, M.; Li, J.; Lu, J.; Ren, T.; Cong, R.; Fahad, S.; Li, X. Effects of fertilization on crop production and nutrient-supplying capacity under rice-oilseed rape rotation system. Sci. Rep. 2017, 7, 1270. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwik-Ziomek, A.; Lemanowicz, J. The content of carbon, nitrogen, phosphorus and sulphur in soil against the activity of selected hydrolases as affected by crop rotation and fertilization. Zemdirb. Agric. 2014, 101, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Roba, T.B. Review on: The effect of mixing organic and inorganic fertilizer on productivity and soil fertility. OALib 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Vašak, F.; Černy, J.; Buranova, Š.; Kulhanek, M.; Balik, J. Soil pH changes in long-term field eksperiments with different fertilizing systems. Soil Water Res. 2015, 10, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Zhu, Z.; Jiang, Y. Long-term impact of fertilization on soil pH and fertility in an apple production system. J. Soil Sci. Plant Nutr. 2018, 18, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Scherer, H.W. Sulfur in soils. J. Plant Nutr. Soil Sci. 2009, 172, 326–335. [Google Scholar] [CrossRef]
- Hemalatha, S.V.; Radhika, K.; Maragatham, S.; Katharine, S.P. Influence of long-term fertilization on soil fertility—A review. J. Agric. Allied Sci. 2013, 2, 30–36. [Google Scholar]
- Murugan, R.; Kumar, S. Influence of long-term fertilisation and crop rotation on changes in fungal and bacterial residues in a tropical rice-field soil. Biol. Fertil. Soils 2013, 49, 847–856. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhao, L.; Zhang, W.; Liu, L. Change of soil microbial community under long-term fertilization in a reclaimed sandy agricultural ecosystem. PeerJ 2019, 7, e6497. [Google Scholar] [CrossRef]
- Elbl, J.; Maková, J.; Javoreková, S.; Medo, J.; Kintl, A.; Lošák, T.; Lukas, V. Response of microbial activities in soil to various organic and mineral amendments as an indicator of soil quality. Agronomy 2019, 9, 485. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Guan, D.W.; Zhou, B.K.; Zhao, B.S.; Ma, M.C.; Qin, J.; Jiang, X.; Chen, S.; Gao, F.; Shen, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Zhong, Y.Q.; Yan, W.M.; Shangguan, Z.P. Impact of long-term N additions upon coupling between soil microbial community structure and activity, and nutrient-use efficiencies. Soil Biol. Biochem. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- Natywa, M.; Sawicka, A.; Wolna-Maruwka, A. Microbial and enzymatic activity in the soil under maize crop in relation to differentiated nitrogen fertilization. Water Environ. Rural Areas 2010, 10, 111–120. [Google Scholar]
