Effects of Physical, Mechanical and Hormonal Treatments of Seed-Tubers on Bud Dormancy and Plant Productivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Varieties and Dormancy-Breaking Treatments
2.2. Abscisic Acid Analysis and Dormancy Release Assessment
2.3. Experimental Design, Field Management, Observations and Sampling
2.4. Statistical Analysis
3. Results
3.1. Experiment 1 (Exp-1)
3.2. Exp-2
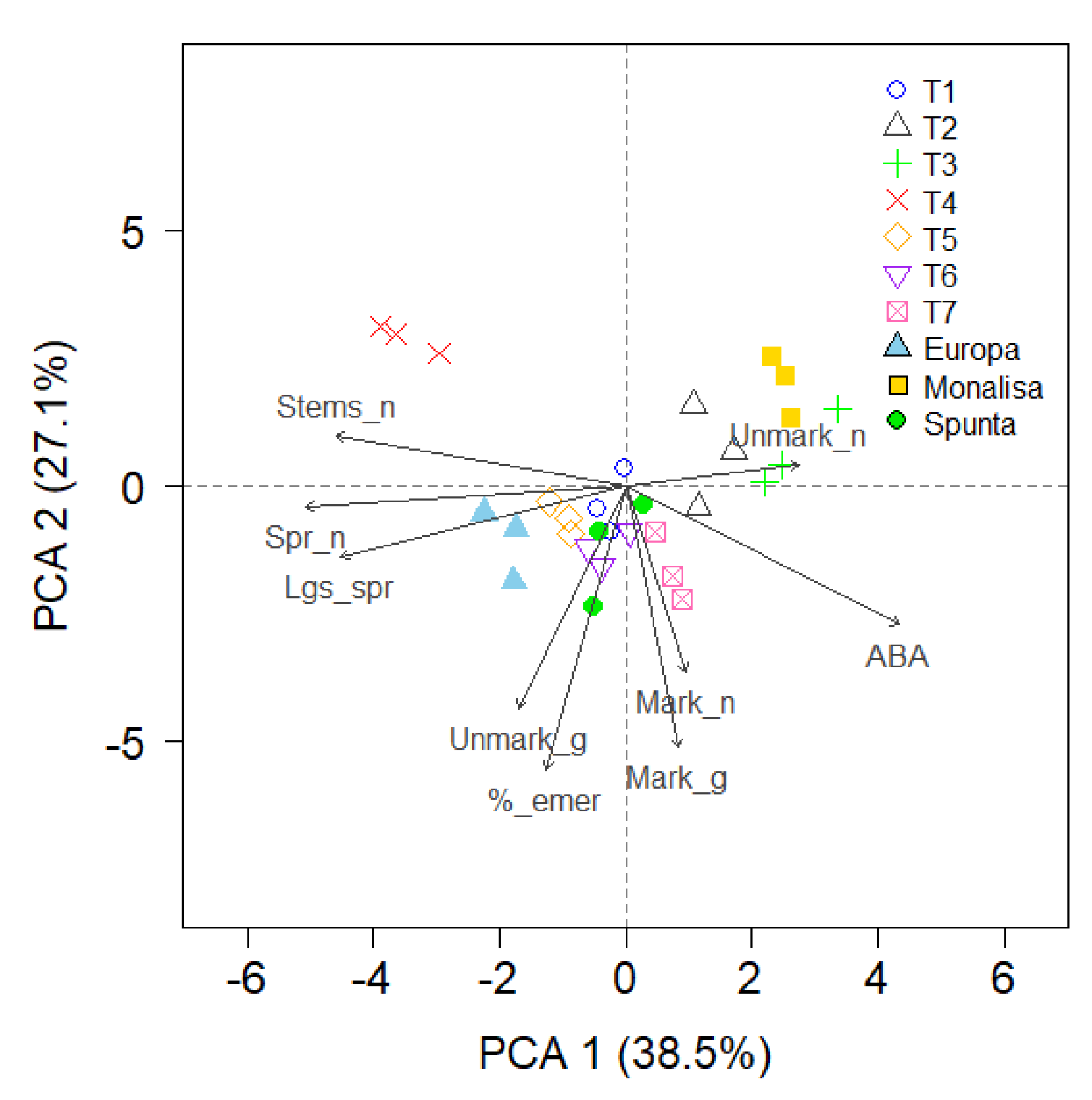
Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, Y.; Onik, J.C.; Hu, X.; Duan, Y.; Lin, Q. Effects of (S)-carvone and gibberellin on sugar accumulation in potatoes during low temperature storage. Molecules 2018, 23, 3118. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Data for Potato. Food and Agriculture Organization of the United Nations, 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 22 June 2019).
