Morpho-Physiology and Antioxidant Enzyme Activities of Transgenic Rice Plant Overexpressing ABP57 under Reproductive Stage Drought Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Treatment
2.3. Morphological Analyses
2.4. Physiological Analyses
2.5. Biochemical Analyses
2.5.1. Proline Content
2.5.2. Antioxidant Enzymes Assay
2.6. Agronomical Traits and Drought Tolerance Indices Determination
2.7. Statistical Analysis
3. Results
3.1. Drought Stress Effects on Morphological Performances
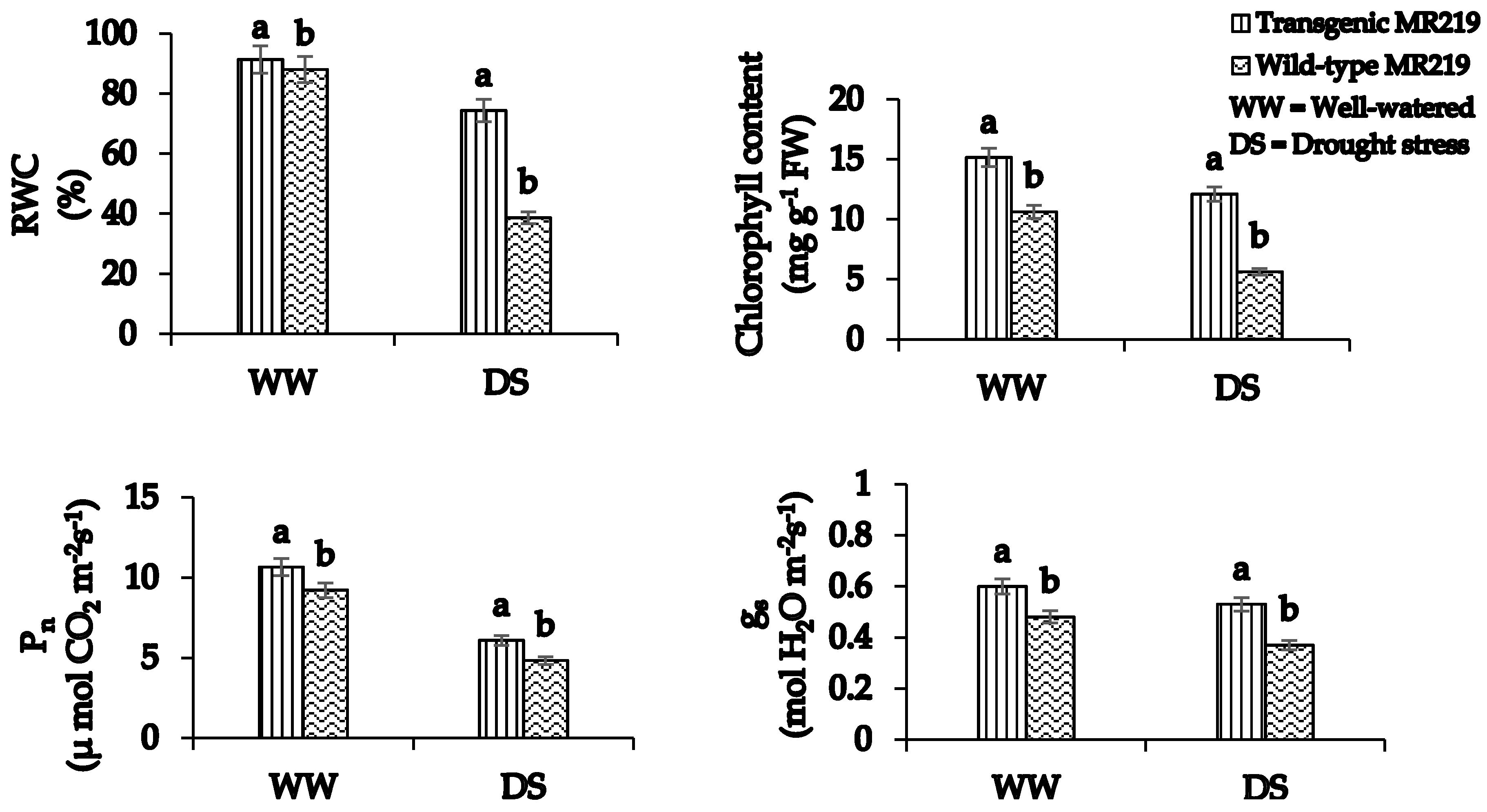
3.2. Drought Stress Effects on Physiological Responses
3.3. Drought Stress Effects on Biochemical Changes
3.4. Drought Stress Effects on Agronomical Performances
3.4.1. Yield and Yield Attributes
3.4.2. Drought Tolerance Indices
3.5. Phenotypic Correlations among Morpho-Physiological, Biochemical and Agronomical Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shao, G.C.; Liu, N.; Zhang, Z.Y. Growth, yield and water use efficiency response of greenhouse-grown hot pepper under Time-Space deficit irrigation. Sci. Hortic. 2010, 126, 172–179. [Google Scholar]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 140, 66–73. [Google Scholar]
- Sokoto, M.B.; Muhammad, A. Response of rice varieties to water stress Sokoto, Sudan Savannah, Nigeria. J. Biosci. Med. 2014, 2, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Tiara, H.; Erik, H.M.; Asgar, A.W. Rice production and climate change: A case study of Malaysian Rice. Pertanika J. Trop. Agric. Sci. 2015, 38, 321–328. [Google Scholar]
- Pandey, V.; Shukla, A. Improving crop yield under drought stress through physiological breeding. Plant Physiol. Bioch. 2016, 1, 331–348. [Google Scholar]
- Carolyn, P.; Lim, A.H.; Farrah, A.; Andi, B.R.; Geetha, M.; Saroj, K.C.; Giulia, R.; Alexandros, G.; Kensuke, F. Impact of extreme drought climate on water security in North Borneo: Case study of Sabah. Water 2020, 12, 1–19. [Google Scholar]
- Kim, Y.S.; Min, J.K.; Kim, D.; Jung, J. A soluble auxin-binding protein, ABP57. J. Biol. Chem. 2001, 276, 10730–10736. [Google Scholar] [CrossRef] [Green Version]
- Morsomme, P.; Boutry, M. The plant plasma membrane H+-ATPase: Structure, function and regulation. BBA Biomembr. 2000, 1465, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef]
- Tan, L.W.; Tan, C.S.; Zuraida, A.R.; Hossein, H.M.; Goh, H.H.; Ismanizan, I.; Zamri, Z. Overexpression of Auxin Binding Protein 57 from rice (Oryza sativa L.) increased drought and salt tolerance in transgenic Arab. Thaliana. Environ. Earth Sci. 2018, 197, 1–9. [Google Scholar]
