Beyond Beer: Hop Shoot Production and Nutritional Composition under Mediterranean Climatic Conditions
Abstract
:1. Introduction
2. Materials and Methods
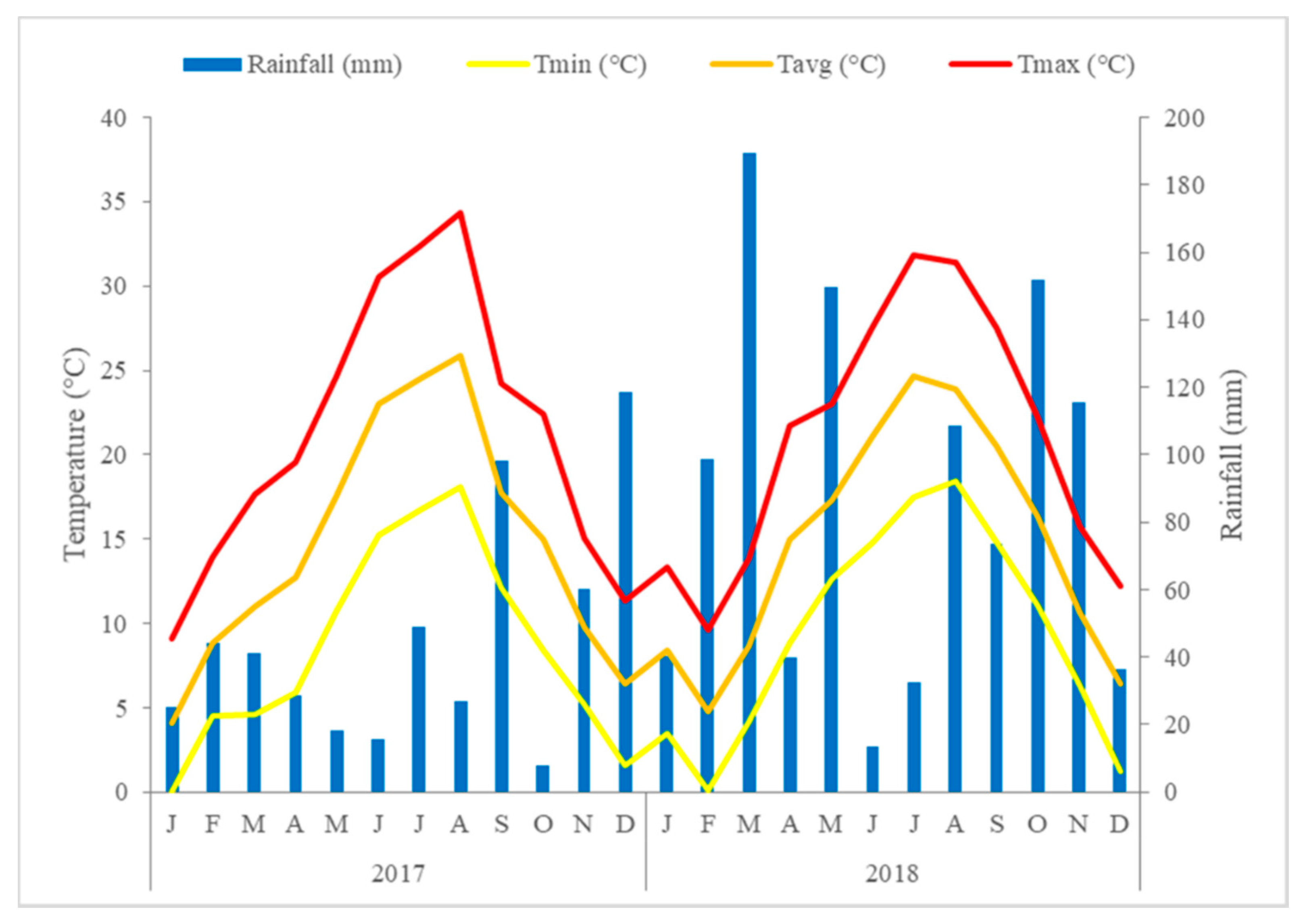
2.1. Location, Experimental Design, and Hopyard Management
2.2. Plant Materials
2.3. Field Measurements
2.4. Laboratory Analysis
2.5. Statistical Analysis
3. Results
3.1. Shoot Yield Characterization
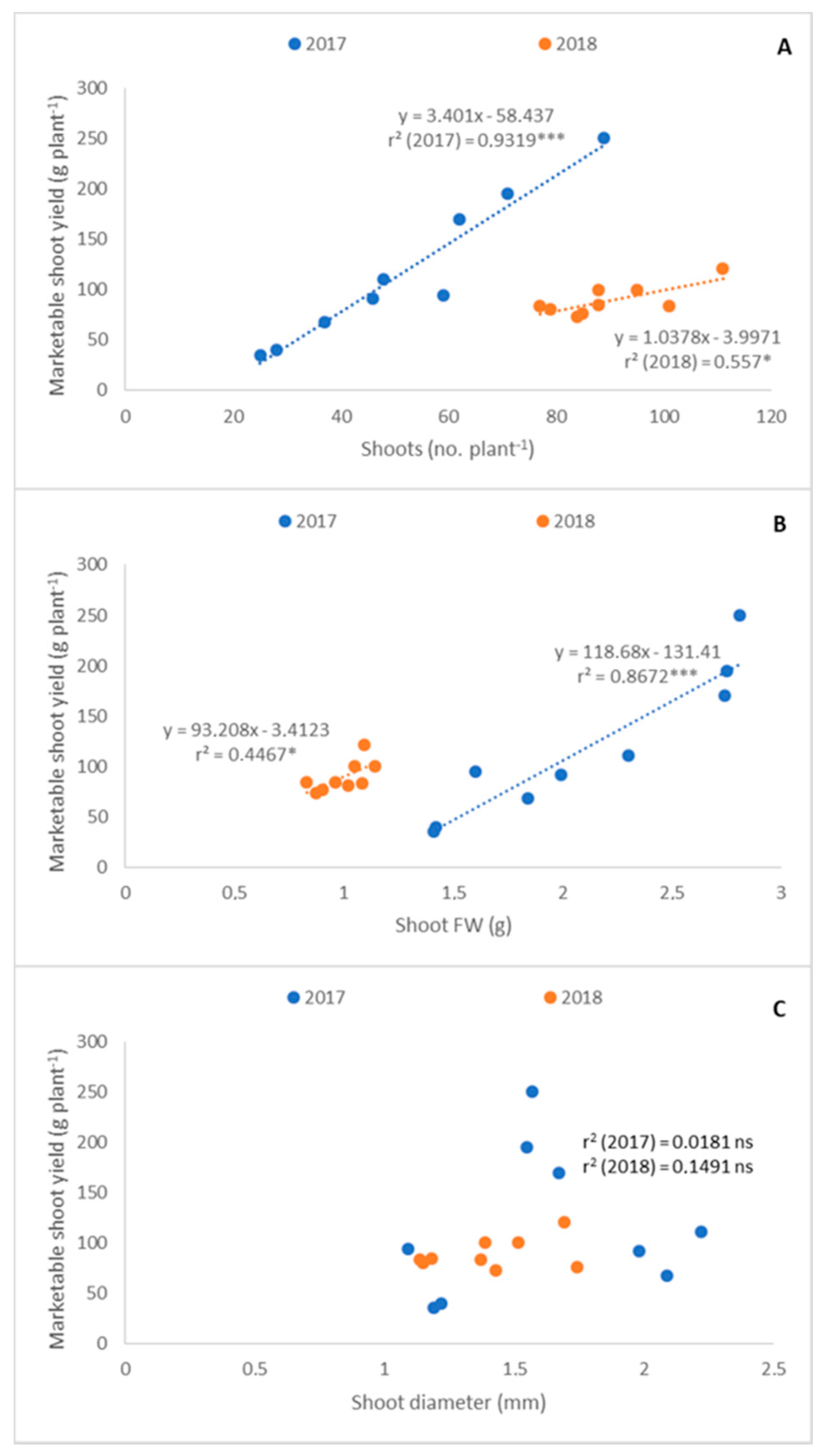
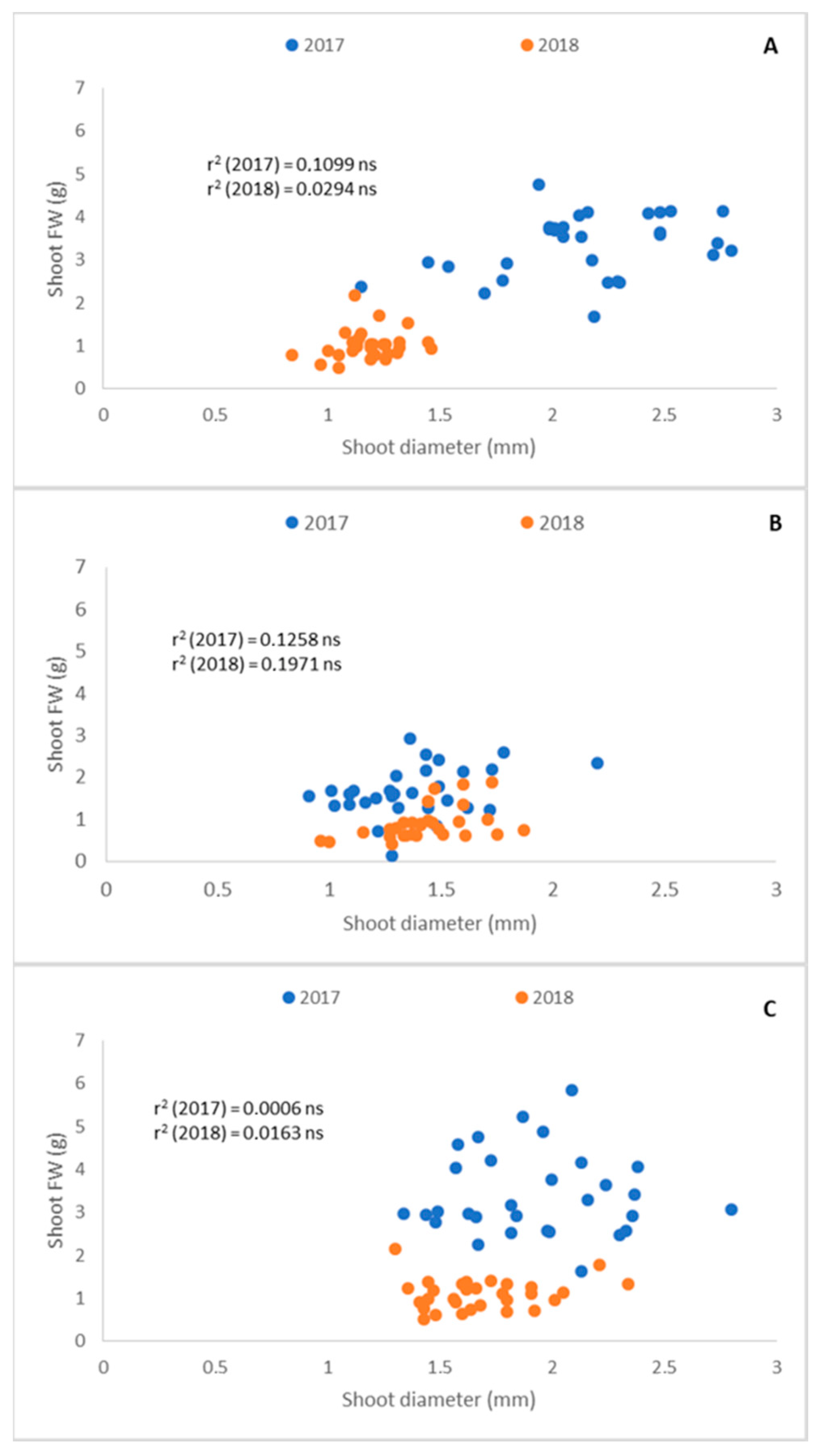
3.2. Relationship among Shoot Traits
3.3. Proximate Composition
4. Discussion
4.1. Shoot Production
4.2. Proximate Composition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haunold, A. Hop Production, Breeding, and Variety Development in Various Countries. J. Am. Soc. Brew. Chem. 1981, 39, 27–34. [Google Scholar] [CrossRef]
- Mahaffee, W.F.; Pethybridge, S. The genus Humulus. In Compendium of Hop Diseases and Pests; Mahaffee, W.F., Pethybridge, S., Gent, D.H., Eds.; The American Phytopatological Society: St. Paul, MN, USA, 2009; pp. 1–5. [Google Scholar]
- Turner, S.F.; Benedict, C.A.; Darby, H.; Hoagland, L.A.; Simonson, P.; Robert Sirrine, J.; Murphy, K.M. Challenges and opportunities for organic hop production in the United States. Agron. J. 2011, 103, 1645–1654. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT Production Quantities of Hops by Country. 2018. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 3 October 2020).