- Cinnadurai, C.; Gopalaswamy, G.; Balachandar, D. Diversity of cultivable Azotobacter in the semi-arid alfisol receiving long-term organic and inorganic nutrient amendments. Ann. Microbiol. 2013, 63, 1397–1404. [Google Scholar] [CrossRef]
- Barabasz, W.; Albinska, D.; Jaskowska, M.; Lipiec, J. Biological effects of mineral nitrogen fertilization on soil microorganisms. Pol. J. Environ. Stud. 2002, 11, 193–198. [Google Scholar]
- Geisseler, D.; Scow, K. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [Green Version]
- Tabatabai, M.A.E.; Weaver, R.I.; Augle, S.; Bottomly, P.J.; Bezdieck, D.; Smith, S.; Tabatabai, M.A.; Wollum, A. Methodes of soil analysis. Part 2. Microbiological and biochemical properties. Soil Sci. Soc. Am. 1994, 5, 775–833. [Google Scholar]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, V.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A. Significance of enzymes and their application in agriculture. In Biocatalysis; Husain, Q., Ullah, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 277–308. [Google Scholar]
- Klose, S.; Tabatabai, M.A. Arylsulfatase activity of microbial biomass in soils. Soil Sci. Soc. Am. J. 1999, 63, 569–574. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walkes, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Wilczewski, E. Soil phosphatase activity and phosphorus content as influenced by catch crops cultivated as green manure. Pol. J. Environ. Stud. 2014, 23, 157–165. [Google Scholar]
- Zhou, X.; Zhang, Y.; Downing, A. Non-linear response of microbial activity across a gradient of nitrogen addition to a soil from the Gurbantunggut Desert, northwestern China. Soil Biol. Biochem. 2012, 47, 67–77. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Liu, S. Effect of long-term mineral fertilizer application on soil enzyme activities and bacterial community composition. Plant Soil Environ. 2018, 64, 571–577. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Marks, M.; Jastrzębska, M.; Kostrzewska, M.K. Long-Term Experiments in Agricultural Studies in Poland; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Poland, 2018; p. 280. [Google Scholar]
- Jaskulska, I. History of the Experimental Station of the University of Science and Technology—70 years of a static fertilisation experiment in Mochełek. In 100 Years of Agricultural Experimentation at the Faculty of Agriculture and Biology Warsaw University of Life Sciences—WULS; Łabętowicz, J., Radecki, A., Eds.; Wydawnictwo SGGW: Warszawa, Poland, 2020; pp. 95–104. [Google Scholar]
- PN-ISO 10390. Chemical and Agricultural Analysis—Determining Soil pH; Polish Standards Committee: Warszawa, Poland, 1997. [Google Scholar]
