Physiological Beneficial Effect of Rhizophagus intraradices Inoculation on Tomato Plant Yield under Water Deficit Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
- (1)
- Prod1: provided by MycAgro (MYCII15ZAV#3, Mycagro Lab sarl, Dijon, France http://www.mycagrolab.com) a granular inoculum containing mineral solid particles (clay, zeolite) with R. intraradices propagules (spores, hyphae pieces) and mycorrhizal root pieces mixed with mineral support powder of roots and fungus R. intraradices (cost 100 € Kg−1);
- (2)
- Prod2: provided by Agrifutur (Agrifutur srl, Alfianello, Italy, http://www.agrifutur.com) a powder formulation containing kaolin as carrier material and propagules of R. intraradices (cost 52 € Kg−1).
- (1)
- The commercial products were homogeneously mixed to the substrate
- (2)
- The commercial products were dissolved in water and provided as irrigation on the germinating seedlings
- (3)
- The commercial products were spread on seedling root blocks before the transplant in the field.
2.2. Assessment of AM Symbiosis Development
2.3. Assessment of Physio-Phenological Traits
2.3.1. Height, Diameter, Phenology, Whole Canopy Leaf Area and Chlorophyll Content
2.3.2. Chlorophyll Fluorescence
2.3.3. Gas Exchange Measurements
2.3.4. Plant Biomass Production, Partitioning, Growth and P Content
2.4. Statistical Analysis
3. Results
3.1. Assessment of AMF Symbiosis Development
3.2. Assessment of Physio-Phenological Traits
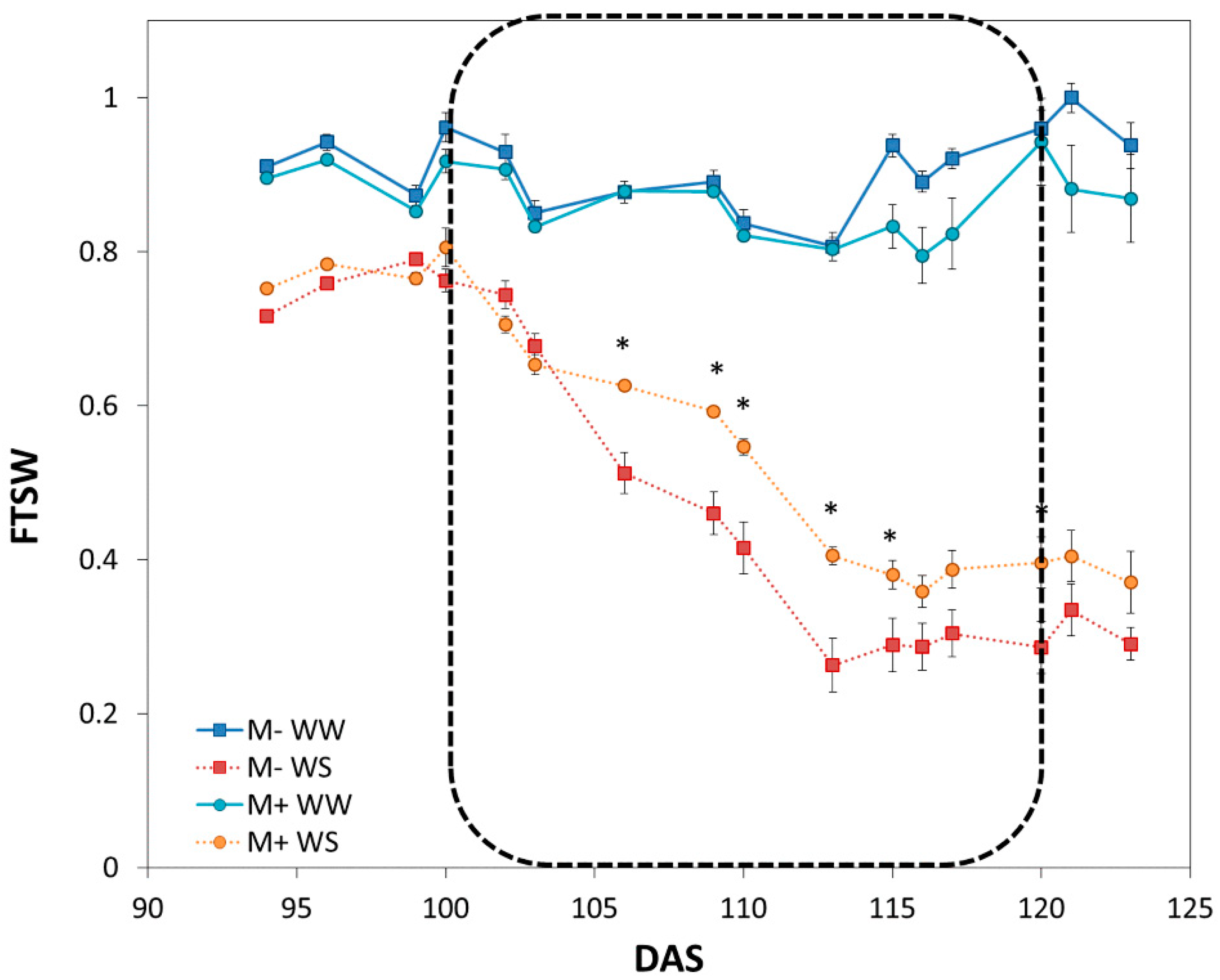
3.2.1. Characterization of Drought Dynamics
3.2.2. Chlorophyll Content and Fluorescence
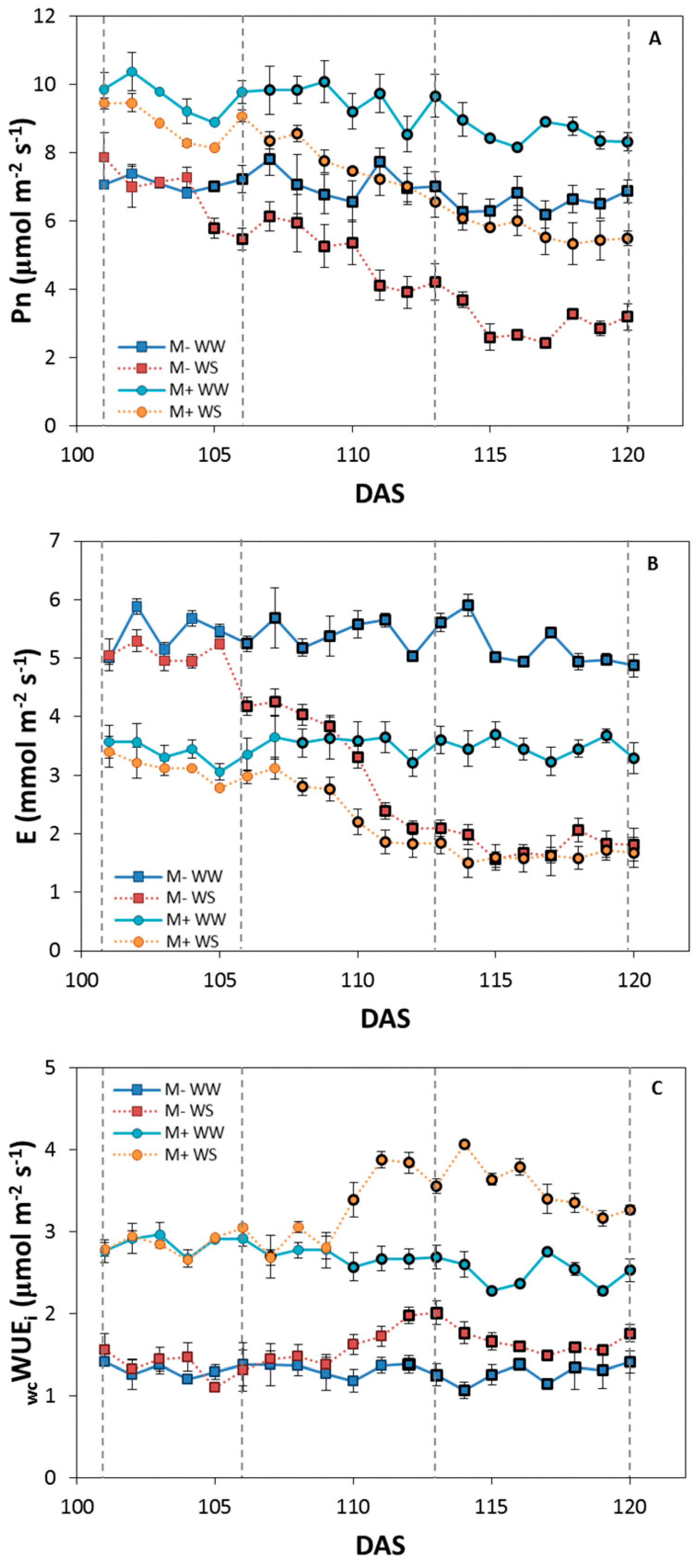
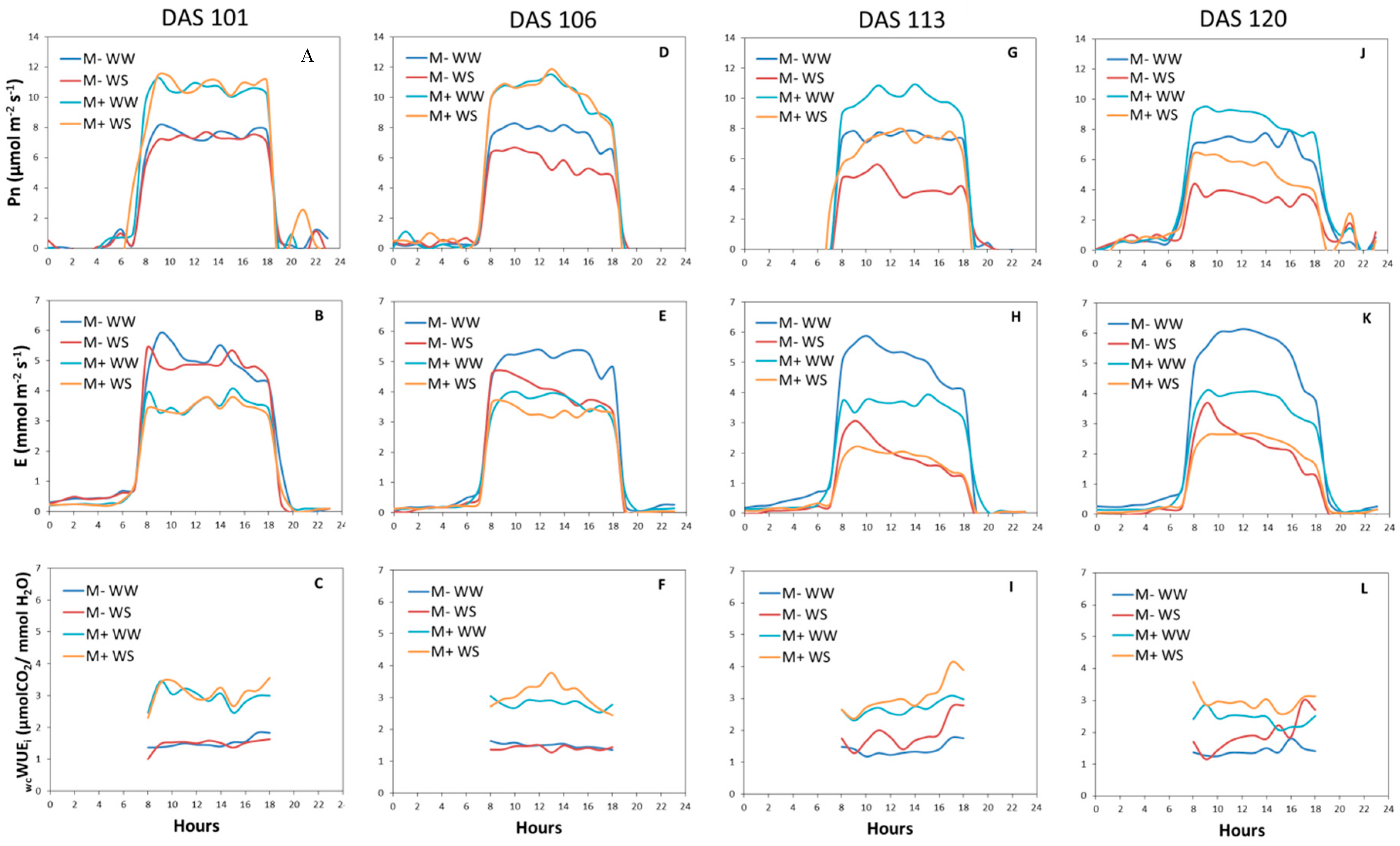
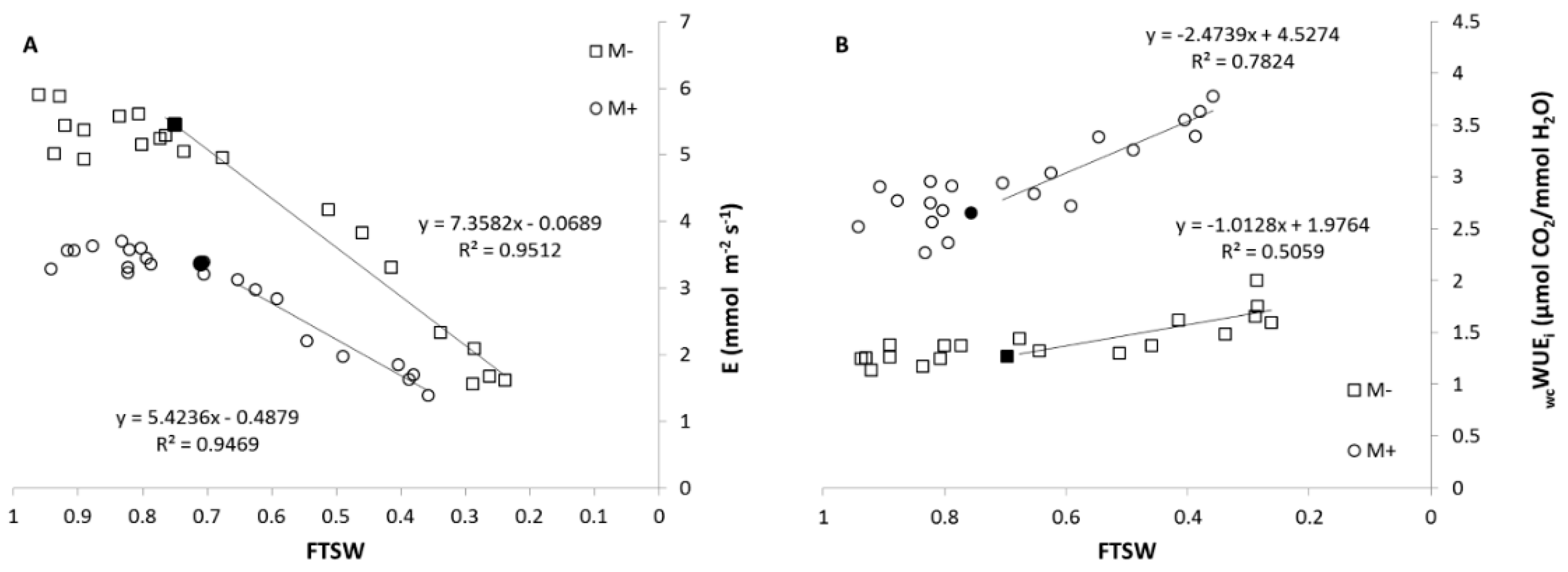
3.2.3. Gas Exchange Measurements
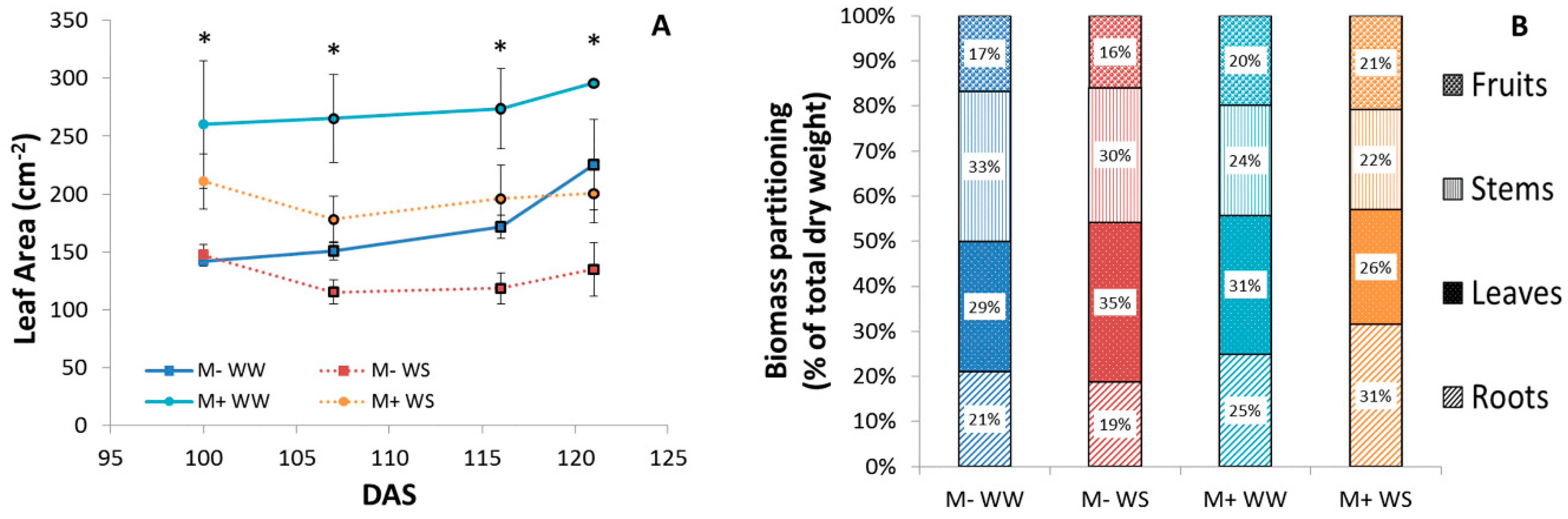
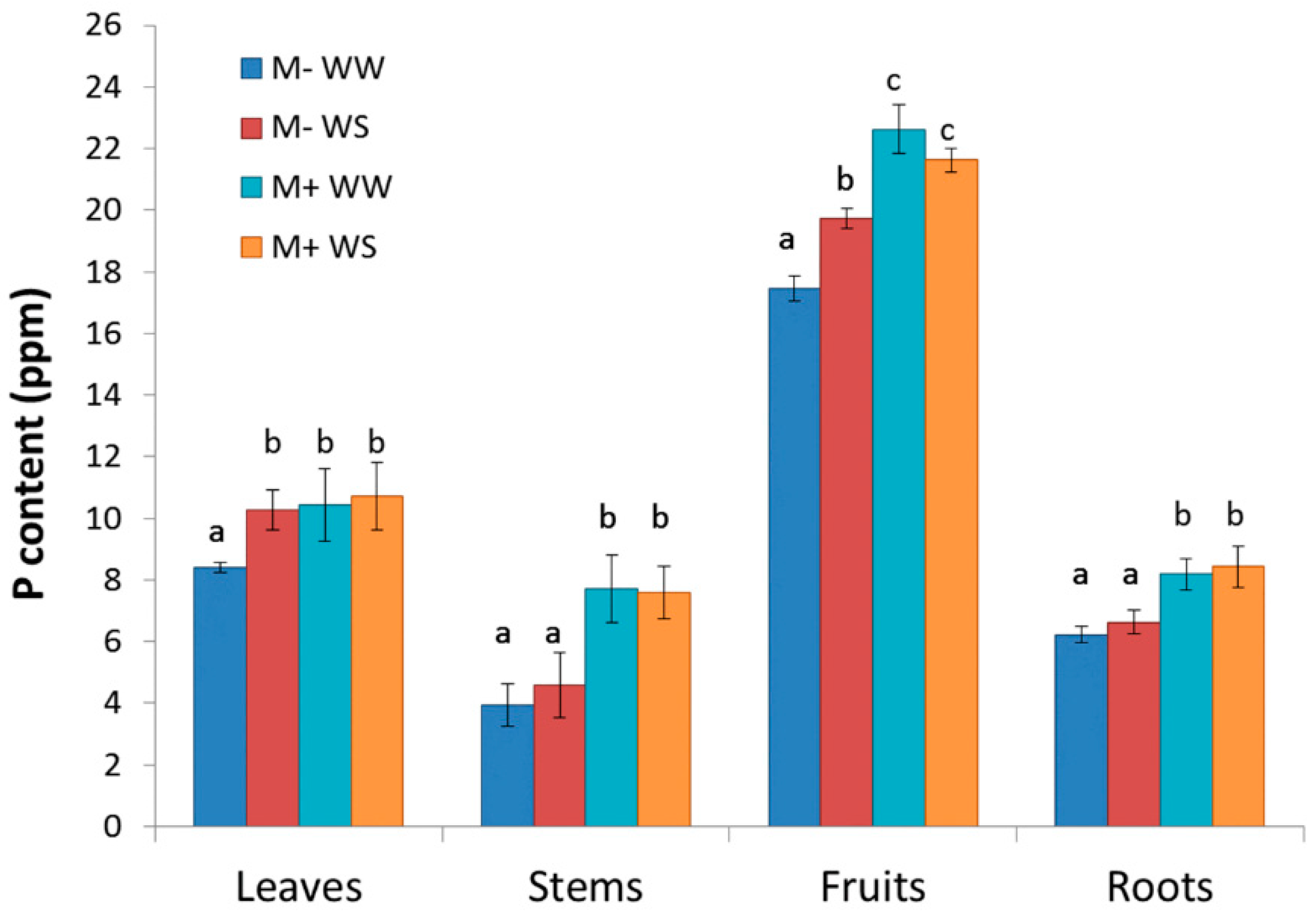
3.2.4. Plant Biometry, Phenology, Biomass Production and Partitioning, Leaf Area, Growth and P Content
4. Discussion
4.1. Assessment of AM Symbiosis Development
4.2. Ecophysiological Assessment of AM Symbiosis in Tomato
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | arbuscular mycorrhizal fungi |
| DAS | days after sowing |
| E | transpiration rate |
| ETR | electron transport rate |
| Fm | maximal fluorescence |
| FTSW | fraction of transpirable soil water |
| Fv/Fm | maximal quantum yield of PSII |
| LAR | leaf area ratio |