- Ierna, A. Tuber yield and quality characteristics of potatoes for off-season crops in a Mediterranean environment. J. Sci. Food Agric. 2010, 90, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Carputo, D.; Conti, S.; Borrelli, C.; Maddaluno, P.; Frusciante, L. Effect of mulching and plant density on out-of-season organic potato growth, yield and quality. Adv. Hortic. Sci. 2013, 27, 115–121. [Google Scholar]
- Li, J.; Huang, W.; Cao, H.; Xiao, G.; Zhou, J.; Xie, C.; Xia, J.; Song, B. Additive and epistatic QTLs underlying the dormancy in a diploid potato population across seven environments. Sci. Hortic. 2018, 240, 578–584. [Google Scholar] [CrossRef]
- Aksenova, N.P.; Sergeeva, L.I.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kolachevskaya, O.O.; Romanov, G.A. Regulation of potato tuber dormancy and sprouting. Russ. J. Plant Physiol. 2013, 60, 301–312. [Google Scholar] [CrossRef]
- Sonnewald, S.; Sonnewald, U. Regulation of potato tuber sprouting. Planta 2014, 239, 27–38. [Google Scholar] [CrossRef]
- Suttle, J.C. Dormancy and sprouting. In Potato Biology and Biotechnology. Advances and Perspectives, 1st ed.; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., MacKerron, D.K.L., Taylor, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 287–309. [Google Scholar]
- Muthoni, J.; Kabira, J.; Shimelis, H.; Melis, R. Regulation of potato tuber dormancy: A review. Aust. J. Crop Sci. 2014, 8, 754–759. [Google Scholar]
- Delaplace, P.; Brostaux, Y.; Fauconnier, M.I.; Du Jardin, P. Potato (Solanum tuberosum L.) tuber physiological age index is a valid reference frame in post-harvest ageing studies. Postharvest Biol. Technol. 2008, 50, 103–106. [Google Scholar] [CrossRef]
- Mustonen, L. Yield formation and quality characteristics of early potatoes during a short growing period. Agric. Food Sci. 2004, 13, 390–398. [Google Scholar] [CrossRef]
- Caldiz, D.O. Physiological age research during the second half of the twentieth century. Potato Res. 2009, 52, 295–304. [Google Scholar] [CrossRef]
- Bisognin, D.A.; Freitas, S.T.; Brackmann, A.; Andriolo, J.L.; Pujol Pereira, E.I.; Muller, D.R.; Bandinelli, M.G. Physiological aging of potato tubers produced during fall and spring growing seasons and stored under different temperatures. Bragantia 2008, 67, 59–65. [Google Scholar] [CrossRef]
- Struik, P.C. Physiological age of seed potato. Nord. Assoc. Agric. Sci. 2006, 2, 3–5. [Google Scholar]
- Pasare, S.A.; Ducreux, L.J.; Morris, W.L.; Campbell, R.; Sharma, S.K.; Roumeliotis, E. The role of the potato (Solanum tuberosum) CCD8 gene in stolon and tuber development. New Phytol. 2013, 198, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Ewing, E.E.; Simko, I.; Omer, E.A.; Davies, P.J. Polygene mapping as a tool to study the physiology of potato tuberization and dormancy. Am. J. Potato Res. 2004, 81, 281–289. [Google Scholar] [CrossRef]
- van Eck, H.J. Genetics of morphological and tuber traits. In Potato Biology and Biotechnology. Advances and Perspectives, 1st ed.; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., MacKerron, D.K.L., Taylor, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 91–111. [Google Scholar]
- Bisognin, D.A.; Manrique-Carpintero, N.C.; Douches, D.S. QTL analysis of tuber dormancy and sprouting in potato. Am. J. Potato Res. 2018, 95, 374–382. [Google Scholar] [CrossRef]
- Mani, F.; Bettaieb, T.; Doudech, N.; Hannachi, C. Physiological mechanisms for potato dormancy release and sprouting: A review. Afr. Crop Sci. J. 2014, 22, 155–174. [Google Scholar]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 2011, 155, 776–796. [Google Scholar] [CrossRef]
- Suttle, J.C. Physiological regulation of potato tuber dormancy. Am. J. Potato Res. 2004, 81, 253. [Google Scholar] [CrossRef]
- Mani, F.; Hannachi, C. Physiology of potato sprouting. J. New Sci. Agric. Biotechnol. 2015, 17, 591–602. [Google Scholar]
- Wróbel, S.; Kęsy, J.; Treder, K. Effect of growth regulators and ethanol on termination of dormancy in potato tubers. Am. J. Potato Res. 2017, 94, 544–555. [Google Scholar] [CrossRef]
- Jansky, S.; Hamernik, A. Rapid cycling of potato tuber generations by overcoming dormancy. Am. J. Potato Res. 2015, 92, 148–152. [Google Scholar] [CrossRef]
- Eshel, D.; Teper-Bamnolker, P. Can loss of apical dominance in potato tuber serve as a marker of physiological age? Plant Signal. Behav. 2012, 7, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Struik, P.C.; Wiersema, S.G. Seed Potato Technology, 1st ed.; Wageningen Academic Pub.: Wageningen, The Netherlands, 1999; p. 382. [Google Scholar]
- Asalfew, G.K. Review on the effect of gibberellic acid on potato (Solanum tuberosum L.) tuber dormancy breaking and sprouting. J. Biol. Agric. Healthc. 2016, 7, 68–79. [Google Scholar]
- United States Department of Agriculture (USDA). Natural Resources Conservation Service (NRCS), Keys to Soil Taxonomy, 11th ed.; Pocahontas Press Inc.: Blacksburg, VA, USA, 2010; p. 338. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Giordano, I.; Mauromicale, G.; Parisi, B.; Pentangelo, A. Coltivazione in Italia. In La Patata, 1st ed.; Frusciante, L., Roversi, G., Eds.; Bayer Crop Science Inc.: Bologna, Italy, 2010; pp. 416–423. [Google Scholar]
- Mallica, G.M.; Baghino, L.; Pisanu, A.B. Confronto varietale sulla patata primaticcia in Sardegna. L’informatore Agrar. 2000, 50, 3–7. [Google Scholar]
- Le Groupe d’Etude et de contrôle des Variétés et des Semences. Bulletin des Varietes Pomme de terre; GEVES: Guyancourt, France, 1993; Available online: https://www.geves.fr/geves/ (accessed on 3 November 2019).
- Allen, E.J.; O’Brien, P.J.; Firman, D. Seed tuber production and management. In the Potato Crop; Harris, P.M., Ed.; Chapman & Hall: London, UK, 1992; pp. 247–291. [Google Scholar]
- van Loon, K.D. The seed potato market. In Potato Biology and Biotechnology, Advances and Perspectives; Vreugdenhil, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 45–52. [Google Scholar]
- Volaire, F.; Seddaiu, G.; Ledda, L.; Lelievre, F. Water deficit and induction of summer dormancy in perennial Mediterranean grasses. Ann. Bot. 2009, 103, 1337–1346. [Google Scholar] [CrossRef]
- Asch, F. Determination of Abscisic Acid by Indirect Enzyme Linked Immunosorbent Assay (ELISA); Technical Report; Laboratory for Agrohydrology and Bioclimatology, Department of Agricultural Sciences, The Royal Veterinary and Agricultural University: Taastrup, Denmark, 2000. [Google Scholar]
- Bahrun, A.; Jensen, C.; Asch, F.; Mogensen, V.O. Drought-induced changes in xylem pH, ionic composition, and ABA concentration act as early signals in field-grown maize (Zea mays L.). J. Exp. Bot. 2002, 53, 251–263. [Google Scholar] [CrossRef]
- Carli, C.; Mihovilovich, E.; Yuldashev, F.; Khalikov, D.; Kadian, M.S. Assessment of dormancy and sprouting behavior of cip elite and advanced clones under different storage conditions in Uzbekistan. Potato Res. 2010, 53, 313–323. [Google Scholar] [CrossRef]
- Johansen, T.J.; Mølmann, J.A.B. Seed potato performance after storage in light at elevated temperatures. Potato Res. 2018, 61, 133–145. [Google Scholar] [CrossRef]
- Little, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D. SAS System for Mixed Models; SAS Institute: Cary, NC, USA, 1996. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org (accessed on 3 November 2019).