- PN-R-04023. Chemical and Agricultural Analysis—Determination of the Content of Available Phosphorus in Mineral Soils; Polish Standards Committee: Warszawa, Poland, 1996. [Google Scholar]
- PN-R-04022. Chemical and Agricultural Analysis—Determination of the Content Available Potassium in Mineral Soils; Polish Standards Committee: Warszawa, Poland, 1996. [Google Scholar]
- PN-R-04020. Chemical and Agricultural Analysis. Determination of the Content Available Magnesium; Polish Standards Committee: Warszawa, Poland, 1994. [Google Scholar]
- Bardsley, C.E.; Lancaster, J.D. Determination of reserve sulfur and soluble sulfates in soils 1. Soil Sci. Soc. Am. J. 1960, 24, 265–268. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p–nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2010. [Google Scholar]
- Gupta, P.; Samant, K.; Sahu, A. Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int. J. Microbiol. 2012, 2012, 578925. [Google Scholar] [CrossRef] [Green Version]
- Crawford, D.L.; Lynch, J.M.; Whipps, J.M.; Ousley, M.A. Isolation and characterization of actinomycetes antagonists of a fungal root pathogen. Appl. Environ. Microbiol. 1996, 59, 3899–3905. [Google Scholar] [CrossRef] [Green Version]
- Statistica, Data Analysis Software System, Version 12; TIBCO Software Inc.: Palo Alto, CA, USA, 2019; Available online: https://www.tibco.com/products/data-science (accessed on 15 January 2019).
- Debreczeni, K.; Kismányoky, T. Acidification of soils in long-term field experiments. Commun. Soil Sci. Plant Anal. 2005, 36, 321–329. [Google Scholar] [CrossRef]
- Zhang, Y.C. Research of Long-Term Fertilization on Soil Acidification. Ph.D. Thesis, Nanjing Agriculture University, Nanjing, China, 2012. [Google Scholar]
- Zhou, X.; Zhou, S.; Xu, G.; Colinet, G. Evolution characteristics and influence factors of acidification in paddy soil of Southern China. Sci. Agric. Sin. 2015, 23, 4811–4817. [Google Scholar]
- Guo, X.; Li, H.; Yu, H.; Li, W.; Ye, Y.; Biswa, A. Drivers of spatio-temporal changes in paddy soil pH in Jiangxi Province, China from 1980 to 2010. Sci. Rep. 2018, 8, 2702. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, J.; Zhu, Q.; Zhang, Z.; Lin, X. pH is the primary determinant of the bacterial community structure in agricultural soils impacted by polycyclic aromatic hydrocarbon pollution. Sci Rep. 2017, 7, 40093. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, W.; Burger, M.; Yang, L.; Gong, P.; Wu, Z. Changes in soil carbon and enzyme activity as a result of different long-term fertilization regimes in a greenhouse field. PLoS ONE 2015, 10, e0118371. [Google Scholar] [CrossRef] [Green Version]