| NPQ | non-photochemical quenching |
| Pn | net photosynthetic rate |
| PPFD | photosynthetic photon flux density |
| PSII | photosystem II |
| qP | photochemical quenching |
| SLA | specific leaf area |
| VPD | photosynthetic photon flux density |
| wcWUEi | whole canopy intrinsic water use efficiency |
| WS | water stress |
| WW | well-watered |
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2015. Available online: http://faostat3.fao.org/download/Q/QC/E (accessed on 14 May 2019).
- Viskelis, P.; Vilkauskaite, G.; Noreika, R.K. Chemical composition, functional properties and consumption of tomatoes. Sodininkyste ir Darzininkyste 2005, 24, 182–192. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2010, 27, 1231–1240. [Google Scholar] [CrossRef]
- Larson, C. Losing arable land, China faces stark choice: Adapt or go hungry. Science 2013, 339, 644–645. [Google Scholar] [CrossRef]
- Campbell, B. Perspectives: Legislating change. Nature 2012, 501, S12–S14. [Google Scholar]
- Barrow, J.R.; Lucero, M.E.; Reyes-Vera, I.; Havstad, K.M. Do symbiotic microbes have a role in plant evolution, performance and response to stress? Commun. Integr. Biol. 2008, 1, 69–73. [Google Scholar] [CrossRef]
- Eisenstein, M. Discovery in a dry spell. Nature 2013, 501, S7–S9. [Google Scholar] [CrossRef]
- Morrissey, J.P.; Dow, J.M.; Mark, G.L.; O’Gara, F. Are microbes at the root of a solution to world food production? EMBO Rep. 2004, 5, 922–926. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Marulanda, A.; Barea, J.M.; Azcón, R. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: Mechanisms related to bacterial effectiveness. J. Plant Growth Regul. 2009, 28, 115–124. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Marasco, R.; Rolli, E.; Vigani, G.; Borin, S.; Sorlini, C.; Ouzari, H.; Zocchi, G.; Daffonchio, D. Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal. Behav. 2013, 8, e26741. [Google Scholar] [CrossRef] [PubMed]
- Chialva, M.; Salvioli di Fossalunga, A.; Daghino, S.; Ghignone, S.; Bagnaresi, P.; Chiapello, M.; Novero, M.; Spadaro, D.; Perotto, S.; Bonfante, P. Native soils with their microbiotas elicit a state of alert in tomato plants. New Phytol. 2018, 220, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Lanfranco, L.; Fiorilli, V.; Gutjahr, C. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol. 2018, 220, 1031–1046. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Ann. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Ezawa, T.; Saito, K. How do arbuscular mycorrhizal fungi handle phosphate? New insight into fine-tuning of phosphate metabolism. New Phytol. 2018, 220, 1116–1121. [Google Scholar] [CrossRef]
