Supplementing Nitrogen in Combination with Rhizobium Inoculation and Soil Mulch in Peanut (Arachis hypogaea L.) Production System: Part I. Effects on Productivity, Soil Moisture, and Nutrient Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Characteristics
2.1.1. Location
2.1.2. Climatic Condition
2.1.3. Soil Characteristics
2.2. Experimental Details
2.2.1. Design and Treatment Details
2.2.2. Fertilizer Application
2.2.3. Variety and Experimental Setup
2.2.4. Irrigation
2.2.5. Harvesting
2.3. Measurements and Analytical Procedures
2.3.1. Yield Attributes and Yield
2.3.2. Microbiological Observations
2.3.3. Nutrient Assessment
2.3.4. Quality Assessment
2.3.5. Determination of Soil Moisture Content
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. Yield-Attributing Characteristics of Peanut were Influenced by Levels of Nitrogen Applied with Rh under Mulch
3.2. Peanut Yield Was Influenced by Different Levels of Nitrogen Fertilizer in Association with Rh and Mulch
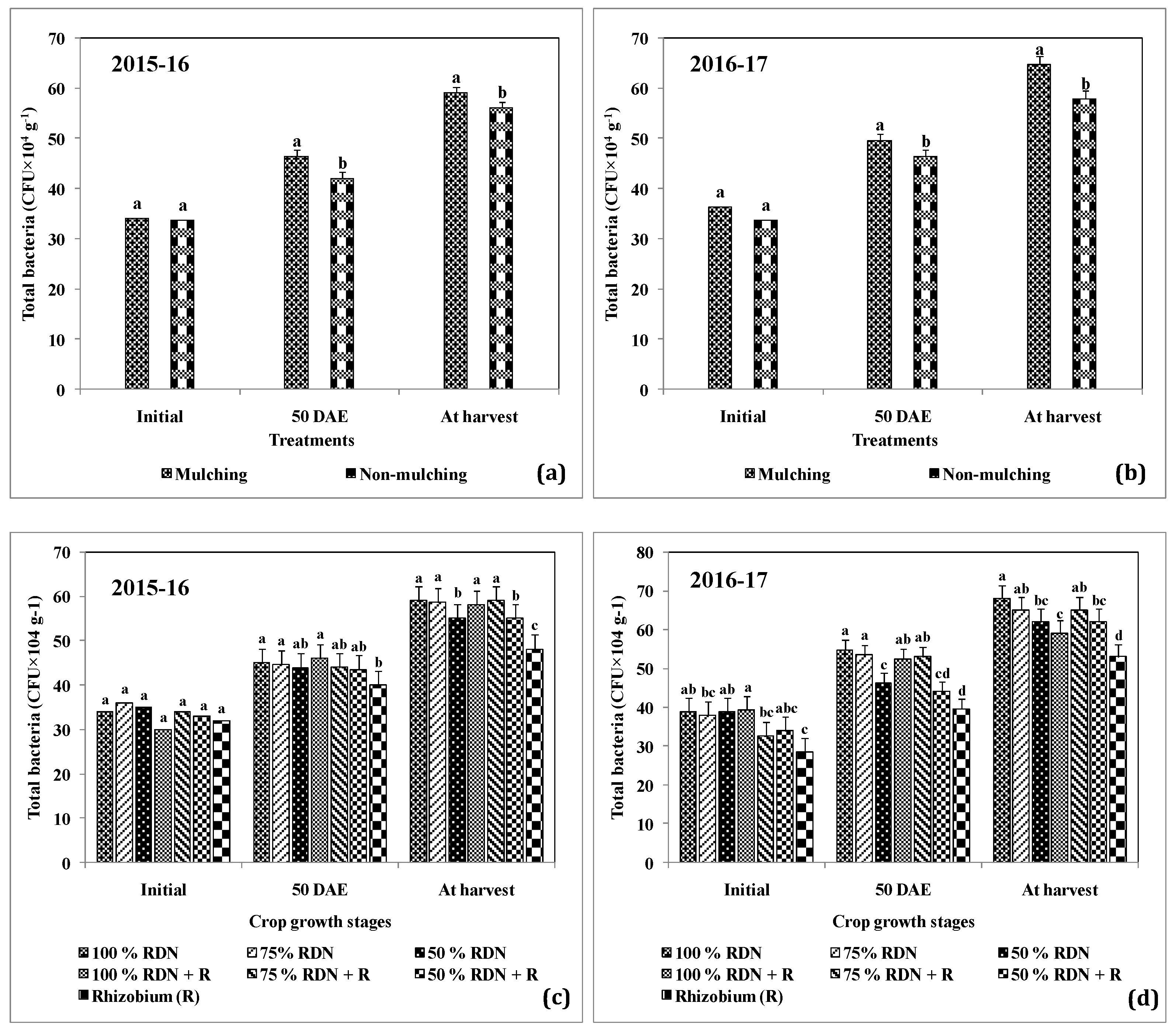
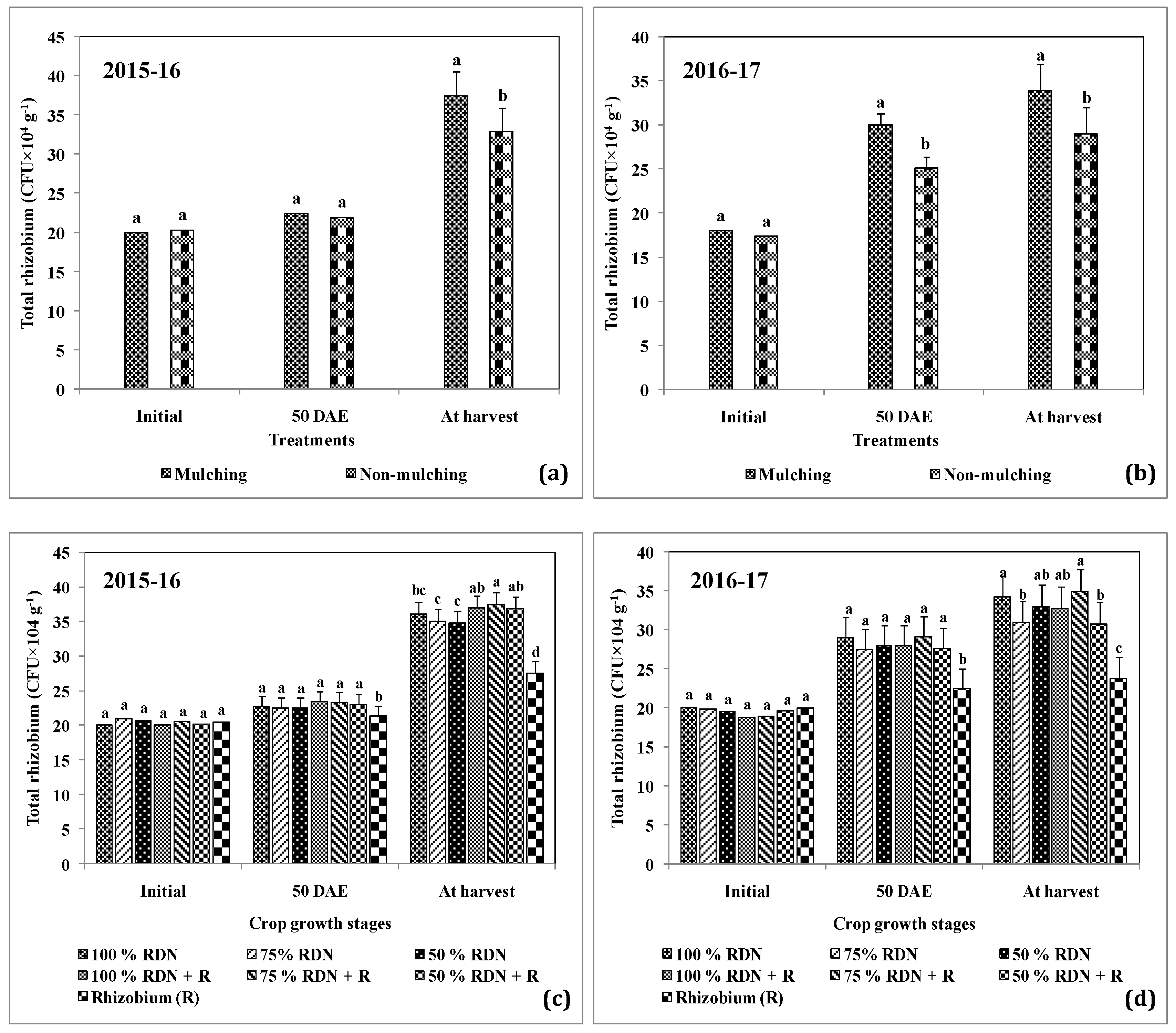
3.3. Supplementation of Different Levels of Nitrogen in Association with Rh and Soil Mulch Influence the Soil Bacterial Populations at Different Growth Stages of Peanut
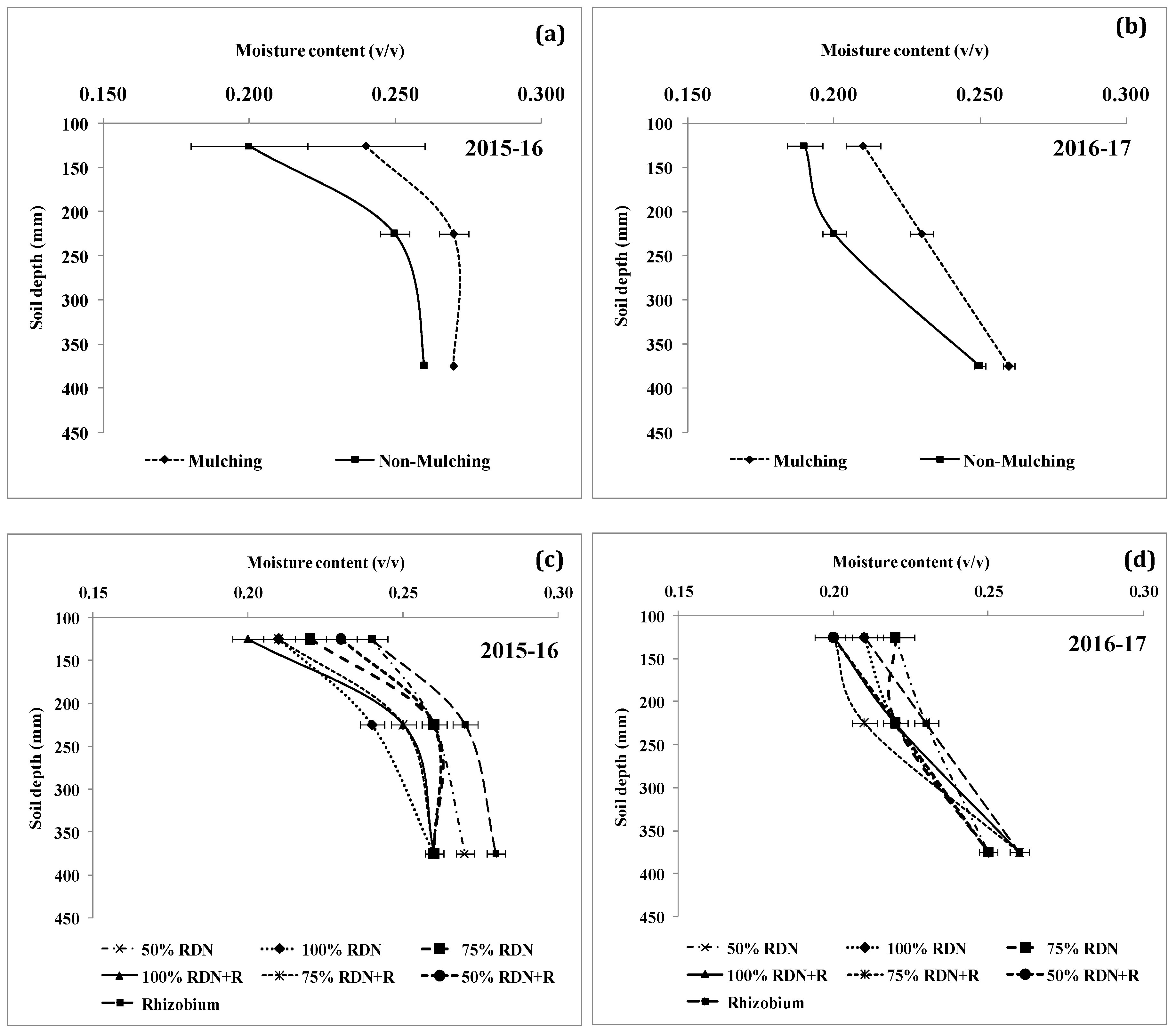
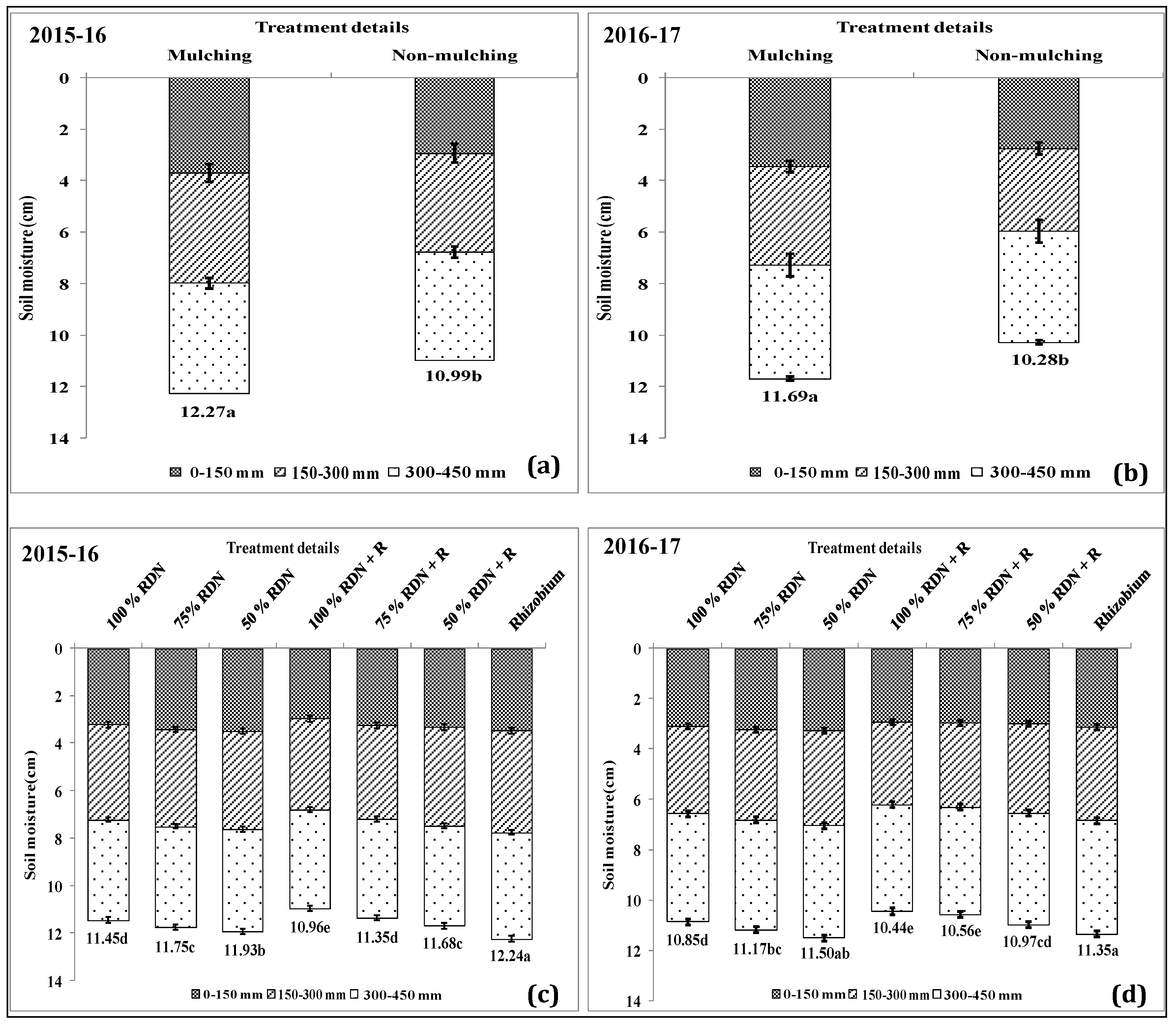
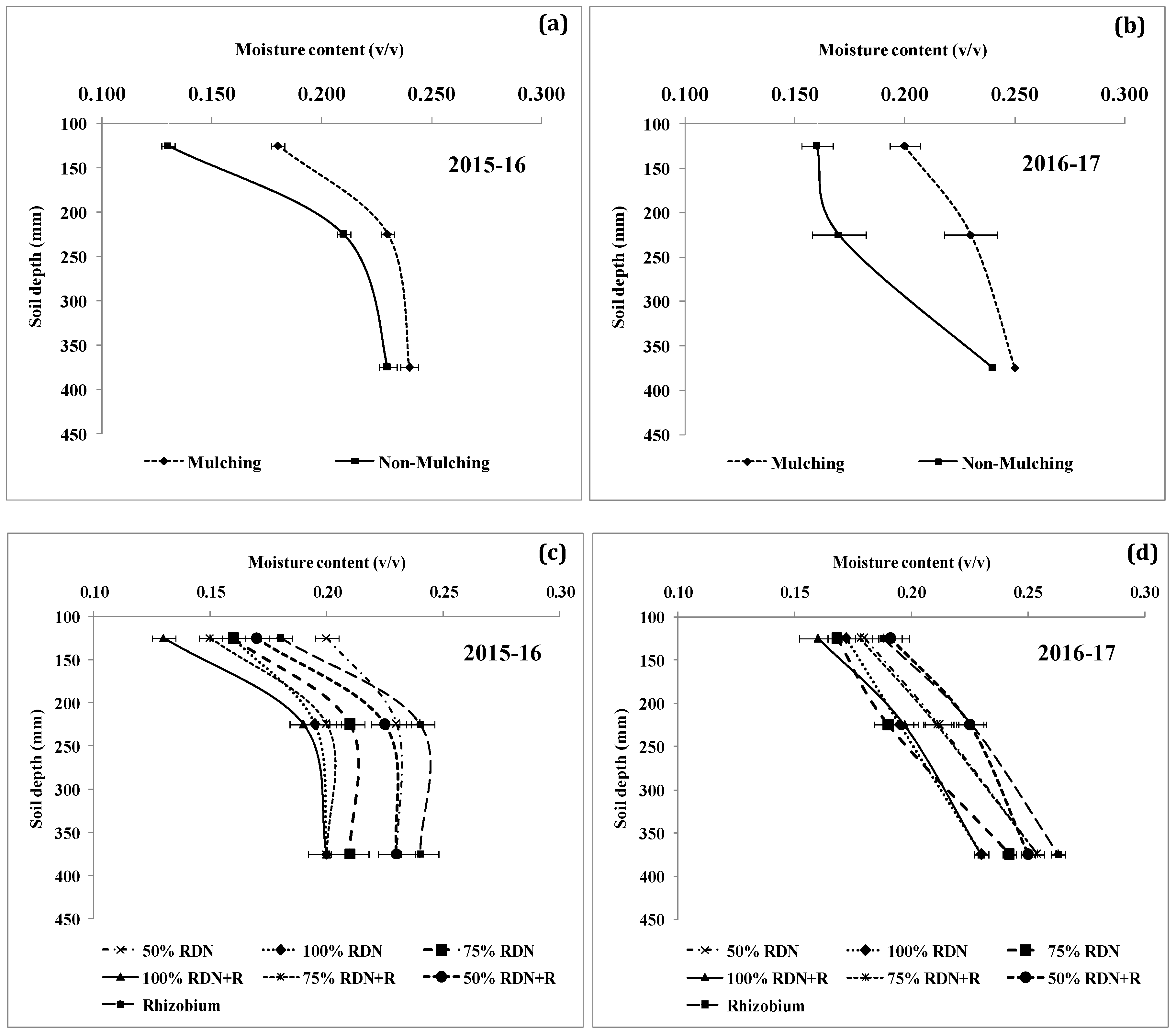
3.4. Soil Moisture Status and Distribution are Influenced by Different Levels of Nitrogen Fertilizer Applied with Rh and Soil Mulch
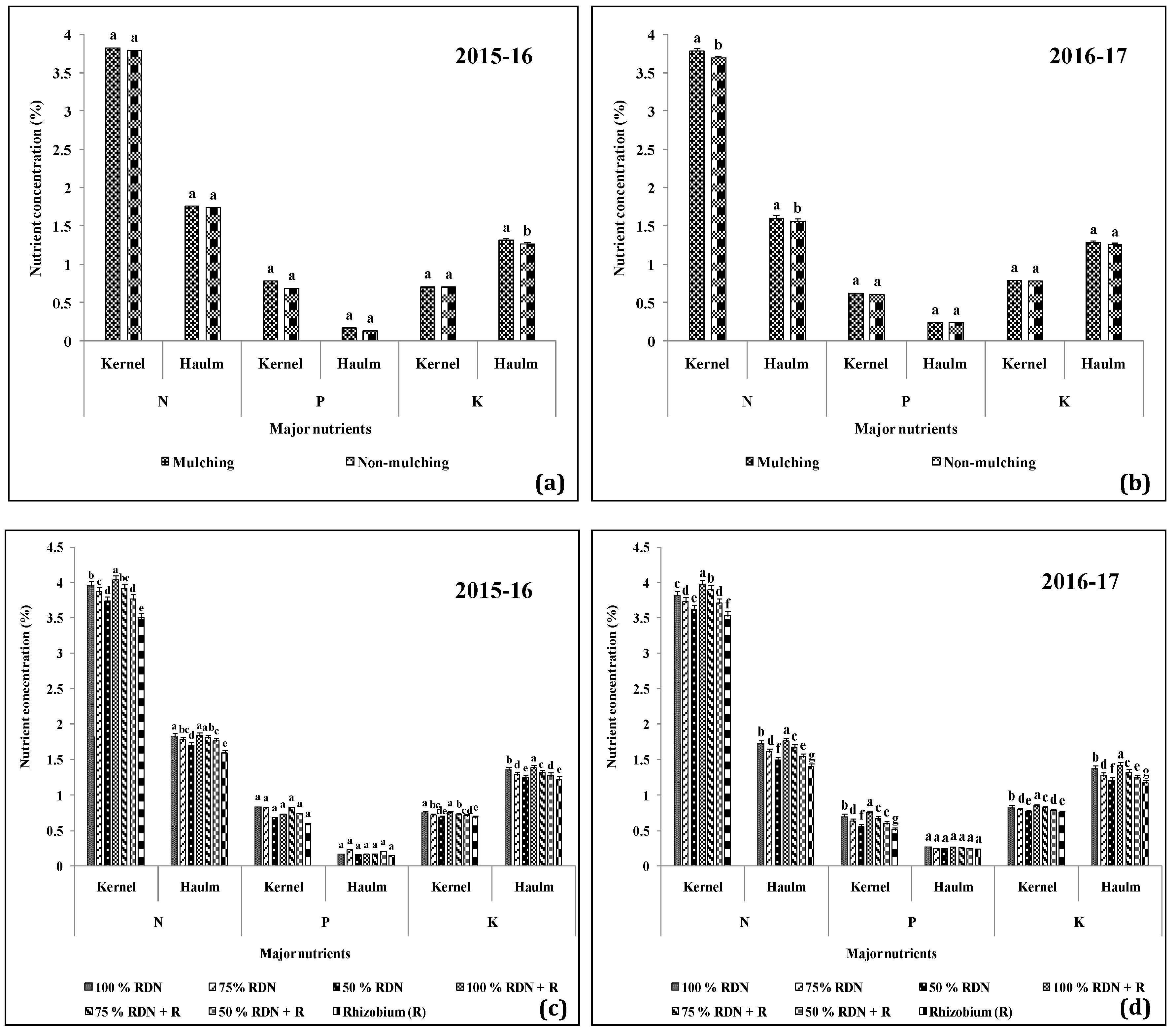
3.5. Nutrient Dynamics are Influenced by Different Levels of Nitrogen in Association with Rh and Soil Mulch
3.6. Nutrient Balance in Peanut is Influenced by the Different Levels of Nitrogen in Association with Rh and Soil Mulch
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jain, N.K.; Meena, H.N.; Bhaduri, D. Improvement in productivity, water-use efficiency, and soil nutrient dynamics of summer peanut (Arachis hypogaea L.) through use of polythene mulch, hydrogel, and nutrient management. Commun. Soil Sci. Plant Anal. 2017, 48, 549–564. [Google Scholar] [CrossRef]
- GOI. AGRICULTURE-Statistical Year Book India; Government of India, Ministry of Statistics and Programme Implementation: New Delhi, India, 2018. Available online: http://www.mospi.gov.in/statistical-year-book-india/2018/177 (accessed on 12 October 2020).
- Irungbam, P.; Pramanick, M.; Shashidhar, K.S. Effect of different nutrient management on growth parameters and yield of summer groundnut in New Alluvial Zone of West Bengal. Ecol. Environ. Conserv. 2016, 22, 39–42. [Google Scholar]
- Rathnakumar, A.L.; Singh, R.; Parmar, D.L.; Misram, J.B. Groundnut: A Crop Profile and Compendium of Notified Varieties of India; Directorate of Groundnut Research: Gujarat, India, 2013; P.B. No. 05. PB 118. [Google Scholar]
- Ojiewo, C.O.; Janila, P.; Bhatnagar-Mathur, P.; Pandey, M.K.; Desmae, H.; Okori, P.; Mwololo, J.; Ajeigbe, H.; Njuguna-Mungai, E.; Muricho, G. Advances in Crop Improvement and Delivery Research for Nutritional Quality and Health Benefits of Groundnut (Arachis hypogaea L.). Front. Plant Sci. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, P.K.; Dayal, D.; Bandyopadhyay, K.K.; Mohanty, M. Evaluation of straw and polythene mulch for enhancing productivity of irrigated summer groundnut. Field Crop Res. 2006, 99, 76–86. [Google Scholar] [CrossRef]