- Lemanowicz, J. Activity of selected enzymes as markers of ecotoxicity in technogenic salinization soils. Environ. Sci. Pollut. Res. 2019, 26, 13014–13024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radziemska, M.; Bęś, A.; Gusiatin, Z.M.; Majewski, G.; Mazur, Z.; Bilgin, A.; Jaskulska, I.; Brtnický, M. Immobilization of potentially toxic elements (PTE) by mineral-based amendments: Remediation of contaminated soils in post-industrial sites. Minerals 2020, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Rashmi, I.; Roy, T.; Kartika, K.S.; Pal, R.; Coumar, V.; Kala, S.; Shinoji, K.C. Organic and inorganic fertilizer contaminants in agriculture: Impact on soil and water resources. In Contaminants in Agriculture; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-term effect of manure and fertilizer on soil organic carbon pools in dryland farming in Northwest China. PLoS ONE 2013, 8, e56536. [Google Scholar] [CrossRef] [Green Version]
- Bitew, Y.; Alemayehu, M. Impact of crop production inputs on soil health: A review. Asian J. Plant Sci. 2017, 16, 109–131. [Google Scholar] [CrossRef] [Green Version]
- Lipiński, W.; Terelak, H.; Motowicka-Terelak, T. Propozycja liczb granicznych zawartości siarki siarczanowej w glebach mineralnych na potrzeby doradztwa nawozowego. Rocz. Glebozn. 2003, 54, 7984. [Google Scholar]
- Förster, S.; Welp, G.; Scherer, H.W. Sulfur specification in bulk soil as influenced by long-term application of mineral and organic fertilizers. Plant Soil Environ. 2012, 58, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X.; Li, Y. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, 0216006. [Google Scholar]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Xu, Y.; Xiao, X.; Li, C.; Li, W.; Cheng, K.; Pan, X.; Sun, G. Impacts of long-term fertilization on the soil microbial communities in double-cropped paddy fields. J. Agric. Sci. 2018, 156, 857–864. [Google Scholar] [CrossRef]
- Pietri, J.C.; Brookes, P.C. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Lee, Y. Effect of pH on soil bacterial diversity. J. Ecol. Environ. 2016, 40, 10. [Google Scholar] [CrossRef] [Green Version]
- Parham, J.A.; Deng, S.P.; Raun, W.R.; Jonson, G.V. Long term cattle manure application in soil, I. Effect on soil phosphorus levels, microbial biomass C, and dehydrogenase and phosphatase activities. Biol. Fertil. Soils 2002, 35, 328–337. [Google Scholar]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Prakash, V.; Kundu, S.; Kumar, N.; Lal Mina, B. Soil enzymatic activity as affected by long term application of farmyard manure and mineral fertilizer under a rainfed soybean–wheat system in N-W Himalaya. Eur. J. Soil Biol. 2008, 44, 309–315. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.; Gong, Y.; Yang, H.; Fan, M.; Kuzyakov, Y. Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a North China Plain soil. Eur. J. Soil Biol. 2014, 60, 112–119. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Series: Soil Biol. Bünemann, E.K., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar]