- Rapparini, F.; Penuelas, J. Mycorrhizal fungi to alleviate drought stress on plant growth. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; Volume 1. [Google Scholar]
- Estrada, B.; Aroca, R.; Maathuis, F.J.M.; Barea, J.M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ. 2013, 36, 1771–1782. [Google Scholar] [CrossRef]
- Estrada, B.; Aroca, R.; Barea, J.M.; Ruiz-Lozano, J.M. Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci. 2013, 201–202, 42–51. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Pedranzani, H.; Tavecchio, N.; Gutierrez, M.; Garbero, M.; Porcel, R.; Ruiz-Lozano, J.M. Differential effects of cold stress on the antioxidant response of mycorrhizal and non-mycorrhizal Jatropha curcas (L.) plants. J. Agric. Sci. 2015, 7, 35–43. [Google Scholar] [CrossRef]
- Pedranzani, H.; Rodrıguez-Rivera, M.; Gutierrez, M.; Porcel, R.; Hause, B.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 2016, 26, 141–152. [Google Scholar]
- Rivero, J.; Gamir, J.; Aroca, R.; Pozo, M.J.; Flors, V. Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 2015, 6, 598. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Mycorrhizal influence on nutrient uptake of citrus exposed to drought stress. Philipp. Agric. Sci. 2009, 92, 33–38. [Google Scholar]
- Bagheri, V.; Shamshiri, M.H.; Shirani, H.; Roosta, H. Nutrient uptake and distribution in mycorrhizal pistachio seedlings under drought stress. J. Agric. Sci. Technol. 2012, 14, 1591–1604. [Google Scholar]
- Huang, Z.; Zou, Z.R.; He, C.X.; He, Z.Q.; Zhang, Z.B.; Li, J.M. Physiological and photosynthetic responses of melon (Cucumis melo L.) seedlings to three Glomus species under water deficit. Plant Soil 2011, 339, 391–399. [Google Scholar] [CrossRef]