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus L., a Very Popular Beer Ingredient and Medicinal Plant: Overview of Its Phytochemistry, Its Bioactivity, and Its Biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Small, E.; Catling, P.M. Canadian Medicinal Crops; NRC Research Press: Ottawa, ON, Canada, 1999; ISBN 0-660-17534-7. [Google Scholar]
- Ruggeri, R.; Loreti, P.; Rossini, F. Exploring the potential of hop as a dual purpose crop in the Mediterranean environment: Shoot and cone yield from nine commercial cultivars. Eur. J. Agron. 2018, 93, 11–17. [Google Scholar] [CrossRef]
- Neve, R.A. Hops; Chapman & Hal: London, UK, 1991; ISBN 9789401053754. [Google Scholar]
- di Tizio, A.; Luczaj, L.J.; Quave, C.L.; Redzic, S.; Pieroni, A. Traditional food and herbal uses of wild plants in the ancient South-Slavic diaspora of Mundimitar/Montemitro (Southern Italy). J. Ethnobiol. Ethnomed. 2012, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hadjichambis, A.C.; Paraskeva-hadjichambi, D.; Della, A.; Elena Giusti, M.; Pasquale, C.D.E.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef]
- Tardio, J.; Pardo-de-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- García Herrera, P. Plantas Silvestres de Consumo Tradicional en España: Caracterización de su Valor Nutricional y Estimación de su Actividad Antifúngica. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Morales Gómez, P. Vegetales Silvestres de uso Alimentario: Determinación de Compuestos Bioactivos y Valoración de la Capacidad Antioxidante. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid Spain, 2011. [Google Scholar]
- Sanchez-Mata, M.C.; Cabrera Loera, R.D.; Morales, P.; Fernandez-Ruiz, V.; Camara, M.; Diez Marqués, C.; Pardo-de-Santayana, M.; Tardio, J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- Benincasa, P.; Tei, F.; Rosati, A. Plant density and genotype effects on wild asparagus (Asparagus acutifolius L.) spear yield and quality. HortScience 2007, 42, 1163–1166. [Google Scholar] [CrossRef] [Green Version]
- D’Antuono, L.F.; Lovato, A. Germination trials and domestication potential of three native species with edible sprouts: Ruscus aculeatus L., Tamus communis L. and Smilax aspera L. Acta Hortic. 2003, 598, 211–218. [Google Scholar] [CrossRef]
- Molina, M.; Pardo-de-Santayana, M.; Tardío, J. Natural Production and Cultivation of Mediterranean Wild Edibles. In Mediterranean Wild Edible Plants; Sánchez-Mata, M.D.C., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 81–107. ISBN 978-1-4939-3329-7. [Google Scholar]
- Kling, J. Protecting medicine’s wild pharmacy. Nat. Plants 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean Wild Edible Plants: Weeds or “New functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarici, E.; Ruggeri, R.; Provenzano, M.E.; Rossini, F. Germination and performance of seven native wildflowers in the Mediterranean landscape plantings. Ital. J. Agron. 2018, 13, 163–171. [Google Scholar] [CrossRef]
- Molina, M.; Tardío, J.; Aceituno-mata, L.; Morales, R.; Reyes-garcía, V.; Pardo-de-santayana, M. Weeds and Food Diversity: Natural Yield Assessment and Future Alternatives for Traditionally Consumed Wild Vegetables. J. Ethnobiol. 2014, 34, 44–67. [Google Scholar] [CrossRef]
- Maietti, A.; Brighenti, V.; Bonetti, G.; Tedeschi, P.; Prencipe, F.P.; Benvenuti, S.; Brandolini, V.; Pellati, F. Metabolite profiling of flavonols and in vitro antioxidant activity of young shoots of wild Humulus lupulus L. (hop). J. Pharm. Biomed. Anal. 2017, 142, 28–34. [Google Scholar] [CrossRef]