- Kinoshita, N.; Wang, H.; Kasahara, H.; Liu, J.; Macpherson, C.; Machida, Y.; Kamiya, Y.; Hannah, M.A.; Chua, N.H. IAA-Ala Resistant3, an evolutionarily conserved target of miR167, mediates Arabidopsis root architecture changes during high osmotic stress. Plant Cell 2012, 24, 3590–3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriwaki, T.; Miyazawa, Y.; Kobayashi, A.; Takahashi, H. Molecular mechanisms of hydrotropism in seedling roots of Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 2012, 100, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kemal, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar]
- Tan, L.W.; Zuraida, A.R.; Goh, H.H.; Hwang, D.J.; Ismanizan, I.; Zamri, Z. Production of transgenic rice (indica cv. MR219) overexpressing Abp57 gene through Agrobacterium-mediated transformation. Sains Malays. 2017, 46, 703–711. [Google Scholar] [CrossRef]
- Tan, L.W. Functional Analysis of Abp57 in Arabidopsis and Transcriptome Profiling of Transgenic Rice Abp57. Ph.D. Thesis, National University of Malaysia, Putrajaya, Malaysia, 2017. [Google Scholar]
- Turner, N.C.; Begg, J.E. Plant-water relations and adaptation to stress. Plant Soil. 1981, 58, 97–131. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Akhtar, K.; Sarwar, G.; Ashraf, M. Role of rooting system in salt tolerance potential of different guar accessions. Agron. Sustain. Dev. 2005, 25, 243–249. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.K. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–208. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method Enzymol. 1984, 105, 121–126. [Google Scholar]
- Zarifth, S.K.; Rafii, M.Y.; Mahmud, T.M.M.; Razi, M.I.; Rahim, A.H. Growth performance and antioxidant enzyme activities of advanced mutant rice genotypes under drought stress condition. Agronomy 2018, 8, 1–15. [Google Scholar]
- Basu, S.; Roychoudhury, A.; Salha, P.P.; Sengupta, D.N. Comparative analysis of some biochemical responses of three indica rice varieties during polyethylene glycol-mediated water stress exhibits distinct varietal differences. Acta Physiol. Plant 2010, 32, 551–563. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars: I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fischer, K.S.; Wood, G. Breeding and selection for drought tolerance in tropical maize. In Proceedings of the Symposium on the Principles and Methods in Crop Improvement for Drought Resistance with Emphasis on Rice, IRRI, Los Banos, Philippines, 4–8 May 1981; CIMMYT: El Batán, México, 1982. [Google Scholar]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environment. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Asian Vegetable Research and Development Centre, Taiwan, 13–18 August 1992; AVRDC Publications: Taipei, Taiwan, 1993; pp. 257–270. [Google Scholar]