- Davies, F.T., Jr.; Portugal-Olalde, V.; Aguilera-Gomez, L.; Alvarado, M.J.; Ferrera-Cerrato, R.C.; Bouton, T.W. Alleviation of drought stress of Chile ancho pepper (Capsicum annuum cv San Luis) with arbuscular mycorrhiza indigenous to Mexico. Sci. Hortic. 2002, 92, 347–359. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and nonmycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jahromi, F.; Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microb. Ecol. 2008, 55, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.R.; Brain, P.; Xu, X.M.; Jeffries, P. Inoculation of drought-stressed strawberry with a mixed inoculum of two arbuscular mycorrhizal fungi: Effects on population dynamics of fungal species in roots and consequential plant tolerance to water deficiency. Mycorrhiza 2015, 25, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Omirou, M.; Ioannides, I.M.; Ehaliotis, C. Mycorrhizal inoculation affects arbuscular mycorrhizal diversity in watermelon roots but leads to improved colonization and plant response under water stress only. Appl. Soil Ecol. 2013, 63, 112–119. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Santhanakrishnan, P.; Balasubramanian, P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 2006, 107, 245–253. [Google Scholar] [CrossRef]
- Wang, B.; Yao, Z.; Zhao, S.; Guo, K.; Sun, J.; Zhang, H. Arbuscular mycorrhizal fungal application to improve growth and tolerance of processing tomato (Lycopersicum esculentum Miller) under drought stress. J. Food Agric. Environ. 2014, 12, 452–457. [Google Scholar]
- Hewitt, E.J. Sand and Water Culture Methods Used in Study of Plant Nutrition, 2nd ed.; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1966; p. 547. [Google Scholar]
- Fracasso, A.; Amaducci, S. Drought tolerance strategies highlighted by two Sorghum bicolor races in a dry-down experiment. J. Plant Physiol. 2015, 190, 1–14. [Google Scholar] [CrossRef]
- Luquet, D.; Clément-Vidal, A.; Fabre, D.; This, D.; Sonderegger, N.; Dingkuhn, M. Orchestration of transpiration, growth and carbohydrate dynamics in rice during a dry-down cycle. Funct. Plant Biol. 2008, 35, 689–704. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche des méthodes d’estimation ayant une signification fonctionnelle. The mycorrhizae: Physiology and genetic. In Mycorrhizae: Physiology and Genetics; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Fracasso, A.; Magnanini, E.; Marocco, A.; Amaducci, S. Real-Time Determination of Photosynthesis, Transpiration, Water-Use Efficiency and Gene Expression of Two Sorghum bicolor (Moench) Genotypes Subjected to Dry-Down. Front. Plant Sci. 2017, 8, 932. [Google Scholar] [CrossRef]