- Bohme, L.; Bohme, F. Soil microbiological and biochemical properties affected by plant growth and different long-term fertilization. Eur. J. Soil Biol. 2006, 42, 1–12. [Google Scholar] [CrossRef]
- Różyło, K.; Bohacz, J. Microbial and enzyme analysis of soil after the agricultural utilization of biogas digestate and mineral mining waste. Int. J. Environ. Sci. Technol. 2020, 17, 1051–1062. [Google Scholar] [CrossRef] [Green Version]
- Filipek-Mazur, B.; Pużyńska, K.; Tabak, M.; Pużyński, S. Enzymatic activity of soil under spelt grown in an organic farming system in Poland’s temperate climate. Agronomy 2020, 10, 930. [Google Scholar] [CrossRef]
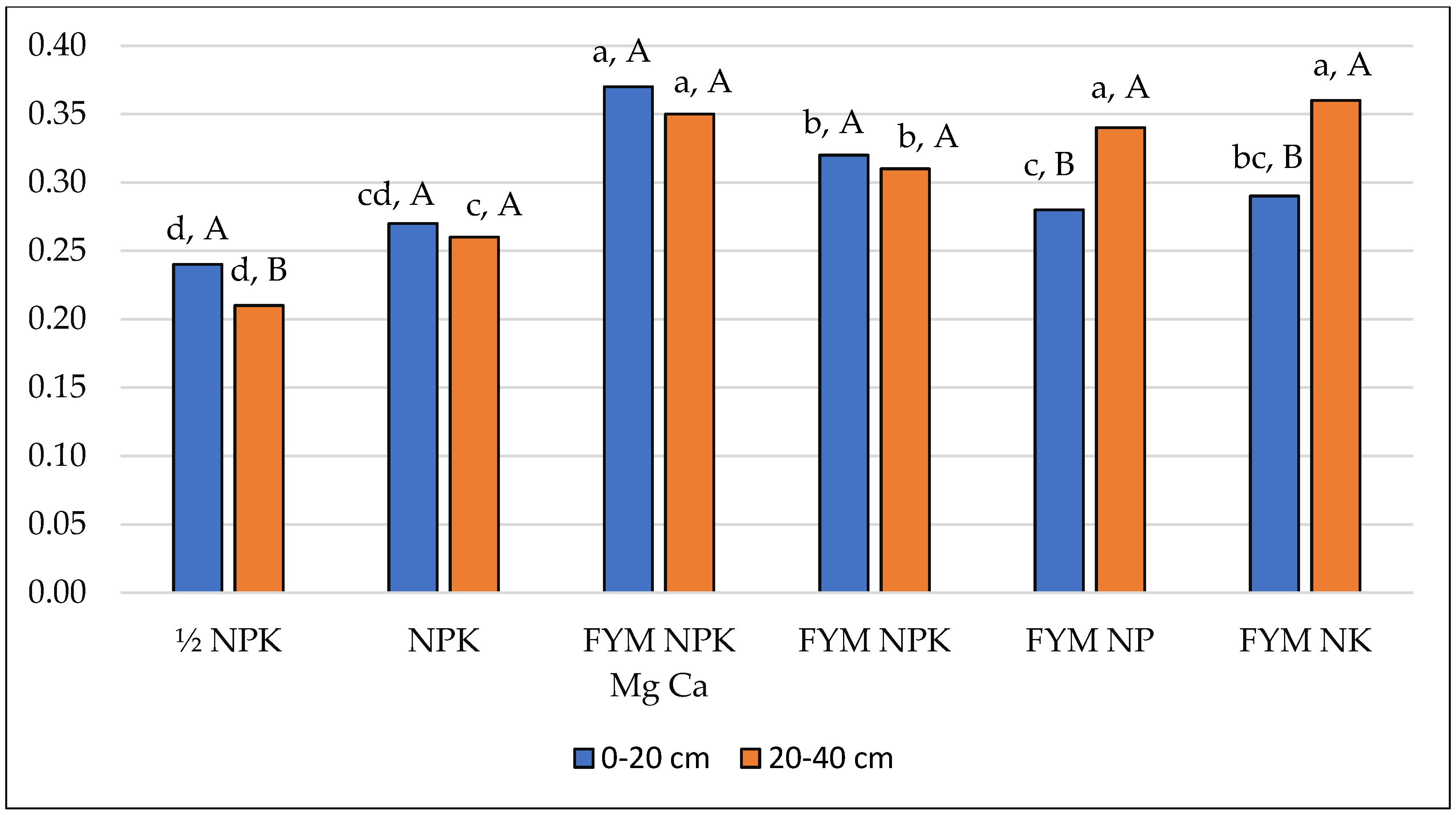

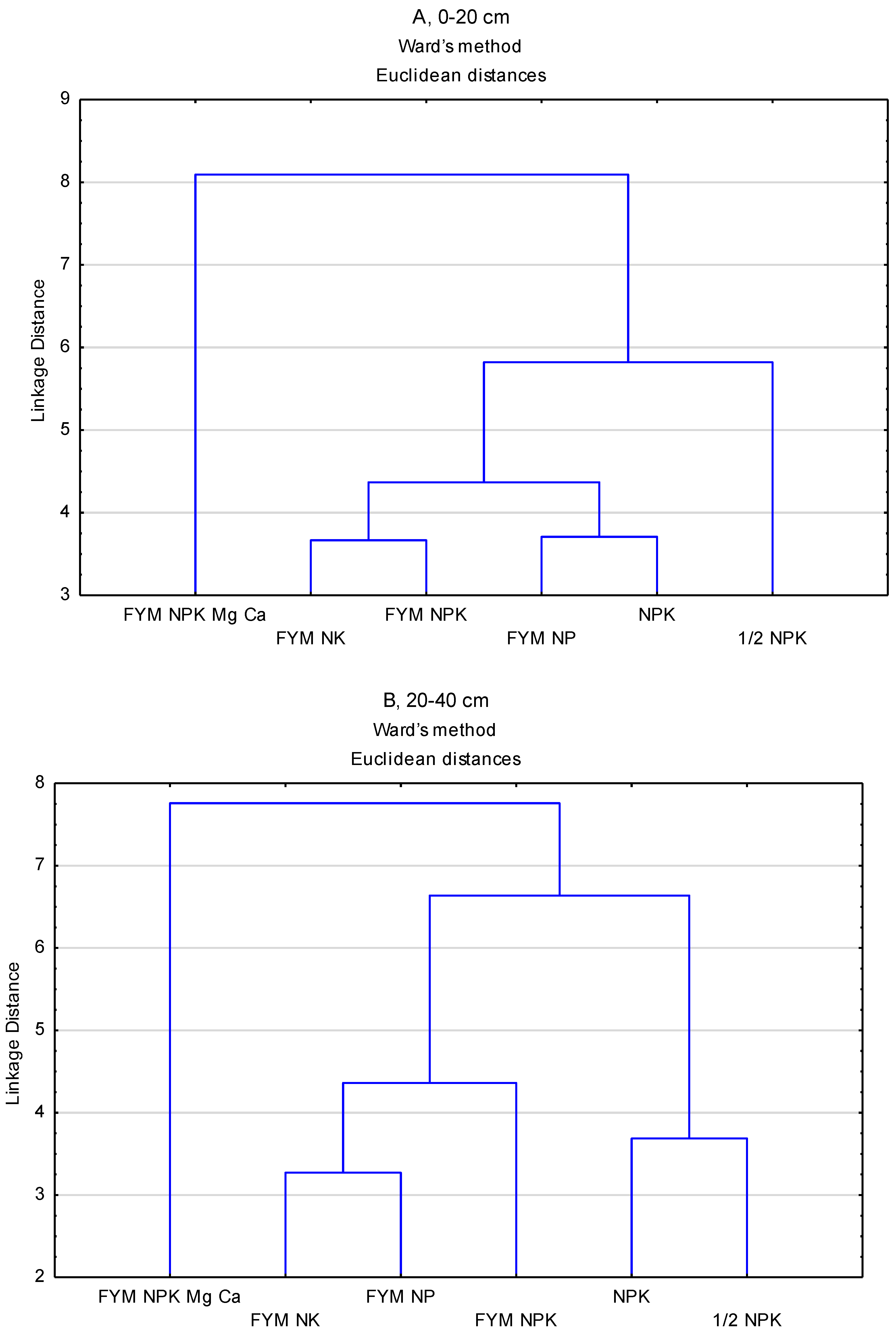
| Depth (cm) | Fertilisation | Mean | |||||
|---|---|---|---|---|---|---|---|
| ½ NPK | NPK | FYM NPK Mg Ca | FYM NPK | FYM NP | FYM NK | ||
| pHKCl | |||||||
| 0–20 | 4.74 (0.17) | 4.44 (0.33) | 5.04 (0.23) | 4.61 (0.29) | 4.24 (0.28) | 4.39 (0.28) | 4.58 (0.35) |
| 20–40 | 4.96 (0.26) | 4.47 (0.38) | 5.21 (0.18) | 4.56 (0.27) | 4.34 (0.29) | 4.36 (0.26) | 4.65 (0.41) |
| Mean | 4.85 (0.23) | 4.46 (0.30) | 5.13 (0.21) | 4.59 (0.25) | 4.28 (0.26) | 4.37 (0.24) | 4.61 (0.38) |
| Mean square: fertilisation = 0.616; depth = 0.046; interaction = 0.019 | |||||||
| Electrical Conductivity—EC (mS cm−1) | |||||||
| 0–20 | 0.183 (0.005) | 0.181 (0.005) | 0.273 (0.004) | 0.225 (0.003) | 0.230 (0.002) | 0.183 (0.005) | 0.212 (0.035) |
| 20–40 | 0.200 (0.002) | 0.262 (0.005) | 0.400 (0.007) | 0.284 (0.005) | 0.189 (0.005) | 0.223 (0.002) | 0.260 (0.068) |
| Mean | 0.192 (0.010) | 0.221 (0.047) | 0.337 (0.073) | 0.254 (0.034) | 0.210 (0.023) | 0.203 (0.024) | 0.236 (0.062) |
| Mean square: fertilisation = 0.012; depth = 0.013; interaction = 0.003 | |||||||
| LSD: fertilisation = 0.016; depth = 0.004; depth/fertilisation = 0.009; fertilisation/depth = 0.019 | |||||||
| Organic Carbon—OC (g C kg−1 dm soil) | |||||||
| 0–20 | 0.46 (0.01) | 0.49 (0.02) | 0.63 (0.04) | 0.57 (0.01) | 0.56 (0.02) | 0.54 (0.01) | 0.54 (0.06) |
| 20–40 | 0.30 (0.02) | 0.28 (0.01) | 0.38 (0.02) | 0.33 (0.02) | 0.32 (0.02) | 0.31 (0.01) | 0.34 (0.04) |
| Mean | 0.38 (0.09) | 0.39 (0.11) | 0.51 (0.14) | 0.45 (0.13) | 0.44 (0.13) | 0.43 (0.12) | 0.44 (0.12) |
| Mean square: fertilisation = 0.017; depth = 0.590; interaction = 0.002 | |||||||