- Hossain, A.B.S.; Sears, A.G.; Cox, T.S.; Paulsen, G.M. Dessication tolerance and its relationship to assimilate partitioning in winter wheat. Crop Sci. 1999, 30, 622–627. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, S.; Ram, T.; Yadaw, R.B.; Mishra, K.K.; Mandal, N.P. Breeding high-yielding drought-tolerant rice: Genetic variations and conventional and molecular approaches. J. Exp. Bot. 2014, 65, 6265–6278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.-K.B.A.; Mohammed, A.A.; Ihsan, M.I. Effect of different drip irrigation regimes on growth, yield and yield components of banana cv. Grand Nain under Gizera condition. In Proceedings of the RUFORUM Third Biennal Conference, Entebbe, Uganda, 24–28 December 2012; RUFORUM: Khartoum, Sudan, 2012. [Google Scholar]
- Akram, H.M.; Ali, A.; Sattar, A.; Rehman, H.S.U.; Bibi, A. Impact of water deficit stress on various physiological and agronomic traits of three basmathi rice (Oryza sativa L.) cultivars. J. Anim. Plant Sci. 2013, 23, 1415–1423. [Google Scholar]
- Yousfi, N.; Slama, I.; Ghnaya, T.; Savoure, A.; Abdelly, C. Effects of water deficit stress on growth, water relations and osmolytes accumulation in Medicago truncatula and M. laciniata populations. CR Biol. 2010, 333, 205–213. [Google Scholar] [CrossRef]
- Monsi, N.; Murata, Y. Development of photosynthetic system as influenced by distribution of matter. In Prediction of Photosynthetic Productivity, Proceedings of the IBP/PP Technical Meeting, Tfebon, Czechoslovakia, 14–21 September 1969; Setlik, I., Ed.; Wageningen Publications: Wageningen, The Netherlands, 1970; pp. 115–129. [Google Scholar]
- Cox, J.A.; Conran, J.G. The effect of water stress on the life cycles of Erodium crinitum Carolin and Erodium cicutarium (L.) L’Helrit ex Aiton (Geraniaceae). Austral. Ecol. 1996, 21, 235–240. [Google Scholar] [CrossRef]
- Kavi-Kishor, P.B.; Sangam, S.; Amrutha, R.N.; Sri-Laxmi, P.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 3. [Google Scholar]
- Munne-Bosch, S.; Jubnay-Mari, T.; Alegre, L. Drought-induced senescence is characterized by a loss of antioxidant defences in chloroplasts. Plant Cell Environ. 2001, 24, 1319–1327. [Google Scholar] [CrossRef]