- Long, S.P.; Hällgren, J.E. Measurement of CO2 assimilation by plants in the field and the laboratory. In Photosynthesis and Production in a Changing Environment: A Field and Laboratory Manual; Hall, D.O., Scurlock, J.M.O., Bolhàr-Nordenkampf, H.R., Leegood, R.C., Long, S.P., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 129–167. [Google Scholar]
- Casadebaig, P.; Debaeke, P.; Lecoeur, J. Thresholds for leaf expansion and transpiration response to soil water deficit in a range of sunflower genotypes. Eur. J. Agron. 2008, 28, 646–654. [Google Scholar] [CrossRef]
- Ma, N.; Yokoyama, K.; Marumoto, T. Effect of peat on mycorrhizal colonization and effectiveness of the arbuscular mycorrhizal fungus Gigaspora margarita. Soil Sci. Plant Nutr. 2007, 53, 744–752. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Schubert, A.; Mazzitelli, M.; Ariusso, O.; Eynard, I. Effects of vesicular-arbuscular mycorrhizal fungi on micropropagated grapevines: Influence of endophyte strain, P fertilization and growth medium. Vitis 1990, 29, 5–13. [Google Scholar]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and Nitrogen Regulate Arbuscular Mycorrhizal Symbiosis in Petunia hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef]
- Ouiziad, F.; Wilde, P.; Schmelzer, E.; Hildebrandt, U.; Bothe, H. Analysis of expression of aquaporins and Na+/H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environ. Exp. Bot. 2006, 57, 177–186. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Flors, V.; García, J.M.; Pozo, M.J. AM symbiosis alters phenolic acid content in tomato roots. Plant Signal. Behav. 2010, 5, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Zouari, I.; Salvioli, A.; Chialva, M.; Novero, M.; Miozzi, L.; Tenore, G.C.; Bagnaresi, P.; Bonfante, P. From root to fruit: RNA-Seq analysis shows that arbuscular mycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genom. 2014, 15, 221. [Google Scholar] [CrossRef]
- Chialva, M.; Zouari, I.; Salvioli, A.; Novero, M.; Vrebalov, J.; Giovannoni, J.J.; Bonfante, P. Gr and hp-1 tomato mutants unveil unprecedented interactions between arbuscular mycorrhizal symbiosis and fruit ripening. Planta 2016, 244, 155–165. [Google Scholar] [CrossRef]