- Jain, N.K.; Jat, R.A.; Yadav, R.S.; Bhaduri, D.; Meena, H.N. Polythene mulching and fertigation in peanut (Arachis hypogaea): Effect on crop productivity, quality, water productivity and economic profitability. Indian J. Agric Sci. 2018, 88, 1168–1178. [Google Scholar]
- Mohapatra, A.K.B.; Dixit, L. Integrated nutrient management in rainy season groundnut (Arachis hypogaea). Indian J. Agron. 2010, 55, 123–127. [Google Scholar]
- Panwar, A.S.; Munda, G.C. Response of groundnut (Arachis hypogaea) to organic and inorganic sources of nutrient supply under mid-hill altitude conditions. Indian J. Agric. Sci. 2007, 77, 814–818. [Google Scholar]
- Jat, N.L.; Jain, N.K.; Choudhary, G.R. Integrated nutrient management in fenugreek (Trigonella foenumgraecum). Indian J. Agron. 2006, 51, 331–333. [Google Scholar]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. J. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Mathenge, C.; Thuita, M.; Masso, C.; Gweyi-Onyango, J.; Vanlauwe, B. Variability of soybean response to Rhizobia inoculant, vermicompost, and a legume-specific fertilizer blend in Siaya County of Kenya. Soil Tillage Res. 2019, 194, 104290. [Google Scholar] [CrossRef]
- Arora, N.K. Plant microbe symbiosis: Fundamentals and advances. In Plant Microbe Symbiosis: Fundamentals and Advances; Springer: New Delhi, India, 2013. [Google Scholar]
- Argaw, A. Effectiveness of Rhizobium inoculation on common bean productivity as determined by inherent soil fertility status. J. Crop Sci. Biotechnol. 2016, 19, 311–322. [Google Scholar] [CrossRef]
- Anandham, R.; Sridar, R.; Nalayini, P.; Poonguzhali, S.; Madhaiyan, M.; Sa, T. Potential for plant growth promotion in groundnut (Arachis hypogaea L.) cv. ALR-2 by co-inoculation of sulfur-oxidizing bacteria and Rhizobium. Microbiol. Res. 2007, 162, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Nievas, F.; Bogino, P.; Nocelli, N.; Giordano, W. Genotypic analysis of isolated peanut-nodulating Rhizobial strains reveals differences among populations obtained from soils with different cropping histories. Appl. Soil Ecol. 2012, 53, 74–82. [Google Scholar] [CrossRef]
- Argaw, A. Development of environmental friendly bioinoculate for peanut (Arachis hypogea L.) production in Eastern Ethiopia. Environ. Syst. Res. 2017, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in Agriculture: Balancing the Cost of an Essential Resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef] [Green Version]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Martín-Closas, L.; Costa, J.; Pelacho, A.M. Agronomic effects of biodegradable films on crop and field environment. In Soil Degradable Bioplastics for a Sustainable Modern Agriculture; Malinconico, M., Ed.; Springer: Berlin, Germany, 2017; pp. 67–104. [Google Scholar] [CrossRef]
- Awe, G.O.; Reichert, J.M.; Timm, L.C.; Wendroth, O.O. Temporal processes of soil water status in a sugarcane field under residue management. Plant Soil 2015, 387, 395–411. [Google Scholar] [CrossRef]
- Luna, L.; Miralles, I.; Andrenelli, M.C.; Gispert, M.; Pellegrini, S.; Vignozzi, N.; Solé-Benet, A. Restoration techniques affect soil organic carbon, glomalin and aggregate stability in degraded soils of a semiarid Mediterranean region. Catena 2016, 143, 256–264. [Google Scholar] [CrossRef]