| LSD: fertilisation = 0.03; depth = 0.01; depth/fertilisation = 0.02; fertilisation/depth = 0.05 | |||||||
| Phosphorus—Pa (mg P kg−1 dm soil) | |||||||
| 0–20 | 110.3 (4.8) | 129.3 (0.2) | 139.6 (2.3) | 158.7 (4.0) | 147.0 (1.2) | 99.6 (0.6) | 130.8 (21.0) |
| 20–40 | 105.4 (5.7) | 117.8 (2.7) | 125.9 (5.8) | 133.4 (1.4) | 126.9 (2.8) | 87.7 (2.3) | 116.2 (16.2) |
| Mean | 107.9 (5.6) | 123.5 (6.4) | 132.8 (8.4) | 146.0 (13.8) | 137.0 (11.0) | 93.7 (6.6) | 123.5 (20.0) |
| Mean square: fertilisation = 3055.881; depth = 2552.387; interaction = 102.699 | |||||||
| LSD: fertilisation = 7.1; depth = 1.3; depth/fertilisation = 3.2; fertilisation/depth = 7.9 | |||||||
| Potassium—Ka (mg K kg−1 dm soil) | |||||||
| 0–20 | 128.8 (1.3) | 153.7 (0.4) | 204.0 (4.3) | 166.9 (1.7) | 87.5 (1.8) | 149.7 (0.5) | 148.4 (37.1) |
| 20–40 | 127.8 (1.5) | 136.9 (0.6) | 164.6 (1.0) | 163.0 (0.1) | 82.8 (0.3) | 136.9 (0.3) | 135.3 (28.5) |
| Mean | 128.3 (1.3) | 145.3 (9.7) | 184.3 (22.9) | 165.0 (2.4) | 85.1 (2.9) | 143.3 (7.4) | 141.9 (33.0) |
| Mean square: fertilisation = 4606.249; depth = 1030.315; interaction = 201.386 | |||||||
| LSD: fertilisation = 4.2; depth = 1.8; depth/fertilisation =4.5; fertilisation/depth = 6.7 | |||||||
| Magnesium—Mga (mg Mg kg−1 dm soil) | |||||||
| 0–20 | 10.5 (0.76) | 12.4 (0.08) | 24.4 (0.03) | 20.5 (0.67) | 18.5 (1.58) | 14.3 (0.01) | 16.8 (4.99) |
| 20–40 | 7.7 (0.11) | 12.3 (0.09) | 17.8 (0.08) | 17.4 (0.02) | 15.7 (0.21) | 12.6 (0.21) | 13.9 (3.58) |
| Mean | 9.1 (1.58) | 12.3 (0.08) | 21.1 (3.52) | 18.9 (1.73) | 17.1 (1.83) | 13.5 (0.89) | 15.3 (4.52) |
| Mean square: fertilisation = 161.408; depth = 96.958; interaction = 9.381 | |||||||
| LSD: fertilisation = 1.0; depth = 0.3; depth/fertilisation = 0.7; fertilisation/depth = 1.3 | |||||||
| Sulphur—S-SO4 (mg S-SO4 kg−1 dm soil) | |||||||
| 0–20 | 12.1 0.18 | 12.3 (0.06) | 14.1 (0.14) | 14.4 (0.52) | 12.5 (0.52) | 16.0 (0.60) | 13.6 (1.46) |
| 20–40 | 11.9 0.24 | 13.8 (0.56) | 14.9 (0.80) | 11.8 (0.21) | 12.9 (0.20) | 12.8 (0.69) | 13.0 (1.18) |
| Mean | 12.0 0.22 | 13.1 (0.86) | 14.5 (0.67) | 13.1 (1.46) | 12.7 (0.42) | 14.4 (1.81) | 13.3 (1.34) |
| Mean square: fertilisation = 7.400; depth = 3.845; interaction = 7.271 | |||||||
| LSD: fertilisation = 0.5; depth = 0.4; depth/fertilisation = 0.9; fertilisation/depth = 1.1 | |||||||
| Depth (cm) | Fertilisation | Mean | |||||
|---|---|---|---|---|---|---|---|
| ½ NPK | NPK | FYM NPK Mg Ca | FYM NPK | FYM NP | FYM NK | ||
| Total Bacteria—Bt (cfu × 106 g−1 dm soil) | |||||||
| 0–20 | 12.9 (0.91) | 5.7 (0.52) | 12.4 (1.35) | 6.4 (0.49) | 4.3 (0.41) | 5.0 (0.89) | 7.8 (3.65) |
| 20–40 | 6.3 (0.52) | 5.6 (1.29) | 10.0 (0.94) | 2.6 (0.47) | 5.0 (0.89) | 4.9 (0.72) | 5.7 (2.40) |