- Fiorilli, V.; Catoni, M.; Miozzi, L.; Novero, M.; Accotto, G.P.; Lanfranco, L. Global and cell-type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytol. 2009, 184, 975–987. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Verhage, A.; Fernandez, I.; Garcia, J.M.; Azcon-Aguilar, C.; Flors, V.; Pozo, M.J. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J. Exp. Bot. 2010, 61, 2589–2601. [Google Scholar] [CrossRef] [PubMed]
- Stemke, J.A.; Santiago, L.S. Consequences of light absorptance in calculating electron transport rate of desert and succulent plants. Photosynthetica 2011, 49, 195–200. [Google Scholar] [CrossRef]
- Yamawaki, K.; Matsumura, A.; Hattori, R.; Tarui, A.; Hossain, M.; Ohashi, Y.; Daimon, H. Effect of inoculation with arbuscular mycorrhizal fungi on growth, nutrient uptake and curcumin production of turmeric (Curcuma longa L.). Agric. Sci. 2013, 4, 66–71. [Google Scholar] [CrossRef][Green Version]
- Boldt, K.; Pörsb, Y. Photochemical processes, carbon assimilation and RNA accumulation of sucrose transporter genes in tomato arbuscular mycorrhiza. J. Plant Physiol. 2011, 168, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, S.A.L.; Domingues, A.P.; Mazzafera, P. Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenate and arsenite stress. Chemosphere 2015, 134, 141–149. [Google Scholar] [CrossRef]
- Millar, J.A.; Ballhorn, D.J. Effect of mycorrhizal colonization and light limitation on growth and reproduction of lima bean (Phaseolus lunatus L.). J. Appl. Bot. Food Qual. 2013, 86, 172–179. [Google Scholar]
- Kaschuk, G.; Thomas, W.; Kuyper, T.W.; Leffelaar, P.A.; Mariangela Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef]
- Amaya-Carpio, F.T.; Davies, T., Jr.; Fox, C. Arbuscular mycorrhizal fungi and organic fertilizer influence photosynthesis, root phosphatase activity, nutrition, and growth of Ipomoea carnea ssp. Fistulosa. Photosynthetica 2009, 47, 1–10. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Liu, W.; Ye, X.F.; Zai, H.F.; Zhao, L.J. Alleviation of salt stress in citrus seedlings inoculated with mycorrhiza: Changes in leaf antioxidant defense systems. Plant Soil Environ. 2010, 56, 470–475. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef]
- Augè, R.M.; Duan, X. Mycorrhizal fungi and non-hydraulic root signals of soil drying. Plant Physiol. 1991, 97, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: London, UK, 1997. [Google Scholar]