- Vidmar, M.; Čeh, B.; Demšar, L.; Ulrih, N.P. White Hop Shoot Production in Slovenia: Total Phenolic, Microelement and Pesticide Residue Content in Five Commercial Cultivars. Food Technol. Biotechnol. 2019, 57, 525–534. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2006. [Google Scholar]
- Rossini, F.; Loreti, P.; Provenzano, M.E.; De Santis, D.; Ruggeri, R. Agronomic performance and beer quality assessment of twenty hop cultivars grown in central Italy. Ital. J. Agron. 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Villari, G.; Borrelli, C.; Russo, G. Effects of crop method and harvest seasons on yield and quality of green asparagus under tunnel in southern italy. Adv. Hortic. Sci. 2012, 26, 51–58. [Google Scholar]
- Molina, M. Producción y Abundancia Natural de Verduras de Hoja, Espárragos y Frutos Carnosos Silvestres de uso Tradicional en España. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Siomos, A.S. The quality of asparagus as affected by preharvest factors. Sci. Hortic. 2018, 233, 510–519. [Google Scholar] [CrossRef]
- Tardío, J.; Sánchez-Mata, M.D.C.; Morales, R.; Molina, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Cruz Matallana-González, M.; Ruiz-Rodríguez, B.M.; Sánchez-Mata, D.; Torija-Isasa, M.E.; et al. Ethnobotanical and Food Composition Monographs of Selected Mediterranean Wild Edible Plants. In Mediterranean Wild Edible Plants—Ethnobotany and Food Composition Tables; Sánchez-Mata, M.D.C., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; p. 478. [Google Scholar]
- Dai, F.J.; Chau, C.F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osuagwu, G.G.E.; Edeoga, H.O. The effect of water stress (drought) on the proximate composition of the leaves of Ocimum gratissimum (L) and Gongronema latifolium (Benth). Int. J. Med. Arom. Plants 2013, 3, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.; Primi, R.; Danieli, P.P.; Ronchi, B.; Rossini, F. Effects of seeding date and seeding rate on yield, proximate composition and total tannins content of two Kabuli chickpea cultivars. Ital. J. Agron. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Zewdie, S.; Olsson, M.; Fetene, M. Effect of drought/irrigation on proximate composition and carbohydrate content of two enset [Ensete ventricosum (Welw.) Cheesman] clones. SINET Ethiop. J. Sci. 2011, 31, 81–88. [Google Scholar] [CrossRef]



| Cultivar | Harvest Time | Brewing Use | Origin |
|---|---|---|---|
| Cascade | M | Dual purpose | US |
| Challenger | L | Dual purpose | UK |
| Hallertauer Magnum | L | Bittering | Germany |
| Year | Cultivar | Emerged Shoots (no. plant−1) | Shoot FW (g) | Shoot DW (g) | Marketable Shoot Yield (g plant−1) | Shoot Diameter (mm) |
|---|---|---|---|---|---|---|
| 2017 | Cascade | 43.67 ± 3.38 | 2.04 ± 1.14 | 0.30 ± 0.018 | 90.08 ± 12.31 | 2.10 ± 0.07 |
| Challenger | 37.33 ± 10.87 | 1.48 ± 0.06 | 0.18 ± 0.009 | 56.56 ± 19.04 | 1.25 ± 0.03 | |
| H. Magnum | 74.00 ± 7.94 | 2.77 ± 0.02 | 0.29 ± 0.003 | 205.21 ± 23.76 | 1.91 ± 0.05 | |
| 2018 | Cascade | 81.33 ± 3.38 | 1.02 ± 0.03 | 0.08 ± 0.003 | 82.74 ± 1.21 | 1.16 ± 0.01 |
| Challenger | 93.33 ± 4.98 | 0.92 ± 0.07 | 0.08 ± 0.007 | 86.26 ± 7.37 | 1.40 ± 0.02 | |
| H. Magnum | 94.67 ± 8.21 | 1.04 ± 0.07 | 0.09 ± 0.007 | 99.08 ± 12.90 | 1.65 ± 0.07 | |
| ANOVA signif. | ns | *** | *** | *** | *** | |