| Mean | 9.6 (3.50) | 5.6 (0.94) | 11.2 (1.67) | 4.5 (2.02) | 4.6 (0.77) | 4.9 (0.79) | 6.8 (3.24) |
| Mean square: fertilisation = 101.960; depth = 74.420; interaction = 23.293 | |||||||
| LSD: fertilisation = 0.7; depth = 0.4; depth/fertilisation = 1.1; fertilisation/depth = 1.4 | |||||||
| Cellulolytic microorganisms—Bc (cfu × 105 g−1 dm soil) | |||||||
| 0–20 | 32.7 (3.06) | 24.3 (1.15) | 37.3 (1.15) | 18.0 (1.00) | 27.3 (2.52) | 18.7 (0.57) | 26.4 (7.37) |
| 20–40 | 33.7 (4.04) | 16.0 (2.00) | 36.0 (1.73) | 18.0 (0.29) | 21.7 (1.15) | 15.0 (1.00) | 23.4 (8.78) |
| Mean | 33.2 (3.35) | 20.2 (4.79) | 36.7 (1.51) | 18.0 (0.66) | 24.5 (3.56) | 16.8 (2.14) | 24.9 (8.13) |
| Mean square: fertilisation = 410.444; depth = 81.000; interaction = 19.133 | |||||||
| LSD: fertilisation = 2.3; depth =1.8; depth/fertilisation = 4.3; fertilisation/depth = 5.3 | |||||||
| Filamentous fungi—Ff (cfu × 104 g−1 dm soil) | |||||||
| 0–20 | 31.7 (2.08) | 26.3 (4.04) | 34.0 (1.73) | 35.0 (0.90) | 30.0 (1.00) | 32.0 (1.00) | 31.5 (3.41) |
| 20–40 | 23.0 (1.73) | 24.7 (1.53) | 30.0 (0.58) | 28.0 (2.00) | 23.7 (1.15) | 22.3 (2.08) | 25.3 (3.25) |
| Mean | 27.3 (5.05) | 25.5 (2.88) | 32.0 (2.32) | 31.5 (4.11) | 26.8 (3.60) | 27.2 (5.49) | 28.4 (4.54) |
| Mean square: fertilisation = 43.311; depth = 348.444; interaction = 13.244 | |||||||
| LSD: fertilisation = 2.9; depth = 1.5; depth/fertilisation = ns; fertilisation/depth = ns | |||||||
| Actinobacteria—Ac (cfu × 105 g−1 dm soil) | |||||||
| 0–20 | 25.0 (3.46) | 9.0 (1.00) | 32.7 (2.52) | 11.0 (1.73) | 15.0 (1.73) | 12.1 (0.12) | 17.5 (8.95) |
| 20–40 | 33.0 (2.65) | 22.6 (1.40) | 24.0 (2.65) | 8.3 (0.58) | 17.0 (1.00) | 19.3 (2.08) | 20.7 (7.84) |
| Mean | 29.0 (5.18) | 15.8 (7.51) | 28.3 (5.28) | 9.7 (1.86) | 16.0 (1.67) | 15.7 (4.19) | 19.1 (8.45) |
| Mean square: fertilisation = 365.304; depth = 95.062; interaction = 97.112 | |||||||
| LSD: fertilisation = 4.8; depth = 1.1; depth/fertilisation = 2.7; fertilisation/depth = 5.6 | |||||||
| Depth (cm) | Fertilisation | Mean | |||||
|---|---|---|---|---|---|---|---|
| ½ NPK | NPK | FYM NPK Mg Ca | FYM NPK | FYM NP | FYM NK | ||
| Alkaline Phosphatase—AlP (mM pNP kg−1 h−1) | |||||||
| 0–20 | 0.237 (0.004) | 0.256 (0.022) | 0.457 (0.007) | 0.329 (0.006) | 0.304 (0.012) | 0.309 (0.006) | 0.315 (0.075) |
| 20–40 | 0.168 (0.004) | 0.218 (0.004) | 0.339 (0.016) | 0.305 (0.012) | 0.333 (0.008) | 0.345 (0.006) | 0.285 (0.066) |
| Mean | 0.203 (0.037) | 0.237 (0.025) | 0.398 (0.040) | 0.317 (0.016) | 0.319 (0.067) | 0.327 (0.014) | 0.300 (0.076) |
| Mean square: fertilisation = 0.043; depth = 0.040; interaction = 0.003 | |||||||