- Ordaz-Ortiz, J.J.; Foukaraki, S.G.; Terry, L.A. Assessing temporal flux of plant hormones in stored processing potatoes using high definition accurate mass spectrometry. Hortic. Res. 2015, 2, 15002. [Google Scholar] [CrossRef][Green Version]
- Carrera, E.; Bou, J.; GarciaMartinez, J.L.; Prat, S. Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J. 2000, 22, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, S.; Podzimska, D.; Voegele, A.; Imbeck, M.; Müller, K.; Linkies, A.; Leubner-Metzger, G. Dose- and tissue-specific interaction of monoterpenes with the gibberellin-mediated release of potato tuber bud dormancy, sprout growth and induction of α-amylases and β-amylases. Planta 2011, 235, 137–151. [Google Scholar] [CrossRef]
- Roumeliotis, E.; Kloosterman, B.; Oortwijn, M.; Kohlen, W.; Bouwmeester, H.J.; Visser, R.G. The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. J. Exp. Bot. 2012, 63, 4539–4547. [Google Scholar] [CrossRef] [PubMed]
- Otroshy, M.; Struik, P.C. Effects of size of normal seed tubers and growth regulator application on dormancy, sprout behaviour, growth vigour and quality of normal seed tubers of different potato cultivars. Res. J. Seed Sci. 2008, 1, 41–50. [Google Scholar] [CrossRef]
- Alamar, M.C.; Tosetti, R.; Landahl, S.; Bermejo, A.; Terry, L.A. Assuring potato tuber quality during storage: A future perspective. Front. Plant Sci. 2017, 8, 2034. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.H.; Beattie, B.; Laurence, R. Intergenerational effects on seed potato physiological ageing. Acta Hort. 2003, 619, 241–249. [Google Scholar] [CrossRef]
- Eremeev, V.; Lõhmus, A.; Lääniste, P.; Jõudu, J.; Talgre, L.; Lauringson, E. The influence of thermal shock and pre-sprouting on formation of some yield structure elements. Acta Agric. Scand. Sect. B Soil Plant Sci. 2008, 58, 35–42. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Aivalakis, G.; Akoumianakis, K.A.; Passam, H.C. Effect of gibberellic acid on the duration of dormancy of potato tubers produced by plants derived from true potato seed. Postharv. Biol. Technol. 2008, 49, 424–430. [Google Scholar] [CrossRef]
- Pande, P.C.; Singh, S.V.; Pandey, S.K.; Singh, B. Dormancy, sprouting behavior and weight loss in Indian potato (Solanum tuberosum) varieties. Indian J. Agric. Sci. 2007, 77, 715–720. [Google Scholar]
- Struik, P. The canon of potato science: 40. Physiological age of seed tubers. Potato Res. 2007, 50, 375–377. [Google Scholar] [CrossRef]
- Caldiz, D.O.; Fernandez, L.V.; Struik, P.C. Physiological age index: A new, simple and reliable index to assess the physiological age of seed potato tubers based on haulm killing date and length of the incubation period. Field Crop. Res. 2001, 69, 69–79. [Google Scholar] [CrossRef]
- Johansen, T.J.; Møllerhagen, P.; Haugland, E. Yield potential of seed potatoes grown at different latitudes in Norway. Acta Agric. Scand. Sect. B Soil Plant Sci. 2008, 58, 132–138. [Google Scholar] [CrossRef]
- Eremeev, V.; Tein, B.; Lääniste, P.; Mäeorg, E.; Kuht, J. The effect of pre-planting thermal treatment of seed tubers on the yield and quality of potato. Agron. Res. 2015, 13, 1193–1201. [Google Scholar]