- Bu, L.; Liu, J.; Zhu, L.; Luo, S.; Chen, X.; Li, S.; Lee, H.R.; Zhao, Y. The effects of mulching on maize growth, yield and water use in a semi-arid region. Agric. Water Manag. 2013, 123, 71–78. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, J.; Luo, S.; Bu, L.; Chen, X.; Li, S. Soil mulching can mitigate soil water deficiency impacts on rainfed maize production in semiarid environments. J. Integr. Agric. 2015, 14, 58–66. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, X.G.; Fu, T.; Wang, L.; Turner, N.C.; Siddique, K.H.M.; Li, F.M. Multi-site assessment of the effects of plastic-film mulch on the soil organic carbon balance in semiarid areas of China. Agric. For. Meteorol. 2016, 228–229, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Rana, L.; Banerjee, H.; Dutta, S.K.; Ray, K.; Majumdar, K.; Sarkar, S. Management practices of macronutrients for potato for smallholder farming system at alluvial soil (Entisols) of India. Arch. Agron. Soil Sci. 2017, 63, 1963–1976. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H.J. Methods in Legume-Rhizobium Technology; NifTAL: Hawaii, HI, USA, 1985; p. 367. [Google Scholar]
- Kundu, R.; Mondal, M.; Garai, S.; Poddar, R.; Banerjee, S. Efficacy of Herbicides against Broad-spectrum Weed Floras and Their Effect on Non-target Soil Micro-organisms and Productivity in Sugarcane (Saccharum sp.). Curr. J. Appl. Sci. Technol. 2020, 39, 23–32. [Google Scholar] [CrossRef]
- Das, T.K.; Sakhuja, P.K.; Zelleke, H. Herbicide efficacy and non-target toxicity in high land rainfed maize of Eastern Ethiopia. Int. J. Pest Manag. 2010, 56, 315–325. [Google Scholar] [CrossRef]
- IRRI. Statistical tool for agricultural research, Version 2.0.1. In User’s manual. Biometrics and Breeding Informatics, PBGB, Division; International Rice Research Institute: Los Bãnos, Philippines, 2014. [Google Scholar]
- Negi, S.; Sing, R.V.; Dwivedi, O.K. Effect of Biofertilizers, nutrient sources and lime on growth and yield of garden pea. Legume Res. 2006, 29, 282–285. [Google Scholar]
- Anikwe, M.A.N.; Obi, M.E.; Agbim, N.N. Effect of crop and soil management practices on soil compatibility in maize and groundnut plots in a Paleustult in Southeastern Nigeria. Plant Soil. 2003, 253, 457–465. [Google Scholar] [CrossRef]
- Khan, A.R. Mulching effect on soil physical properties and peanut production. Italian J. Agron. 2002, 6, 113–118. [Google Scholar]
- Namvar, A.; Seyed, S.R.; Khandan, T.; Jafari, M.M. Seed inoculation and inorganic nitrogen fertilization effects on some physiologicaland agronomical traits of chickpea (Cicer arietinum L.) in irrigated condition. J. Central Eur. Agric. 2013, 14, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Caliskan, S.I.; Ozkaya, I.; Caliskan, M.E.; Arslan, M. The effect of nitrogen and iron fertilization on growth, yield, and fertilizer use efficiency of soybean in Mediterranean type soil. Field Crops Res. 2008, 108, 126–132. [Google Scholar] [CrossRef]
- Togay, N.; Togay, Y.; Cimrin, K.M.; Turan, M. Effect of Rhizobium inoculation, sulfur, and phosphorus application on yield, yield components, and nutrient uptake in chickpea (Cicer aretinum L.). Afr. J. Biotechnol. 2008, 7, 776–782. [Google Scholar]
- Nalayini, P.; Anandham, R.; Sankaranarayanan, K.; Rajendran, T.P. Polyethylene mulching for enhancing crop productivity and water use efficiency in cotton (Gossypium hirsutum) and maize (Zea mays) cropping system. Indian J. Agron. 2009, 54, 409–514. [Google Scholar]
- Hamaguchi, H.; Yamamoto, N.; Takeda, A.; Masumura, T.; Sugimoto, T.; Azuma, T. Nitrogen fertilization affects yields and storage compound contents in seeds of field-grown soybeans cv Enrei (Glycine max L.) and its super-nodulating mutant En-b0-1 through changing N2 fixation activity of the plants. Soil Sci. Plant Nutr. 2019, 66, 299–307. [Google Scholar] [CrossRef]