- Senguttuvel, P.; Vijayalakshmi, C.; Thiyagarajan, K.; Kannanbapu, J.R.; Kota, S.; Padmavathi, G.; Gheeta, S.; Sritharan, N.; Viraktamath, B.C. Changes in photosynthesis, chlorophyll fluorescence, gas exchange parameters and osmotic potential to salt stress during early seedling stage in rice (Oryza sativa L.). SABRAO J. Breed. Genet 2014, 46, 120–135. [Google Scholar]
- Zheng, Y.X.; Wu, J.C.; Cao, F.L.; Zhang, Y.P. Effects of water stress on photosynthetic activity, dry mass partitioning and some associated metabolic changes in four provenances of neem (Azadirachta indica A. Juss). Photosynthetica 2010, 48, 361–369. [Google Scholar] [CrossRef]
- Abdell-Nasser, L.E.; Abdel-Aal, A.E. Effect of elevated CO2 and drought on proline metabolism and growth of safflower (Carthamus mareoticus L.) seedlings without improving water status. PJBS 2002, 5, 523–528. [Google Scholar]
- Anjum, S.A.; Farooq, M.; Xie, X.Y.; Liu, X.J.; Ijaz, M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- Lum, M.S.; Hanafi, M.M.; Rafii, M.Y.; Akmar, A.S.N. Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. J. Anim. Plant. Sci. 2014, 24, 1487–1493. [Google Scholar]
- Mehlhorn, H.; Lelandais, M.; Korth, H.G.; Foyer, C.H. Ascorbate is the natural substrate for plant peroxidases. FEBS 1996, 378, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Sezen, S.M.; Yazar, A.; Eker, S. Effect of drip irrigation regimes on yield and quality of field grown bell pepper. Agric. Water Manag. 2006, 81, 115–131. [Google Scholar] [CrossRef]
- Jaimez, R.E.; Rada, F.; Garcia-Nunez, C. The effect of irrigation frequency on water and carbon relations in three cultivars of sweet pepper (Capsicum Chinese Jacq) in a tropical semiarid region. Sci. Hortic. 1999, 81, 301–308. [Google Scholar] [CrossRef]
- Lafitte, H.R.; Blum, A.; Atlin, G. Using secondary traits to help identify drought-tolerant genotypes. In Breeding Rice for Drought-Prone Environments; Fisher, K.S., Lafitte, R., Fukai, S., Atlin, G., Hardy, B., Eds.; IRRI: Los Banos, Philippines, 2003; pp. 37–48. [Google Scholar]
- Raman, A.; Verulkar, S.B.; Mandal, N.P.; Variar, M.; Shukla, V.D.; Dwivedi, J.L.; Singh, B.N.; Singh, O.N.; Swain, P.; Mall, A.K.; et al. Drought yield index to select high yielding rice lines under different drought stress severities. Rice 2012, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]

| SSI Value | Rate |
|---|---|
| Less than 0.50 | Highly drought-tolerant |
| 0.51–0.75 | Drought-tolerant |
| 0.76–1.00 | Moderately drought-tolerant |
| More than 1.00 | Drought-susceptible |
| Source of Variation | df | Mean Square | |||||