- Blauer, J.M.; Knowles, L.O.; Knowles, N.R. Manipulating stem number, tuber set and size distribution in specialty potato cultivars. Am. J. Potato Res. 2013, 90, 470–496. [Google Scholar] [CrossRef]
- Herman, D.J.; Knowles, L.O.; Knowles, N.R. Differential sensitivity of genetically related potato cultivars to treatments designed to alter apical dominance, tuber set and size distribution. Am. J. Potato Res. 2016, 93, 331–349. [Google Scholar] [CrossRef]
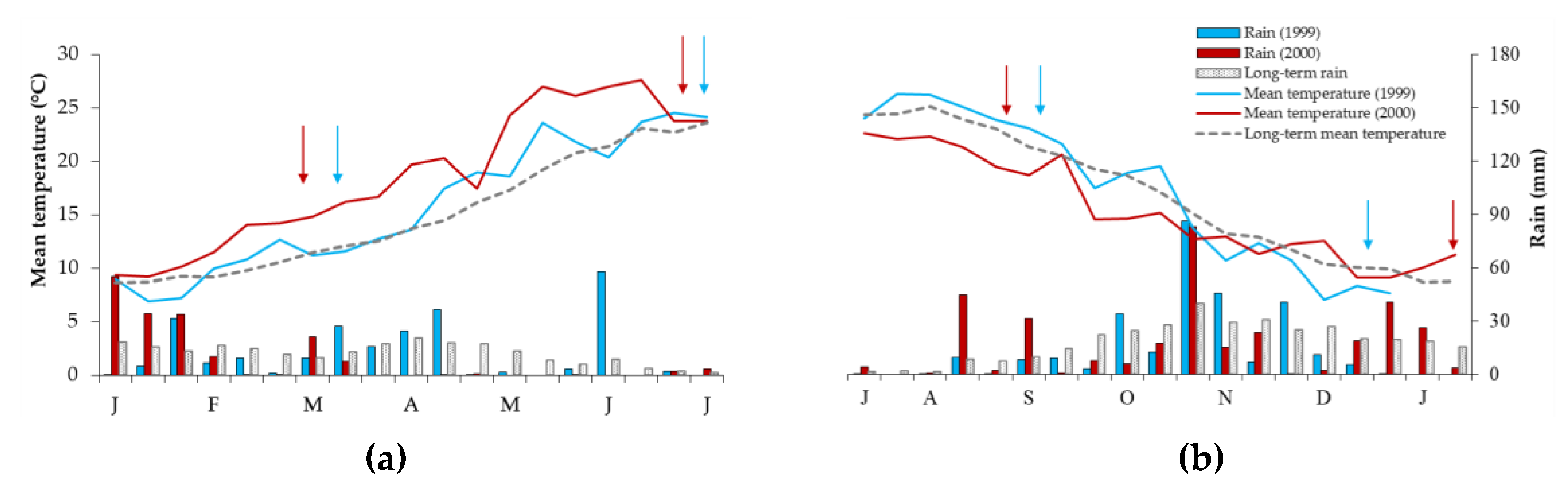

| Treatments | Dark Condition | Light Diffuse Condition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Duration (days) | Temperature (°C) | Duration (days) | Temperature (°C) | Duration (days) | Temperature (°C) | Duration (days) | Temperature (°C) | Duration (days) | GA3 | ||
| (ppm) | (DAH) | |||||||||||
| T1 | 20 | 38 | - | - | - | - | - | - | - | - | - | |
| T2 | 10 | 10 | 20 | 4 | 10 | 10 | 20 | 4 | 10 | 10 | - | - |
| T3 | 2 | 10 | 20 | 4 | 2 | 10 | 20 | 4 | 2 | 10 | - | - |
| T4 | 38 | 10 | 20 | 28 | - | - | - | - | - | - | - | - |
| T5 | 20 | 38 | - | - | - | - | - | - | - | - | 10 | 39 |
| T6 | 10 | 10 | 20 | 4 | 10 | 10 | 20 | 4 | 10 | 10 | 10 | 39 |
| T7 | 2 | 10 | 20 | 4 | 2 | 10 | 20 | 4 | 2 | 10 | 10 | 39 |
| Haulms | Tubers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut | Harvest | Post-Harvest Storage | Treatments | Temperature | Duration (days) | GA3 | Mechanical | Temperature (°C) | Duration (days) | ||||
| (DAP) | (DAP) | Temperature (°C) | Duration (days) | Light Condition | (ppm) | (DAH) | |||||||
| Removed (R) | 90 | 100 | 20 | 25 | Dark | R-T1 | 20 | 58 | - | - | - | - | - |
| R-T2 | 20 | 58 | 10 | 79 | - | - | - | ||||||
| Undisturbed (U) | - | 125 | - | - | - | U-T3 | 20 | 58 | - | - | - | - | |
| U-T4 | 20 | 58 | 10 | 54 | - | - | - | ||||||
| U-T5 | 20 | 48 | - | - | - | 32 | 10 | ||||||
| U-T6 | 20 | 53 | - | - | - | 38 | 5 | ||||||
| U-T7 | 20 | 48 | - | - | Cut in half | 20 | 10 | ||||||
| Treatments | 9-Jul | 16-Aug | 23-Aug | 30-Aug | 06-Sep | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | |
| T1 | – | 3.8 ± 0.1 | 9.3 ± 0.5 | 5.0 ± 0.4 | 2.5 ± 0.1 | 3.3 ± 0.2 | 2.7 ± 0.2 | 2.0 ± 0.04 | 3.0 ± 0.1 | 2.8 ± 0.4 | 2.5 ± 0.1 | 2.9 ± 0.1 | 2.5 ± 0.1 | ||
| T2 | – | 5.7 ± 0.2 | 10.3 ± 1.0 | 6.0 ± 0.2 | 4.0 ± 0.1 | 4.0 ± 0.1 | 3.3 ± 0.1 | 3.0 ± 0.1 | 3.5 ± 0.02 | 3.0 ± 0.03 | 2.5 ± 0.1 | 3.4 ± 0.2 | 2.6 ± 0.02 | ||
| T3 | – | 6.5 ± 0.3 | 8.3 ± 0.3 | 5.3 ± 0.2 | 5.5 ± 0.2 | 4.8 ± 0.1 | 6.0 ± 0.4 | 4.5 ± 0.1 | 4.0 ± 0.1 | 4.5 ± 0.1 | 3.2 ± 0.2 | 3.6 ± 0.2 | 3.3 ± 0.01 | ||
| T4 | – | 3.7 ± 0.3 | 4.7 ± 0.4 | 3.5 ± 0.1 | – | – | – | – | – | – | – | – | – | ||
| T5 | – | – | – | – | 2.8 ± 0.6 | 3.8 ± 0.3 | 3.0 ± 0.1 | 2.5 ± 0.04 | 3.5 ± 0.1 | 2.7 ± 0.1 | 2.4 ± 0.2 | 2.8 ± 0.1 | 2.5 ± 0.1 | ||
| T6 | – | – | – | – | 3.8 ± 0.2 | 4.8 ± 0.1 | 3.7 ± 0.1 | 3.0 ± 0.1 | 3.3 ± 0.1 | 2.8 ± 0.1 | 2.6 ± 0.1 | 3.0 ± 0.1 | 2.4 ± 0.1 | ||
| T7 | – | – | – | – | 5.3 ± 0.5 | 5.3 ± 0.2 | 5.8 ± 0.2 | 4.3 ± 0.1 | 3.8 ± 0.1 | 4.7 ± 0.3 | 3.7 ± 0.1 | 3.3 ± 0.2 | 3.4 ± 0.04 | ||
| Significance | p > F | p > F | p > F | p > F | p > F | ||||||||||
| Variety (V) | 0.385 | 0.028 | 0.932 | 0.213 | 0.098 | ||||||||||
| Treatment (T) | – | 0.825 | <0.001 | 0.041 | 0.004 | ||||||||||
| V × T | – | 0.776 | 0.888 | 0.249 | 0.071 | ||||||||||