| LSD: fertilisation = 0.018; depth = 0.006; depth/fertilisation = 0.015; fertilisation/depth = 0.024 | |||||||
| Acid Phosphatase—AcP (mM pNP kg−1 h−1) | |||||||
| 0–20 | 0.972 (0.007) | 0.949 (0.009) | 1.252 (0.053) | 1.040 (0.018) | 1.079 (0.019) | 1.071 (0.023) | 1.060 (0.104) |
| 20–40 | 0.812 (0.036) | 0.824 (0.017) | 0.978 (0.015) | 0.993 (0.011) | 0.977 (0.035) | 0.965 (0.040) | 0.925 (0.082) |
| Mean | 0.892 (0.095) | 0.887 (0.073) | 1.115 (0.161) | 1.017 (0.029) | 1.028 (0.063) | 1.018 (0.069) | 0.993 (0.115) |
| Mean square: fertilisation = 0.031; depth = 0.110; interaction = 0.006 | |||||||
| LSD: fertilisation = 0.103; depth = 0.022; depth/fertilisation = 0.055; fertilisation/depth = 0.121 | |||||||
| Arylsulphatase—ArS (μM pNP g−1 h−1) | |||||||
| 0–20 | 0.113 (0.018) | 0.183 (0.027) | 0.208 (0.035) | 0.194 (0.013) | 0.232 (0.014) | 0.225 (0.008) | 0.193 (0.045) |
| 20–40 | 0.060 (0.003) | 0.070 (0.008) | 0.239 (0.010) | 0.218 (0.006) | 0.208 (0.002) | 0.169 (0.004) | 0.161 (0.073) |
| Mean | 0.087 (0.032) | 0.127 (0.063) | 0.224 (0.029) | 0.206 (0.016) | 0.220 (0.016) | 0.197 (0.029) | 0.177 (0,062) |
| Mean square: fertilisation = 0.026; depth = 0.012; interaction = 0.006 | |||||||
| LSD: fertilisation = 0.024; depth = 0.009; depth/fertilisation = 0.023; fertilisation/depth = 0.034 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaskulska, I.; Lemanowicz, J.; Breza-Boruta, B.; Siwik-Ziomek, A.; Radziemska, M.; Dariusz, J.; Białek, M. Chemical and Biological Properties of Sandy Loam Soil in Response to Long-Term Organic–Mineral Fertilisation in a Warm-Summer Humid Continental Climate. Agronomy 2020, 10, 1610. https://doi.org/10.3390/agronomy10101610
Jaskulska I, Lemanowicz J, Breza-Boruta B, Siwik-Ziomek A, Radziemska M, Dariusz J, Białek M. Chemical and Biological Properties of Sandy Loam Soil in Response to Long-Term Organic–Mineral Fertilisation in a Warm-Summer Humid Continental Climate. Agronomy. 2020; 10(10):1610. https://doi.org/10.3390/agronomy10101610
Chicago/Turabian StyleJaskulska, Iwona, Joanna Lemanowicz, Barbara Breza-Boruta, Anetta Siwik-Ziomek, Maja Radziemska, Jaskulski Dariusz, and Mateusz Białek. 2020. "Chemical and Biological Properties of Sandy Loam Soil in Response to Long-Term Organic–Mineral Fertilisation in a Warm-Summer Humid Continental Climate" Agronomy 10, no. 10: 1610. https://doi.org/10.3390/agronomy10101610
APA StyleJaskulska, I., Lemanowicz, J., Breza-Boruta, B., Siwik-Ziomek, A., Radziemska, M., Dariusz, J., & Białek, M. (2020). Chemical and Biological Properties of Sandy Loam Soil in Response to Long-Term Organic–Mineral Fertilisation in a Warm-Summer Humid Continental Climate. Agronomy, 10(10), 1610. https://doi.org/10.3390/agronomy10101610