- Asante, M.; Ahiabor, B.D.K.; Atakora, W.K. Growth, Nodulation, and Yield Responses of Groundnut (Arachis hypogaea L.) as Influenced by Combined Application of Rhizobium Inoculant and Phosphorus in the Guinea Savanna Zone of Ghana. Int. J. Agron. 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Subrahmaniyan, K.; Kalaiselvan, P.; Balasubramanian, T.N.; Zhou, W. Crop productivity and soil properties as affected by polyethylene film mulch and land configurations in groundnut (Arachis hypogaea L.). Arch. Agron. Soil Sci. 2006, 52, 79–103. [Google Scholar] [CrossRef]
- Hasan, M.; Sahid, I.B. Evaluation of Rhizobium inoculation in combination with phosphorus and nitrogen fertilization on groundnut growth and yield. J. Agron. 2016, 15, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Ravankar, H.N.; Topre, R.K.; Desmukh, M.S.; Desmukh, P.W. Effect of polythene mulch on dry matter, yield and uptake of nutrient by summer groundnut. Legume Res. 2003, 26, 73–74. [Google Scholar]
- Shaikh, A.A.; Nimbalkar, C.A.; Jawale, S.M. Effect of irrigation scheduling and mulches on yield contributing characters of summer groundnut. J. Maharastra Agric. Univ. 2004, 299, 163–166. [Google Scholar]
- Acharya, C.L.; Hati, K.M.; Bandyopadhyay, K.K. Mulches. In Encyclopedia of Soils in the Environment; Hillel, D., Rosenzweig, C., Pawlson, D.S., Scow, K.M., Sorger, M.J., Sparks, D.L., Hatfield, J., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 521–532. [Google Scholar]
- Argaw, A. Integrating inorganic NP application and Brady Rhizobium inoculation to minimize production cost of peanut (Arachis hypogea L.) in eastern Ethiopia. Agric. Food Secur. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Singh, G.P.; Singh, P.L.; Panwar, A.S. Response of groundnut (Arachis hypogaea) to biofertilizers, organic and inorganic sources of nutrient in North-East India. Legume Res. 2011, 34, 196–201. [Google Scholar]
- Sharma, S.; Jat, N.L.; Puniya, M.M.; Shivran, A.C.; Choudhary, S. Fertility levels and biofertilizers on nutrient concentrations, uptake and quality of groundnut. Ann. Agric. Res. 2014, 35, 71–74. [Google Scholar]
- Rathore, P.K.; Kamble, B.M. Integrated nutrient management in summer groundnut. Adv. Plant. Sci. 2008, 21, 329–331. [Google Scholar]
- Sarr, A.; Neyra, M.; Houeibib, M.A.O.; Ndoye, I.; Oihabi, A.; Lesueur, D. Rhizobial populations in soils from natural Acacia senegal and Acacia nilotica forests in Mauritania and the Senegal River valley. Microbial. Ecol. 2005, 50, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.; Zhang, B.; Jin, X.X.; Zhang, P.; Wang, J.K. Long-term effect of plastic film mulching and fertilization on bacterial communities in a brown soil revealed by high through-put sequencing. Arch. Agron. Soil Sci. 2017, 63, 230–241. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Kruse, M.L.; Sawyer, J.E. effect of nitrogen fertilizer application on growing season soil carbon dioxide emission in a corn–soybean rotation. J. Environ. Qual. 2008, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Long, R.; Wang, Q.; Liu, W.; Jing, Z.; Zhang, L. Fertilization and litter effects on the functional group biomass, species diversity of plants, microbial biomass, and enzyme activity of two alpine meadow communities. Plant Soil 2010, 331, 377–389. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Hu, W.; Duan, S.; Sui, Q. High yield technology for groundnut. Int. Arachis Newsl. 1995, 15, 1–22. [Google Scholar]