| Treatments | 16-Aug | 23-Aug | 30-Aug | 06-Sep | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | |
| T1 | 0.7 ± 0.01 | 0.3 ± 0.02 | 0.6 ± 0.01 | 0.8 ± 0.06 | 0.3 ± 0.01 | 0.6 ± 0.01 | 1.7 ± 0.31 | 0.7 ± 0.02 | 0.9 ± 0.05 | 2.7 ± 0.33 | 1.1 ± 0.06 | 2.1 ± 0.08 |
| T2 | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.7 ± 0.01 | 0.3 ± 0.01 | 0.4 ± 0.01 | 2.5 ± 0.50 | 0.7 ± 0.02 | 1.6 ± 0.08 |
| T3 | 0.0 ± 0.01 | 0.0 ± 0.01 | 0.0 ± 0.01 | 0.0 ± 0.02 | 0.0 ± 0.01 | 0.0 ± 0.02 | 0.7 ± 0.01 | 0.1 ± 0.01 | 0.7 ± 0.03 | 2.1 ± 0.12 | 0.6 ± 0.01 | 1.3 ± 0.06 |
| T4 | 4.9 ± 0.22 | 0.7 ± 0.01 | 4.8 ± 0.24 | 5.3 ± 0.10 | 0.7 ± 0.24 | 4.7 ± 0.22 | 5.6 ± 2.94 | 1.3 ± 0.81 | 4.3 ± 1.30 | 5.5 ± 0.76 | 1.3 ± 0.19 | 3.8 ± 0.17 |
| T5 | – | – | – | 0.9 ± 0.01 | 0.4 ± 0.01 | 0.7 ± 0.01 | 3.1 ± 0.40 | 0.9 ± 0.01 | 2.0 ± 0.53 | 3.5 ± 0.48 | 1.2 ± 0.06 | 3.2 ± 0.20 |
| T6 | – | – | – | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.01 | 2.3 ± 0.25 | 0.4 ± 0.01 | 1.7 ± 0.90 | 3.2 ± 0.60 | 1.6 ± 0.08 | 2.7 ± 0.16 |
| T7 | – | – | – | 0.0 ± 0.01 | 0.0 ± 0.01 | 0.1 ± 0.01 | 2.2 ± 0.48 | 0.6 ± 0.01 | 1.6 ± 0.42 | 3.3 ± 0.36 | 1.4 ± 0.07 | 2.3 ± 0.86 |
| Significance | p > F | p > F | p > F | p > F | ||||||||
| Variety (V) | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| Treatment (T) | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| V × T | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| Treatments | 16-Aug | 23-Aug | 30-Aug | 06-Sep | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | |
| T1 | 16.4 ± 2.13 | 0.9 ± 0.01 | 10.6 ± 3.05 | 15.1 ± 1.07 | 1.7 ± 0.01 | 10.8 ± 2.21 | 15.8 ± 2.78 | 2.6 ± 0.55 | 9.7 ± 0.82 | 17.9 ± 2.22 | 4.3 ± 0.36 | 11.4 ± 2.01 |
| T2 | 1.1 ± 0.09 | 0.8 ± 0.01 | 1.0 ± 0.01 | 1.4 ± 0.02 | 1.1 ± 0.01 | 1.2 ± 0.01 | 2.1 ± 0.20 | 1.4 ± 0.46 | 1.4 ± 0.11 | 5.1 ± 0.71 | 2.6 ± 0.38 | 4.3 ± 0.44 |
| T3 | 0.2 ± 0.01 | 0.1 ± 0.01 | 0.0 ± 0.01 | 0.3 ± 0.01 | 0.2 ± 0.01 | 0.0 ± 0.01 | 1.7 ± 0.72 | 0.4 ± 0.01 | 1.6 ± 0.51 | 4.8 ± 0.98 | 1.7 ± 0.05 | 3.3 ± 0.03 |
| T4 | 23.7 ± 7.88 | 2.0 ± 0.44 | 12.6 ± 2.53 | 23.7 ± 4.11 | 2.8 ± 0.71 | 13.7 ± 2.76 | 20.3 ± 2.33 | 4.0 ± 0.26 | 13.0 ± 1.25 | 19.9 ± 2.34 | 4.2 ± 0.74 | 11.4 ± 1.91 |
| T5 | – | – | – | 7.2 ± 1.20 | 3.0 ± 0.34 | 4.5 ± 1.71 | 12.9 ±1.98 | 5.0 ± 0.79 | 11.4 ± 2.18 | 16.0 ± 3.12 | 5.7 ± 0.88 | 15.1 ± 1.79 |
| T6 | – | – | – | 0.8 ± 0.01 | 0.6 ± 0.01 | 0.8 ± 0.01 | 4.7 ± 0.44 | 1.8 ± 0.11 | 6.3 ± 1.00 | 8.6 ± 0.45 | 4.5 ± 0.09 | 13.5 ± 2.01 |
| T7 | – | – | – | 0.0 ± 0.01 | 0.5 ± 0.01 | 0.4 ± 0.01 | 3.8 ± 0.51 | 2.6 ± 0.09 | 5.0 ± 0.75 | 7.6 ± 0.61 | 5.6 ± 0.33 | 8.8 ± 1.08 |
| Significance | p > F | p > F | p > F | p > F | ||||||||
| Variety (V) | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| Treatment (T) | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| V × T | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| Treatments | 06-Oct | 12-Oct | 22-Oct | 29-Oct | 10-Nov | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | |
| T1 | 5 ± 0.72 | 0 ± 0.01 | 16 ± 1.41 | 40 ± 4.35 | 0 ± 0.01 | 47 ± 6.15 | 93 ± 14.6 | 47 ± 2.98 | 73 ± 12.0 | 100 ± 22.3 | 93 ± 21.0 | 73 ± 12.3 | 100 ± 11.6 | 93 ± 11.1 | 80 ± 8.74 |
| T2 | 0 ± 0.01 | 0 ± 0.01 | 5 ± 0.63 | 13 ± 1.19 | 0 ± 0.01 | 13 ± 1.11 | 93 ± 11.3 | 20 ± 2.07 | 87 ± 12.7 | 93 ± 8.64 | 87 ± 8.84 | 87 ± 9.6 | 93 ± 8.12 | 100 ± 7.01 | 87 ± 6.11 |