- De Groot, C.C.; Marcelis, L.F.M.; van den Boogaard, R.; Lambers, H. Growth and dry-mass partitioning in tomato as affected by phosphorus nutrition and light. Plant Cell Environ. 2001, 24, 1309–1317. [Google Scholar] [CrossRef]
- Poulton, J.L.; Koide, R.T.; Stephenson, A.G. Effects of mycorrhizal infection and soil phosphorus availability on in vitro and in vivo pollen performance in Lycopersicon esculentum (Solanaceae). Am. J. Bot. 2001, 88, 1786–1793. [Google Scholar] [CrossRef]
- Alloush, G.A.; Zeto, S.K.; Clark, R.B. Phosphorus source, organic matter, and arbuscular mycorrhiza effects on growth and mineral acquisition of chickpea grown in acidic soil. J. Plant Nutr. 2010, 23, 1351–1369. [Google Scholar] [CrossRef]
- Tamayo, E.; Gómez-Gallego, T.; Azcón-Aguilar, C.; Ferrol, N. Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front. Plant Sci. 2014, 5, 547. [Google Scholar] [CrossRef] [PubMed]
- Karandashov, V.; Bucher, M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 2005, 10, 22–29. [Google Scholar] [CrossRef]
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef]


| Name | Cost (€/kg) | Concentration in Exp1 | Amount of Inoculum per Seedling (gr Seedling −1) | N of Seedling that Could be Inoculated with 1kg of Formulation | Cost per Seedling (€ Seedling−1) | Cost per Hectare (40,000 Plant ha−1) (€ ha−1) |
|---|---|---|---|---|---|---|
| Prod1 | 100 | 1% | 0.05 | 20,000 | 0.01 | 200 |
| 5% | 0.23 | 4348 | 0.02 | 920 | ||
| Prod2 | 52 | 1% | 0.05 | 20,000 | 0 | 104 |
| 5% | 0.23 | 4348 | 0.01 | 478.4 |
| Variable | Inoculatio | Water Treatment | ANOVA | |||
|---|---|---|---|---|---|---|
| WW | WS | Inoculation | Water Treatment | I × W | ||
| Plant Height (cm) | M- | 59.2 | 63.4 | F (8.3) p = 0.015 | F (0.09) p = 0.77 | F (1.6) p = 0.24 |
| M+ * | 52.5 | 45.8 | ||||
| A | A | |||||
| Stem Diameter (mm) | M- | 6.27 | 6.89 | F (2.11) p = 0.18 | F (4.82) p = 0.05 | F (0.02) p = 0.96 |
| M+ | 6.61 | 7.15 | ||||
| A | B | |||||
| N Flower Branches Plants−1 | M- | 2 | 1.3 | F (4.97) p = 0.047 | F (7.2) p = 0.021 | F (0.8) p = 0.39 |
| M+ * | 3 | 1.6 | ||||
| A | B | |||||
| N Fruit Flower Branch−1 | M- | 2.3 | 2 | F (6.88) p = 0.024 | F (0.27) p = 0.61 | F (1.85) p = 0.21 |
| M+ * | 0.8 | 1.5 | ||||
| A | A | |||||
| Mean Single Fruit Fresh Weight (g) | M- | 12.4 | 17.7 | F (5.03) p = 0.046 | F (0.07) p = 0.79 | F (0.5) p = 0.49 |
| M+ * | 25.3 | 22.8 | ||||
| A | A | |||||