- Chen, H.; Liu, J.; Zhang, A.; Chen, J.; Cheng, G.; Sun, B.; Pi, X.; Dyck, M.; Si, B.; Zhao, Y. Effects of straw and plastic film mulching on greenhouse gas emissions in Loess Plateau, China: A field study of 2 consecutive wheat-maize rotation cycles. Sci. Total Environ. 2017, 579, 814–824. [Google Scholar] [CrossRef]
- Tu, C.; Ristaino, J.B.; Hu, S. Soil microbial biomass and activity in organic tomato farming systems: Effects of organic inputs and straw mulching. Soil Biol. Biochem. 2006, 38, 247–255. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Lloyd, J.; Herms, D.A.; Hoitink, H.A.J.; Michel, J.F.C. Effects of mulching and fertilization on soil nutrients, microbial activity and Rhizosphere bacterial community structure determined by analysis of TRFLPs of PCR-amplified 16S rRNA genes. Appl. Soil Ecol. 2002, 21, 31–48. [Google Scholar] [CrossRef]
- Liu, M.; Hu, F.; Chen, X.; Huang, Q.; Jiao, J.; Zhang, B.; Li, H. Organic amendments with reduced chemical fertilizer promote soil microbial development and nutrient availability in a subtropical paddy field: The influence of quantity, type and application time of organic amendments. Appl. Soil Ecol. 2009, 42, 166–175. [Google Scholar] [CrossRef]
- Mondal, M.; Skalicky, M.; Garai, S.; Hossain, A.; Sarkar, S.; Banerjee, H.; Kundu, R.; Brestic, M.; Barutcular, C.; Erman, M.; et al. Supplementing nitrogen in combination with rhizobium inoculation and soil mulch in peanut (Arachis hypogaea L.) production system: Part II. Effect on phenology, growth, yield attributes, pod quality, profitability and nitrogen use efficiency. Agronomy 2020, 10, 1513. [Google Scholar] [CrossRef]
- Jiang, R.; Li, X.; Zhou, M.; Li, H.J.; Zhao, Y.; Yi, J.; Cui, L.L.; Li, M.; Zhang, J.G.; Qu, D. Plastic film mulching on soil water and maize (Zea mays L.) yield in a ridge cultivation system on Loess Plateau of China. Soil Sci. Plant Nutr. 2016, 62, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Xie, Y.; Jiang, H.; Wu, B.; Niu, J. Soil water status and root distribution across the rooting zone in maize with plastic film mulching. Field Crops Res. 2014, 156, 40–47. [Google Scholar] [CrossRef]
- Mulumba, L.N.; Lal, R. Mulching effects on selected soil physical properties. Soil Tillage Res. 2008, 98, 106–111. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.K.; Halder, S.; Mondal, K.; Singh, K.C.; Nandi, R.; Ghosh, P.K. Response of Lentil (Lens culinaries) to Post-rice Residual Soil Moisture under Contrasting Tillage Practices. Agric. Res. 2018, 7, 463–479. [Google Scholar] [CrossRef]
- Zhang, S.; Sadars, V.; Chen, X.; Zhang, F. Water use efficiency of dryland maize in the Loess Plateau of China in response to crop management. Field Crops Res. 2014, 163, 55–63. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Hoang, M.T.; Wain, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crop Res. 2006, 95, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Subrahmaniyan, K.; Kalaiselvan, P.; Balasubramanian, T.N. Microclimate variations in relation to different types of polyethylene-film mulch on growth and yield of groundnut (Arachis hypogaea). Indian J. Agron. 2008, 53, 184–188. [Google Scholar]
- Ronner, E.; Franke, A.C.; Vanlauwe, B.; Dianda, M.; Edeh, E.; Ukem, B.; Giller, K.E. Understanding variability in soybean yield and response to P-fertilizer and Rhizobium inoculants on farmers’ fields in northern Nigeria. Field Crop. Res. 2016, 186, 133–145. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization—A global meta-analysis. Front. Plant Sci. 2018, 12, 2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subrahmaniyan, K.; Kalaiselvan, P.; Balasubramanian, T.N.; Zhou, W. Soil properties and yield of groundnut associated with herbicides, plant geometry and plastic mulch. Commun. Soil Sci. Plan. 2008, 39, 1206–1234. [Google Scholar] [CrossRef]