| T3 | 0 ± 0.01 | 0 ± 0.01 | 4 ± 0.11 | 13 ± 1.02 | 0 ± 0.01 | 27 ± 1.67 | 73 ± 9.12 | 33 ± 1.91 | 80 ± 11.4 | 87 ± 17.1 | 93 ± 24.3 | 93 ± 13.7 | 87 ± 17.7 | 100 ± 14.6 | 100 ± 10.2 |
| T4 | 71 ± 12.1 | 2 ± 0.08 | 58 ± 9.33 | 67 ± 11.4 | 13 ± 2.01 | 67 ± 8.36 | 73 ± 4.57 | 20 ± 1.46 | 80 ± 9.36 | 73 ± 3.79 | 27 ± 3.17 | 80 ± 11.2 | 73 ± 3.11 | 33 ± 1.50 | 80 ± 10.1 |
| T5 | 80 ± 14.6 | 29 ± 1.44 | 78 ± 11.0 | 80 ± 13.3 | 73 ± 11.9 | 80 ± 10.9 | 87 ± 10.2 | 80 ± 15.2 | 87 ± 8.44 | 93 ± 10.4 | 87 ± 12.1 | 93 ± 18.4 | 93 ± 7.12 | 100 ± 8.91 | 93 ± 10.3 |
| T6 | 36 ± 1.75 | 18 ± 3.52 | 80 ± 11.4 | 87 ± 10.1 | 53 ± 8.01 | 87 ± 16.7 | 93 ± 17.4 | 93 ±15.5 | 73 ± 18.2 | 93 ± 9.04 | 100 ± 19.1 | 87 ± 14.5 | 100 ± 25.0 | 100 ± 15.2 | 87 ± 9.61 |
| T7 | 33 ± 1.83 | 9 ± 0.51 | 42 ± 6.62 | 100 ± 21.4 | 47 ± 4.17 | 73 ± 8.84 | 100 ± 16.0 | 93 ± 16.0 | 93 ± 16.9 | 100 ± 11.6 | 93 ± 12.8 | 93 ± 8.74 | 100 ± 8.0 | 93 ± 9.45 | 100 ± 7.65 |
| Significance | p > F | p > F | p > F | p > F | p > F | ||||||||||
| Variety (V) | <0.001 | 0.002 | 0.008 | 0.088 | 1.527 | ||||||||||
| Treatment (T) | <0.001 | 0.035 | 0.019 | 0.033 | 0.047 | ||||||||||
| V × T | <0.001 | 1.645 | 0.072 | 0.577 | 0.645 | ||||||||||
| Treatments | 12-Oct | 25-Oct | 29-Oct | 10-Nov | 13-Dec | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | |
| T1 | 0.0 ± 0.01 | 0.0 ± 0.01 | 1.0 ± 0.01 | 0.9 ± 0.01 | 0.6 ± 0.01 | 1.2 ± 0.09 | 1.3 ± 0.02 | 0.9 ± 0.01 | 1.2 ± 0.07 | 1.7 ± 0.14 | 1.6 ± 0.09 | 1.3 ± 0.05 | 1.5 ± 0.21 | 1.3 ± 0.10 | 1.3 ± 0.07 |
| T2 | 0.0 ± 0.01 | 0.0 ± 0.01 | 0.6 ± 0.01 | 0.9 ± 0.01 | 0.5 ± 0.01 | 1.2 ± 0.07 | 1.2 ± 0.01 | 1.1 ± 0.02 | 1.3 ± 0.08 | 1.6 ± 0.11 | 1.6 ± 0.13 | 1.4 ± 0.06 | 1.4 ± 0.09 | 1.2 ± 0.08 | 1.3 ± 0.09 |
| T3 | 0.0 ± 0.01 | 0.0 ± 0.01 | 0.3 ± 0.01 | 0.6 ± 0.01 | 0.5 ± 0.01 | 1.0 ± 0.09 | 1.1 ± 0.50 | 1.0 ± 0.02 | 1.1 ± 0.13 | 1.5 ± 0.13 | 1.3 ± 0.05 | 1.3 ± 0.10 | 1.3 ± 0.07 | 1.2 ± 0.08 | 1.2 ± 0.10 |
| T4 | 2.3 ± 0.11 | 0.1 ± 0.01 | 1.4 ± 0.11 | 2.8 ± 0.12 | 0.4 ± 0.01 | 1.5 ± 0.06 | 2.9 ± 0.14 | 0.6 ± 0.01 | 1.5 ± 0.05 | 3.0 ± 0.15 | 0.6 ± 0.01 | 1.5 ± 0.11 | 2.4 ± 0.11 | 1.7 ± 0.11 | 2.0 ± 0.13 |
| T5 | 2.0 ± 0.10 | 0.8 ± 0.05 | 1.3 ± 0.09 | 2.3 ± 0.10 | 1.2 ± 0.02 | 1.4 ± 0.14 | 2.2 ± 0.11 | 1.5 ± 0.08 | 1.4 ± 0.11 | 2.3 ± 0.10 | 7.5 ± 0.22 | 1.4 ± 0.09 | 1.8 ± 0.12 | 1.4 ± 0.07 | 1.3 ± 0.11 |
| T6 | 1.6 ± 0.05 | 0.7 ± 0.07 | 1.6 ± 0.08 | 1.9 ± 0.11 | 1.7 ± 0.14 | 1.7 ± 0.12 | 2.0 ± 0.09 | 1.8 ± 0.08 | 1.7 ± 0.09 | 2.1 ± 0.08 | 1.8 ± 0.09 | 1.7 ± 0.11 | 1.9 ± 0.21 | 1.4 ± 0.07 | 1.6 ± 0.09 |
| T7 | 1.2 ± 0.05 | 0.7 ± 0.08 | 1.1 ± 0.04 | 1.6 ± 0.08 | 1.4 ± 0.11 | 1.5 ± 0.08 | 1.9 ± 0.15 | 1.5 ± 0.08 | 1.5 ± 0.12 | 2.0 ± 0.07 | 1.7 ± 0.12 | 1.5 ± 0.12 | 1.8 ± 0.14 | 1.1 ± 0.01 | 1.3 ± 0.08 |
| Significance | p > F | p > F | p > F | p > F | p > F | ||||||||||
| Variety (V) | 0.003 | 0.017 | 0.020 | 0.009 | 0.009 | ||||||||||
| Treatment (T) | 0.043 | 0.048 | 0.022 | 0.038 | 0.047 | ||||||||||
| V × T | 0.025 | 0.015 | 1.438 | 0.047 | 0.065 | ||||||||||
| Treatments | Marketable Yield | Unmarketable Yield | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tuber Number (no. plant−1) | Tuber Weight (g plant−1) | Tuber Number (no. plant−1) | Tuber Weight (g plant−1) | |||||||||
| Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | Eur | Mon | Spu | |
| T1 | 5.1 ± 0.4 | 5.1 ± 0.1 | 4.2 ± 0.1 | 268.9 ± 21 | 212.6 ± 21 | 352.0 ± 16 | 0.6 ± 0.01 | 0.1 ± 0.01 | 0.5 ± 0.01 | 27.8 ± 1.1 | 5.2 ± 0.1 | 99.6 ± 7.4 |
| T2 | 5.7 ± 0.5 | 4.1 ± 0.2 | 4.6 ± 0.1 | 259.3 ± 15 | 204.2 ± 18 | 301.8 ± 15 | 0.4 ± 0.01 | 0.1 ± 0.01 | 0.7 ± 0.01 | 23.0 ± 1.3 | 5.4 ± 0.3 | 64.0 ± 5.1 |
| T3 | 5.2 ± 0.4 | 4.9 ± 0.4 | 4.3 ± 0.4 | 242.9 ± 20 | 224.6 ± 15 | 307.6 ± 18 | 0.6 ± 0.01 | 0.1 ± 0.01 | 0.3 ± 0.01 | 45.5 ± 1.4 | 2.0 ± 0.1 | 38.8 ± 3.6 |
| T4 | 4.6 ± 0.3 | 1.2 ± 0.0 | 3.0 ± 0.1 | 227.2 ± 12 | 51.4 ± 5.1 | 278.1 ± 20 | 0.8 ± 0.01 | 0.0 ± 0.01 | 0.2 ± 0.01 | 39.5 ± 1.4 | 3.6 ± 0.1 | 28.2 ± 2.0 |
| T5 | 5.7 ± 0.1 | 4.1 ± 0.4 | 4.0 ± 0.2 | 275.8 ± 14 | 272.9 ± 18 | 347.9 ± 25 | 0.4 ± 0.01 | 0.1 ± 0.01 | 0.3 ± 0.01 | 35.6 ± 1.6 | 8.4 ± 0.5 | 47.3 ± 5.7 |
| T6 | 6.2 ± 0.2 | 5.0 ± 0.1 | 4.0 ± 0.1 | 354.5 ± 24 | 316.0 ± 23 | 395.5 ± 21 | 0.7 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.01 | 59.4 ± 2.0 | 1.5 ± 0.1 | 32.6 ± 3.8 |
| T7 | 5.0 ± 0.1 | 4.5 ± 0.1 | 4.3 ± 0.1 | 247.1 ± 22 | 470.8 ± 28 | 314.3 ± 24 | 0.6 ± 0.01 | 0.1 ± 0.01 | 0.4 ± 0.01 | 49.2 ± 1.4 | 0.0 ± 0.1 | 48.8 ± 4.4 |
| Significance | p >F | p > F | p > F | p > F | ||||||||
| Variety (V) | 0.028 | 0.018 | 0.003 | <0.001 | ||||||||
| Treatment (T) | 0.037 | 0.031 | 0.317 | 0.076 | ||||||||
| V × T | <0.001 | <0.001 | 0.190 | 0.087 | ||||||||
| Treatments | 5-Jun | 30-Jun | 18-Aug | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ari | Mon | Spu | Ari | Mon | Spu | Ari | Mon | Spu | |
| Removed | 8.8 ± 1.4 | 9.8 ± 2.2 | 9.3 ± 2.1 | 4.3 ± 0.9 | 5.5 ± 0.5 | 4.7 ± 0.4 | 2.8 ± 0.1 | 3.0 ± 0.1 | 3.3 ± 0.1 |
| Undisturbed | – | – | – | 5.3 ± 1.0 | 5.3 ± 0.4 | 5.3 ± 0.5 | 2.3 ± 0.1 | 2.5 ± 0.1 | 2.3 ± 0.1 |
| Significance | p > F | p > F | p > F | ||||||
| Variety (V) | 0.212 | 0.079 | 0.641 | ||||||
| Treatment (T) | – | 0.726 | 0.047 | ||||||
| V × T | – | 0.064 | 0.907 | ||||||
| Treatments | 24-Aug | 29-Aug | ||||
|---|---|---|---|---|---|---|
| Ari | Mon | Spu | Ari | Mon | Spu | |
| R-T1 | 2.3 ± 0.1 | 3.0 ± 0.2 | 3.5 ± 0.2 | 2.3 ± 0.1 | 2.0 ± 0.1 | 2.3 ± 0.2 |
| R-T2 | – | – | – | 2.5 ± 0.1 | 2.0 ± 0.1 | 1.8 ± 0.1 |
| U-T3 | 2.3 ± 0.1 | 2.5 ± 0.1 | 2.3 ± 0.1 | 1.5 ± 0.1 | 2.0 ± 0.1 | 1.3 ± 0.1 |
| U-T4 | – | – | – | 1.8 ± 0.1 | 2.0 ± 0.1 | 1.5 ± 0.1 |
| U-T5 | 1.8 ± 0.1 | 2.3 ± 0.1 | 2.5 ± 0.1 | 2.0 ± 0.1 | 2.3 ± 0.1 | 2.5 ± 0.3 |
| U-T6 | – | – | – | 1.3 ± 0.1 | 2.3 ± 0.1 | 2.0 ± 0.1 |
| U-T7 | 2.0 ± 0.1 | 2.3 ± 0.1 | 2.0 ± 0.1 | 1.0 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 |
| Significance | p > F | p > F | ||||
| Variety (V) | 0.517 | 0.385 | ||||
| Treatment (T) | 0.033 | 0.029 | ||||
| V × T | 0.835 | 0.273 | ||||
| Treatments | Sprouting Activity (0–5) | Plant Emergence (%) | ||||
|---|---|---|---|---|---|---|
| Ari | Mon | Spu | Ari | Mon | Spu | |
| R-T1 | 3.0 ± 0.3 | 1.0 ± 0.1 | 2.0 ± 0.1 | 31.3 ± 4.3 | 0.0 ± 0.01 | 0.0 ± 0.01 |
| R-T2 | 2.0 ± 0.1 | 1.0 ± 0.1 | 2.0 ± 0.1 | 35.3 ±5.1 | 0.0 ± 0.01 | 18.0 ± 2.7 |
| U-T3 | 3.0 ± 0.2 | 1.0 ± 0.1 | 4.0 ± 0.2 | 40.0 ±6.0 | 0.0 ± 0.01 | 20.0 ± 4.4 |
| U-T4 | 5.0 ± 0.3 | 2.0 ± 0.1 | 5.0 ± 0.3 | 71.3 ± 10.2 | 0.0 ± 0.01 | 48.7 ± 7.8 |