| Mean Total Fresh Weight of Fruit (g) | M- | 52.9 a | 39.7 d | F (0.77) p = 0.40 | F (2.86) p = 0.12 | F (10.04) p = 0.008 |
| M+ * | 46.7 c | 50.7 b | ||||
| Variable | Inoculation | Water Treatment | ANOVA | |||
|---|---|---|---|---|---|---|
| WW | WS | Inoculation F (1,11) P | Water Treatment F (1,11) P | I × W F (2,11) P | ||
| ABG Dry Matter (g plant−1) | M- | 8.00 | 8.26 | F (7.37) p = 0.026 | F (0.90) p = 0.36 | F (2.07) p = 0.18 |
| M+ * | 7.31 | 6.02 | ||||
| A | A | |||||
| BG Dry Matter (g plant−1) | M- | 1.22 | 1.12 | F (9.55) p = 0.015 | F (0.33) p = 0.58 | F (0.004) p = 0.95 |
| M+ * | 0.73 | 0.65 | ||||
| A | A | |||||
| Root/ Shoot | M- | 0.15 | 0.14 | F (5.92) p = 0.040 | F (0.058) p = 0.81 | F (0.49) p = 0.5 |
| M+ * | 0.10 | 0.11 | ||||
| A | A | |||||
| Total Dry Matter (g plant−1) | M- | 9.22 | 9.39 | F (8.58) p = 0.018 | F (0.83) p = 0.39 | F (1.32) p = 0.28 |
| M+ * | 8.04 | 6.66 | ||||
| A | A | |||||
| SLA (cm2 g−1) | M- | 87.7 | 78.3 | F (4.98) p = 0.047 | F (0.14) p = 0.71 | F (2.63) p = 0.14 |
| M+ * | 118.8 | 127.8 | ||||
| A | A | |||||
| LAR (cm2 g−1) | M- | 31.4 | 23.5 | F (7.11) p = 0.028 | F (6.24) p = 0.037 | F (0.02) p = 0.87 |
| M+ * | 40.9 | 32.02 | ||||
| A | B | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fracasso, A.; Telò, L.; Lanfranco, L.; Bonfante, P.; Amaducci, S. Physiological Beneficial Effect of Rhizophagus intraradices Inoculation on Tomato Plant Yield under Water Deficit Conditions. Agronomy 2020, 10, 71. https://doi.org/10.3390/agronomy10010071
Fracasso A, Telò L, Lanfranco L, Bonfante P, Amaducci S. Physiological Beneficial Effect of Rhizophagus intraradices Inoculation on Tomato Plant Yield under Water Deficit Conditions. Agronomy. 2020; 10(1):71. https://doi.org/10.3390/agronomy10010071
Chicago/Turabian StyleFracasso, Alessandra, Luca Telò, Luisa Lanfranco, Paola Bonfante, and Stefano Amaducci. 2020. "Physiological Beneficial Effect of Rhizophagus intraradices Inoculation on Tomato Plant Yield under Water Deficit Conditions" Agronomy 10, no. 1: 71. https://doi.org/10.3390/agronomy10010071
APA StyleFracasso, A., Telò, L., Lanfranco, L., Bonfante, P., & Amaducci, S. (2020). Physiological Beneficial Effect of Rhizophagus intraradices Inoculation on Tomato Plant Yield under Water Deficit Conditions. Agronomy, 10(1), 71. https://doi.org/10.3390/agronomy10010071