|---|---|---|---|---|---|---|---|
| FLA (cm2) | NOT | PH (cm) | CLDW (g plant−1) | PDW (g plant−1) | TDW (g plant−1) | ||
| Replications (R) | 2 | 0.02 | 0.25 | 2.27 | 0.57 | 2.46 | 0.31 |
| Water treatments (W) | 1 | 458.80 * | 126.75 | 61.20 | 102.55 | 1017.71 * | 1864.76 * |
| R × W | 2 | 2.77 | 0.25 | 0.96 | 0.03 | 1.97 | 6.37 |
| Genotypes (G) | 1 | 705.33 * | 630.75 * | 8.17 | 225.51 * | 4186.94 * | 6541.27 * |
| G × W | 1 | 83.21 * | 0.75 | 3.31 | 12.94 | 185.57 | 337.61 |
| Error | 4 | 0.27 | 1.75 | 0.74 | 0.91 | 1.68 | 3.04 |
| Genotype/Treatment | FLA (cm2) | NOT | PH (cm) | CLDW (g plant−1) | PDW (g plant−1) | TDW (g plant−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | DS | WW | DS | WW | DS | WW | DS | WW | DS | WW | DS | |
| MR219 transgenic line | 41.5 a | 34.4 a | 28 a | 21 a | 95.1 b | 91.6 a | 20.5 a | 16.8 a | 94.7 a | 84.1 a | 115.2 a | 100.9 a |
| Wild-type MR219 | 31.4 b | 13.8 b | 13 b | 7 b | 97.8 a | 92.2 a | 13.9 b | 6.0 b | 65.2 b | 38.9 b | 79.1 b | 43.6 b |
| Mean | 36.4 | 24.1 | 21 | 14 | 96.4 | 91.9 | 17.2 | 11.4 | 79.9 | 61.5 | 97.2 | 72.2 |
| CV (%) | 15.3 | 47.0 | 40.3 | 55.1 | 1.66 | 1.38 | 21.1 | 52.2 | 20.3 | 40.3 | 20.4 | 43.5 |
| Source of Variation | df | Mean Square | |||
|---|---|---|---|---|---|
| RWC (%) | Chlorophyll Content (mg g−1 FW) | Pn (µ mol CO2 m−2s−1) | gs (mol H2O m−2s−1) | ||
| Replications (R) | 2 | 9.33 | 0.19 | 0.01 | 0.01 |
| Water treatments (W) | 1 | 3300.08 * | 53.78 * | 60.21 * | 0.02 * |
| R × W | 2 | 4.33 | 0.14 | 0.01 | 0.01 |
| Genotypes (G) | 1 | 1140.75 * | 76.93 * | 5.50 * | 0.04 * |
| G × W | 1 | 784.08 * | 36.04 * | 0.02 | 0.06 * |
| Error | 4 | 2.17 | 0.06 | 0.01 | 0.01 |
| Source of Variation | df | Mean Square | |||
|---|---|---|---|---|---|
| Proline Content (mg g−1) | CAT (Units mg Protein−1) | APX (Units mg Protein) | GPX (Units mg Protein−1) | ||
| Replications (R) | 2 | 0.07 | 0.01 | 0.06 | 0.03 |
| Water treatments (W) | 1 | 0.97 * | 7.24 * | 4.94 * | 26.58 * |
| R × W | 2 | 0.02 | 0.01 | 0.02 | 0.02 |
| Genotypes (G) | 1 | 0.37 * | 2.22 * | 2.41 * | 16.10 * |
| G × W | 1 | 0.24 * | 2.22 * | 1.79 * | 12.20 * |
| Error | 4 | 0.04 | 0.03 | 0.06 | 0.03 |
| Source of Variation | df | Mean Square | ||
|---|---|---|---|---|
| Grain Yield (g plant−1) | 1000-Grain Weight (g) | Spikelets/Panicle | ||
| Replications (R) | 2 | 3.82 | 0.01 | 3.00 |
| Water treatments (W) | 1 | 2679.94 * | 45.86 * | 602.08 * |
| R × W | 2 | 0.82 | 0.01 | 2.33 |
| Genotypes (G) | 1 | 4918.73 * | 83.32 * | 1850.08 * |
| G × W | 1 | 1625.18 * | 19.92 * | 352.08 * |