- Vessey, J.K. Benefits of inoculating legume crops with Rhizobia in the northern Great Plains. Crop. Manag. 2004, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Marra, L.M.; de OliveiraII, S.M.; Soares, C.R.F.S.; de Souza, M.F.M. Solubilisation of inorganic phosphates by inoculant strains from tropical legumes. Sci. Agric. 2011, 68, 603–609. [Google Scholar] [CrossRef] [Green Version]
- Werner, D.; Newton, W.E. Nitrogen Fixation in Agriculture, Forestry, Ecology, and Environment; Springer: New York, NY, USA, 2005. [Google Scholar]
- Liu, Y.Y.; Sun, Y.; Heinz, M.S.; Yan, R.; Zhou, Z.X.; Wang, Y.J.; Yu, F.H. Do invasive alien plants differ from non-invasives in dominance and nitrogen uptake in response to variation of abiotic and biotic environments under global anthropogenic change? Sci. Total Environ. 2019, 672, 634–642. [Google Scholar] [CrossRef]


| Properties | Seasons | Analysis Methods | |
|---|---|---|---|
| 2015–2016 | 2016–2017 | ||
| Physical properties | |||
| Sand (%) | 46.6 | 47.9 | Hydrometer method [26] |
| Silt (%) | 30.1 | 31.5 | |
| Clay (%) | 23.3 | 21.2 | |
| Chemical properties | |||
| pH | 7.3 | 7.2 | Glass electrode Beckman pH meter (in 1:2.5: Soil:Water) [26] |
| Organic Carbon (%) | 0.53 | 0.50 | Walkley and Black method [26] |
| Available N (kg ha−1) | 195.3 | 213.7 | Alkaline Permanganate method [26] |
| Available P (kg ha−1) | 19.8 | 23.9 | 0.5 M NaHCO3 extractable Olsen’s Colorimetric method [26] |
| Available K (kg ha−1) | 143.3 | 136.7 | Flame photometric method [26] |
| Treatment Details | Peg Plant−1 | Pod Plant−1 | SMK (%) | 100 Kernel Weight (g) | ||||
|---|---|---|---|---|---|---|---|---|
| 2015–2016 | 2016–2017 | 2015–2016 | 2016–2017 | 2015–2016 | 2016–2017 | 2015–2016 | 2016–2017 | |
| Mulching | ||||||||
| 100% RDN | 29.30 abc | 29.90 abcd | 24.60 abc | 25.20 bc | 93.30 ab | 92.50 ab | 45.50 ab | 46.00 ab |
| 75% RDN | 30.40 ab | 31.20 ab | 23.30 bc | 23.75cd | 91.70 ab | 91.60 ab | 44.90 abc | 45.30 abcd |
| 50% RDN | 27.40 c | 29.40 bcd | 19.00 ef | 19.42 fg | 88.40 b | 90.20 bc | 43.30 c | 44.80 abcd |
| 100% RDN + Rh | 30.10 ab | 31.30 ab | 25.20 ab | 30.57 ab | 94.20 ab | 93.50 ab | 46.10 ab | 45.90 abc |
| 75% RDN + Rh | 31.40 ab | 30.90 abc | 25.90 ab | 25.40 bc | 93.30 ab | 93.10 ab | 45.90 ab | 46.30 ab |
| 50% RDN + Rh | 28.20 bc | 28.60cd | 20.90 de | 21.10 ef | 88.70 b | 89.90 bc | 44.80 abc | 45.20 abcd |
| Rhizobium (Rh) | 22.21 d | 21.90 f | 13.20 g | 14.93 h | 82.70 de | 83.60 f | 44.20 abc | 43.70 d |
| Non-mulching | ||||||||
| 100% RDN | 27.21 c | 27.90 de | 20.50 de | 22.94 de | 87.60 bc | 88.10 cde | 44.60 abc | 45.10 abcd |
| 75% RDN | 26.82 c | 27.70 de | 19.60 ef | 20.30 fg | 86.90 bc | 87.30de | 43.30 bc | 44.30 abcd |
| 50% RDN | 24.30 d | 23.20 f | 17.80 f | 16.70 h | 83.60 de | 88.20 cde | 43.00 c | 44.00 bcd |
| 100% RDN + Rh | 29.24 abc | 28.90 bcd | 22.30 cd | 26.64 b | 88.60 b | 89.00 cd | 45.00 abc | 45.30 abcd |
| 75% RDN + Rh | 28.30 bc | 29.10 bcd | 20.80 de | 23.69 cd | 87.20 bc | 88.00 cde | 44.60 abc | 44.80 abcd |
| 50% RDN + Rh | 27.90 bc | 26.00 e | 18.70 ef | 18.95 g | 85.00 cd | 86.30 e | 43.30 c | 43.80 cd |
| Rhizobium (Rh) | 24.00 d | 21.90 f | 11.40 g | 10.20 i | 81.90 e | 82.90 f | 43.00 c | 43.70 d |