| U-T5 | 2.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 20.0 ± 4.5 | 0.0 ± 0.01 | 4.7 ± 3.5 |
| U-T6 | 4.0 ± 0.2 | 1.0 ± 0.1 | 2.0 ± 0.1 | 38.0 ± 5.8 | 0.0 ± 0.01 | 2.0 ± 0.1 |
| U-T7 | 5.0 ± 0.3 | 2.0 ± 0.1 | 2.0 ± 0.1 | 58.0 ± 6.6 | 0.0 ± 0.01 | 22.0 ± 5.2 |
| Significance | p > F | p > F | ||||
| Variety (V) | <0.001 | <0.001 | ||||
| Treatment (T) | <0.001 | 0.0013 | ||||
| V × T | 0.0831 | 0.2191 | ||||
| Treatments | Marketable Yield | Unmarketable Yield | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tuber Number (no. plant−1) | Tuber Weight (g plant−1) | Tuber Number (no. plant−1) | Tuber Weight (g plant−1) | |||||||||
| Ari | Mon | Spu | Ari | Mon | Spu | Ari | Mon | Spu | Ari | Mon | Spu | |
| R-T1 | 4.1 ± 1.0 | 4.0 ± 1.0 | 2.7 ± 1.0 | 578.7 ± 34 | 428.9 ± 41 | 487.6 ± 44 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.6 ± 0.01 | 31.2 ± 4.3 | 25.8 ± 3.0 | 102.2 ± 12 |
| R-T2 | 5.2 ± 1.4 | 4.7 ± 1.3 | 3.2 ± 1.0 | 586.6 ± 45 | 520.1 ± 49 | 440.8 ± 39 | 0.4 ± 0.01 | 0.2 ± 0.01 | 0.8 ± 0.01 | 24.9 ± 3.8 | 6.3 ± 2.3 | 113.8 ± 9 |
| U-T3 | 4.7 ± 1.0 | 3.8 ± 1.0 | 3.6 ± 1.0 | 628.1 ± 66 | 491.7 ± 37 | 489.3 ± 45 | 0.3 ± 0.01 | 0.0 ± 0.01 | 0.8 ± 0.01 | 2.1 ± 0.1 | 0.2 ± 0.01 | 105.7 ± 11 |
| U-T4 | 4.8 ± 1.0 | 5.8 ± 1.6 | 5.1 ± 1.4 | 605.5 ± 60 | 649.3 ± 59 | 622.8 ± 64 | 0.2 ± 0.01 | 4.9 ± 0.56 | 1.3 ± 0.01 | 4.7 ± 1.9 | 11.4 ± 2.4 | 102.2 ± 19 |
| U-T5 | 4.9 ± 1.5 | 2.9 ± 0.9 | 5.4 ± 1.5 | 618.8 ± 58 | 388.6 ± 38 | 717.8 ± 68 | 0.5 ± 0.01 | 0.3 ± 0.01 | 1.4 ± 0.09 | 63.5 ± 5.6 | 9.0 ± 1.6 | 114.7 ± 11 |
| U-T6 | 5.0 ± 1.5 | 2.7 ± 0.5 | 4.0 ± 1.0 | 618.1 ± 54 | 393.2 ± 29 | 685.9 ± 65 | 0.4 ± 0.01 | 0.1 ± 0.01 | 1.0 ± 0.01 | 38.0 ± 4.7 | 31.8 ± 4.5 | 103.9 ± 10 |
| U-T7 | 4.7 ± 0.9 | 3.6 ± 1.0 | 3.0 ± 1.0 | 521.7 ± 42 | 459.1 ± 46 | 651.1 ± 64 | 0.5 ± 0.01 | 0.2 ± 0.01 | 0.5 ± 0.01 | 9.5 ± 2.2 | 3.2 ± 1.0 | 104.3 ± 8 |
| Significance | p > F | p > F | p > F | p > F | ||||||||
| Variety (V) | <0.001 | 0.042 | 0.552 | <0.001 | ||||||||
| Treatment (T) | <0.001 | 0.032 | 0.031 | 0.142 | ||||||||
| V × T | <0.001 | 0.057 | 0.425 | 0.119 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deligios, P.A.; Rapposelli, E.; Mameli, M.G.; Baghino, L.; Mallica, G.M.; Ledda, L. Effects of Physical, Mechanical and Hormonal Treatments of Seed-Tubers on Bud Dormancy and Plant Productivity. Agronomy 2020, 10, 33. https://doi.org/10.3390/agronomy10010033
Deligios PA, Rapposelli E, Mameli MG, Baghino L, Mallica GM, Ledda L. Effects of Physical, Mechanical and Hormonal Treatments of Seed-Tubers on Bud Dormancy and Plant Productivity. Agronomy. 2020; 10(1):33. https://doi.org/10.3390/agronomy10010033
Chicago/Turabian StyleDeligios, Paola A., Emma Rapposelli, Massimiliano G. Mameli, Limbo Baghino, Gian Mario Mallica, and Luigi Ledda. 2020. "Effects of Physical, Mechanical and Hormonal Treatments of Seed-Tubers on Bud Dormancy and Plant Productivity" Agronomy 10, no. 1: 33. https://doi.org/10.3390/agronomy10010033
APA StyleDeligios, P. A., Rapposelli, E., Mameli, M. G., Baghino, L., Mallica, G. M., & Ledda, L. (2020). Effects of Physical, Mechanical and Hormonal Treatments of Seed-Tubers on Bud Dormancy and Plant Productivity. Agronomy, 10(1), 33. https://doi.org/10.3390/agronomy10010033