| Error | 4 | 2.54 | 0.02 | 1.33 |
| Genotype/Treatment | Grain Yield (g plant−1) | 1000-Grain Weight (g) | Spikelets/Panicle | |||
|---|---|---|---|---|---|---|
| WW | DS | WW | DS | WW | DS | |
| MR219 transgenic line | 91.77a | 85.15a | 29.19a | 27.86a | 81a | 78a |
| Wild-type MR219 | 74.55b | 21.39b | 26.50b | 20.01b | 67b | 42b |
| Mean | 83.16 | 53.27 | 27.85 | 23.94 | 74 | 60 |
| CV (%) | 11.55 | 65.58 | 5.31 | 17.96 | 10.41 | 32.56 |
| Treatment | Transgenic MR219 | Wild-Type MR219 |
|---|---|---|
| Well-watered plant | ||
| Mean yield (g) | 91.77a | 74.55b |
| RY (g) | 1.00 | 0.81 |
| Mean RY (g) | 0.91 | |
| Drought stress plant | ||
| Mean yield (g) | 88.15a | 21.39b |
| RY (g) | 1.00 | 0.25 |
| Mean RY (g) | 0.63 | |
| Genotype | REI | MPI | MRP | TOL | STI | SSI | DTE (%) |
|---|---|---|---|---|---|---|---|
| Transgenic MR219 | 1.76 (1) | 88.43 (1) | 2.70 (1) | 6.61 (1) | 0.03 (1) | 0.20 (1) | 92.81 (1) |
| Wild-type MR219 | 0.36 (2) | 47.97 (2) | 1.30 (2) | 53.16 (2) | 0.01 (2) | 1.98 (2) | 28.69 (2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamarudin, Z.S.; Shamsudin, N.A.A.; Othman, M.H.C.; Shakri, T.; Tan, L.-W.; Sukiran, N.L.; Isa, N.M.; Rahman, Z.A.; Zainal, Z. Morpho-Physiology and Antioxidant Enzyme Activities of Transgenic Rice Plant Overexpressing ABP57 under Reproductive Stage Drought Condition. Agronomy 2020, 10, 1530. https://doi.org/10.3390/agronomy10101530
Kamarudin ZS, Shamsudin NAA, Othman MHC, Shakri T, Tan L-W, Sukiran NL, Isa NM, Rahman ZA, Zainal Z. Morpho-Physiology and Antioxidant Enzyme Activities of Transgenic Rice Plant Overexpressing ABP57 under Reproductive Stage Drought Condition. Agronomy. 2020; 10(10):1530. https://doi.org/10.3390/agronomy10101530
Chicago/Turabian StyleKamarudin, Zarifth Shafika, Noraziyah Abd Aziz Shamsudin, Muhamad Hafiz Che Othman, Tasneem Shakri, Lay-Wen Tan, Noor Liyana Sukiran, Nurulhikma Md Isa, Zuraida Ab Rahman, and Zamri Zainal. 2020. "Morpho-Physiology and Antioxidant Enzyme Activities of Transgenic Rice Plant Overexpressing ABP57 under Reproductive Stage Drought Condition" Agronomy 10, no. 10: 1530. https://doi.org/10.3390/agronomy10101530
APA StyleKamarudin, Z. S., Shamsudin, N. A. A., Othman, M. H. C., Shakri, T., Tan, L.-W., Sukiran, N. L., Isa, N. M., Rahman, Z. A., & Zainal, Z. (2020). Morpho-Physiology and Antioxidant Enzyme Activities of Transgenic Rice Plant Overexpressing ABP57 under Reproductive Stage Drought Condition. Agronomy, 10(10), 1530. https://doi.org/10.3390/agronomy10101530