| F-test | ||||||||
| Mulching (M) | ** | ** | ** | ** | ** | * | * | * |
| Nitrogen (N) | ** | ** | * | ** | * | * | ** | ** |
| M × N | * | * | * | * | ** | ** | * | ** |
| Treatments | Pod Yield (t ha−1) | Kernel Yield (t ha−1) | Haulm Yield (t ha−1) | Oil Yield (t ha−1) | Protein Yield (t ha−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2015–2016 | 2016–2017 | 2015–2016 | 2016–2017 | 2015–2016 | 2016–2017 | 2015–2016 | 2016–2017 | 2015–2016 | 2016–2017 | |
| Mulching | ||||||||||
| 100% RDN | 3.65 b | 3.83 ab | 2.58 bc | 2.80 ab | 4.36 bc | 4.47 ab | 1.21 b | 1.35 b | 0.66 b | 0.71 b |
| 75% RDN | 3.49 bc | 3.60 ab | 2.45 cd | 2.50 cde | 4.18 cd | 4.24 ab | 1.10 c | 1.15 cd | 0.60 c | 0.62 cd |
| 50% RDN | 3.32 c | 3.46 ab | 2.31 de | 2.38 def | 4.02 d | 4.11 ab | 0.97 ef | 1.02 ef | 0.54 de | 0.56 efg |
| 100% RDN + Rh | 3.87 ab | 3.96 ab | 2.88 ab | 2.99 ab | 4.62 ab | 4.58 ab | 1.38 ab | 1.46 ab | 0.75 ab | 0.78 ab |
| 75% RDN + Rh | 3.70 ab | 3.75 ab | 2.63 b | 2.62 bc | 4.47 ab | 4.41 ab | 1.20 b | 1.22 c | 0.66 b | 0.65 c |
| 50% RDN + Rh | 3.56 b | 3.62 ab | 2.49 bc | 2.53 cd | 4.29 bc | 4.27 ab | 1.06 d | 1.09 de | 0.60 c | 0.60def |
| Rhizobium (Rh) | 1.68 h | 1.59 ab | 1.14 j | 1.08 j | 2.22 i | 2.12 ab | 0.44 j | 0.42 h | 0.24 i | 0.23 j |
| Without mulching | ||||||||||
| 100% RDN | 3.00 de | 3.20 ab | 2.09 fg | 2.22 fgh | 3.67 ef | 3.90 ab | 0.98 def | 1.07 de | 0.54 de | 0.57 defg |
| 75% RDN | 2.84 efg | 3.05 ab | 1.96 ghi | 2.11 ghi | 3.48 fgh | 3.72 ab | 0.88 gh | 0.97 f | 0.49 fg | 0.52 gh |
| 50% RDN | 2.68 g | 2.88 ab | 1.82 i | 1.92 i | 3.31 h | 3.65 ab | 0.77 i | 0.83 g | 0.43 h | 0.45 i |
| 100% RDN + Rh | 3.11 d | 3.29 ab | 2.18 ef | 2.30 efg | 3.77 e | 3.93 ab | 1.04 cde | 1.13 cd | 0.57 cd | 0.61 cde |
| 75% RDN + Rh | 2.94 def | 3.17 ab | 2.07 fgh | 2.20 fgh | 3.60 efg | 3.90 ab | 0.94 fg | 1.03 ef | 0.52 ef | 0.55 fg |
| 50% RDN + Rh | 2.77 fg | 2.90 ab | 1.91 hi | 2.01 hi | 3.43 gh | 3.69 ab | 0.81 hi | 0.88 g | 0.46 gh | 0.48 hi |
| Rhizobium (Rh) | 1.39 i | 1.29 ab | 0.91 k | 0.86 k | 1.90 j | 1.87 ab | 0.35 k | 0.34 h | 0.19 j | 0.19 j |
| F-test | ||||||||||
| Mulching (M) | ** | ** | ** | ** | ** | ** | ** | * | * | ** |
| Nitrogen (N) | ** | ** | ** | ** | ** | ** | ** | * | ** | ** |
| M × N | * | ns | * | * | ** | ns | ** | * | * | * |
| Treatments | Initial N (kg ha−1) (A) | Fertilizer N Added (kg ha−1) (B) | Total N (kg ha−1) (A + B) | Total N Uptake (kg ha−1) (C) | Expected Balance {(A + B) − C} (D) | Post-Harvest Soil N Status (kg ha−1) (E) | Apparent Balance, Gain (E − D) or Loss (D − E) (F) | Actual Gain (E − A)/Loss (A − E) (G) |
|---|---|---|---|---|---|---|---|---|
| Mulching | ||||||||
| 100% RDN | 195.3 | 25.0 | 220.3 | 180.8 | 39.5 | 201.4 | 161.9 | 6.15 |
| 75% RDN | 195.3 | 18.7 | 214.0 | 168.3 | 45.79 | 192.4 | 146.7 | −2.8 |
| 50% RDN | 195.3 | 12.5 | 207.8 | 152.9 | 54.86 | 183.6 | 128.6 | −11.8 |
| 100% RDN + Rh | 195.3 | 25.00 | 220.3 | 199.8 | 20.50 | 205.6 | 185 | 10.2 |
| 75% RDN + Rh | 195.3 | 18.7 | 214.0 | 182.9 | 31.12 | 207.1 | 176.00 | 11.8 |
| 50% RDN + Rh | 195.3 | 12.5 | 207.8 | 169.9 | 37.88 | 196.3 | 158.4 | 1.0 |
| Rhizobium (Rh) | 195.3 | 0 | 195.3 | 74.8 | 120.54 | 176.5 | 56.0 | −18.8 |
| Non-mulching | ||||||||
| 100% RDN | 195.3 | 25.00 | 220.3 | 150.2 | 70.13 | 207.2 | 137.0 | 11.9 |
| 75% RDN | 195.3 | 18.7 | 214.0 | 138.0 | 75.96 | 200.3 | 124.4 | 5.0 |
| 50% RDN | 195.3 | 12.5 | 207.8 | 125.0 | 82.71 | 192.5 | 109.8 | −2.8 |