| LSD (p < 0.05) | - | 0.25 | 0.03 | 0.25 | 0.16 | |
| Treatments | Emerged Shoots (no. plant−1) | Shoot FW (g) | Shoot DW (g) | Marketable Shoot Yield (g plant−1) | Shoot Diameter (mm) |
|---|---|---|---|---|---|
| Cultivar | |||||
| Cascade | 62.50 ± 8.69 | 1.53 ± 0.24 | 0.19 ± 0.048 | 86.41 ± 5.77 | 1.63 ± 0.21 |
| Challenger | 65.33 ± 13.62 | 1.20 ± 0.13 | 0.13 ± 0.024 | 71.41 ± 11.29 | 1.32 ± 0.04 |
| H. Magnum | 84.33 ± 6.89 | 1.91 ± 0.39 | 0.19 ± 0.045 | 152.15 ± 26.63 | 1.78 ± 0.07 |
| ANOVA signif. | * | *** | *** | *** | *** |
| LSD (p < 0.05) | 16.17 | 0.18 | 0.02 | 34.18 | 0.12 |
| Year | |||||
| 2017 | 51.67 ± 6.93 | 2.10 ± 0.19 | 0.26 ± 0.019 | 117.28 ± 24.42 | 1.75 ± 0.13 |
| 2018 | 89.78 ± 3.62 | 0.99 ± 0.04 | 0.08 ± 0.004 | 89.36 ± 4.97 | 1.40 ± 0.07 |
| ANOVA signif. | *** | *** | *** | * | *** |
| Year | Cultivar | Moisture (%) | Ash (% DM) | EE (% DM) | CP (% DM) | CF (% DM) |
|---|---|---|---|---|---|---|
| 2017 | Cascade | 85.5 ± 0.1 | 11.64 ± 0.21 | 3.88 ± 0.07 | 27.04 ± 0.46 | 16.48 ± 0.06 |
| Challenger | 87.6 ± 0.4 | 10.56 ± 0.25 | 4.03 ± 0.04 | 21.56 ± 0.14 | 14.15 ± 0.08 | |
| H. Magnum | 89.4 ± 0.6 | 10.73 ± 0.28 | 6.30 ± 0.12 | 26.75 ± 0.83 | 12.23 ± 0.34 | |
| 2018 | Cascade | 91.9 ± 0.05 | 8.71 ± 0.18 | 1.71 ± 0.02 | 30.01 ± 0.14 | 11.27 ± 0.28 |
| Challenger | 91.4 ± 0.05 | 8.10 ± 0.14 | 2.05 ± 0.02 | 27.38 ± 1.50 | 10.36 ± 0.15 | |
| H. Magnum | 90.9 ± 0.05 | 8.17 ± 0.07 | 2.61 ± 0.02 | 30.00 ± 0.44 | 11.26 ± 0.28 | |
| ANOVA signif. | ** | ns | *** | ns | *** | |
| LSD (p < 0.05) | 1.06 | - | 0.20 | - | 0.66 | |
| Treatments | Moisture (%) | Ash (% DM) | EE (% DM) | CP (% DM) | CF (% DM) |
|---|---|---|---|---|---|
| Cultivar | |||||
| Cascade | 88.68 ± 1.83 | 10.18 ± 0.67 | 2.79 ± 0.49 | 28.52 ± 0.70 | 13.88 ± 1.17 |
| Challenger | 89.48 ± 1.09 | 9.33 ± 0.56 | 3.04 ± 0.44 | 24.47 ± 1.47 | 12.26 ± 0.85 |
| H. Magnum | 90.13 ± 0.49 | 9.45 ± 0.59 | 4.45 ± 0.83 | 28.37 ± 0.84 | 11.75 ± 0.29 |
| ANOVA signif. | * | *** | *** | *** | *** |
| LSD (p < 0.05) | 0.87 | 0.37 | 0.14 | 1.70 | 0.46 |
| Year | |||||
| 2017 | 87.50 ± 0.74 | 10.98 ± 0.21 | 4.73 ± 0.39 | 25.11 ± 0.93 | 14.29 ± 0.62 |
| 2018 | 91.35 ± 0.18 | 8.33 ± 0.12 | 2.12 ± 0.13 | 29.13 ± 0.63 | 10.96 ± 0.19 |
| ANOVA signif. | *** | *** | *** | *** | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossini, F.; Virga, G.; Loreti, P.; Provenzano, M.E.; Danieli, P.P.; Ruggeri, R. Beyond Beer: Hop Shoot Production and Nutritional Composition under Mediterranean Climatic Conditions. Agronomy 2020, 10, 1547. https://doi.org/10.3390/agronomy10101547
Rossini F, Virga G, Loreti P, Provenzano ME, Danieli PP, Ruggeri R. Beyond Beer: Hop Shoot Production and Nutritional Composition under Mediterranean Climatic Conditions. Agronomy. 2020; 10(10):1547. https://doi.org/10.3390/agronomy10101547
Chicago/Turabian StyleRossini, Francesco, Giuseppe Virga, Paolo Loreti, Maria Elena Provenzano, Pier Paolo Danieli, and Roberto Ruggeri. 2020. "Beyond Beer: Hop Shoot Production and Nutritional Composition under Mediterranean Climatic Conditions" Agronomy 10, no. 10: 1547. https://doi.org/10.3390/agronomy10101547
APA StyleRossini, F., Virga, G., Loreti, P., Provenzano, M. E., Danieli, P. P., & Ruggeri, R. (2020). Beyond Beer: Hop Shoot Production and Nutritional Composition under Mediterranean Climatic Conditions. Agronomy, 10(10), 1547. https://doi.org/10.3390/agronomy10101547