| 100% RDN + Rh | 195.3 | 25.0 | 220.3 | 157.9 | 62.37 | 212.5 | 150.1 | 17.2 |
| 75% RDN + Rh | 195.3 | 18.7 | 214.0 | 146.8 | 67.20 | 216.8 | 149.6 | 21.5 |
| 50% RDN + Rh | 195.3 | 12.5 | 207.8 | 131.6 | 76.23 | 205.2 | 129 | 9.9 |
| Rhizobium (Rh) | 195.3 | 0 | 195.3 | 62.5 | 132.79 | 183.24 | 50.4 | −12.0 |
| Treatments | Initial N (kg ha−1) (A) | Fertilizer N Added (kg ha−1) (B) | Total N (kg ha−1) (A + B) | Total N Uptake (kg ha−1) (C) | Expected Balance {(A + B) − C} (D) | Post-Harvest Soil N Status (kg ha−1) (E) | Apparent Balance, Gain (E − D) or Loss (D − E) (F) | Actual Gain (E − A)/Loss (A − E) (G) |
|---|---|---|---|---|---|---|---|---|
| Mulching | ||||||||
| 100% RDN | 213.7 | 25.0 | 238.7 | 192.5 | 46.2 | 207.12 | 160.9 | −6.6 |
| 75% RDN | 213.7 | 18.7 | 232.4 | 169.6 | 62.9 | 205.2 | 142.3 | −8.5 |
| 50% RDN | 213.7 | 12.5 | 226.2 | 154.8 | 71.4 | 194.5 | 123.0 | −19.2 |
| 100% RDN + Rh | 213.7 | 25.0 | 238.7 | 208.1 | 30.6 | 217.2 | 186.6 | 3.5 |
| 75% RDN + Rh | 213.7 | 18.7 | 232.4 | 182.6 | 49.8 | 215.3 | 165.4 | 1.6 |
| 50% RDN + Rh | 213.7 | 12.5 | 226.2 | 167.1 | 59.0 | 200.6 | 141.6 | −13.0 |
| Rhizobium (Rh) | 213.7 | 0 | 213.7 | 71.0 | 142.6 | 182.9 | 40.3 | −30.8 |
| Non-mulching | ||||||||
| 100% RDN | 213.7 | 25.0 | 238.7 | 159.01 | 79.7 | 212.6 | 132.9 | −1.0 |
| 75% RDN | 213.7 | 18.7 | 232.4 | 143.7 | 88.8 | 215.2 | 126.7 | 1.5 |
| 50% RDN | 213.7 | 12.5 | 226.2 | 128.4 | 97.8 | 200.8 | 103 | −12.9 |
| 100% RDN + Rh | 213.7 | 25.0 | 238.7 | 167.6 | 71.0 | 223.4 | 152.3 | 9.7 |
| 75% RDN + Rh | 213.7 | 18.7 | 232.4 | 157.3 | 75.1 | 220.2 | 145.0 | 6.5 |
| 50% RDN + Rh | 213.7 | 12.5 | 226.2 | 137.7 | 88.5 | 209.5 | 121 | −4.2 |
| Rhizobium (Rh) | 213.7 | 0 | 213.7 | 59.6 | 154.1 | 188.6 | 34.4 | −25.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, M.; Skalicky, M.; Garai, S.; Hossain, A.; Sarkar, S.; Banerjee, H.; Kundu, R.; Brestic, M.; Barutcular, C.; Erman, M.; et al. Supplementing Nitrogen in Combination with Rhizobium Inoculation and Soil Mulch in Peanut (Arachis hypogaea L.) Production System: Part I. Effects on Productivity, Soil Moisture, and Nutrient Dynamics. Agronomy 2020, 10, 1582. https://doi.org/10.3390/agronomy10101582
Mondal M, Skalicky M, Garai S, Hossain A, Sarkar S, Banerjee H, Kundu R, Brestic M, Barutcular C, Erman M, et al. Supplementing Nitrogen in Combination with Rhizobium Inoculation and Soil Mulch in Peanut (Arachis hypogaea L.) Production System: Part I. Effects on Productivity, Soil Moisture, and Nutrient Dynamics. Agronomy. 2020; 10(10):1582. https://doi.org/10.3390/agronomy10101582
Chicago/Turabian StyleMondal, Mousumi, Milan Skalicky, Sourav Garai, Akbar Hossain, Sukamal Sarkar, Hirak Banerjee, Rajib Kundu, Marian Brestic, Celaleddin Barutcular, Murat Erman, and et al. 2020. "Supplementing Nitrogen in Combination with Rhizobium Inoculation and Soil Mulch in Peanut (Arachis hypogaea L.) Production System: Part I. Effects on Productivity, Soil Moisture, and Nutrient Dynamics" Agronomy 10, no. 10: 1582. https://doi.org/10.3390/agronomy10101582
APA StyleMondal, M., Skalicky, M., Garai, S., Hossain, A., Sarkar, S., Banerjee, H., Kundu, R., Brestic, M., Barutcular, C., Erman, M., EL Sabagh, A., & Laing, A. M. (2020). Supplementing Nitrogen in Combination with Rhizobium Inoculation and Soil Mulch in Peanut (Arachis hypogaea L.) Production System: Part I. Effects on Productivity, Soil Moisture, and Nutrient Dynamics. Agronomy, 10(10), 1582. https://doi.org/10.3390/agronomy10101582












