Effects of Low Water Availability on Root Placement and Shoot Development in Landraces and Modern Barley Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Traits
2.4. Statistical Analysis
3. Results
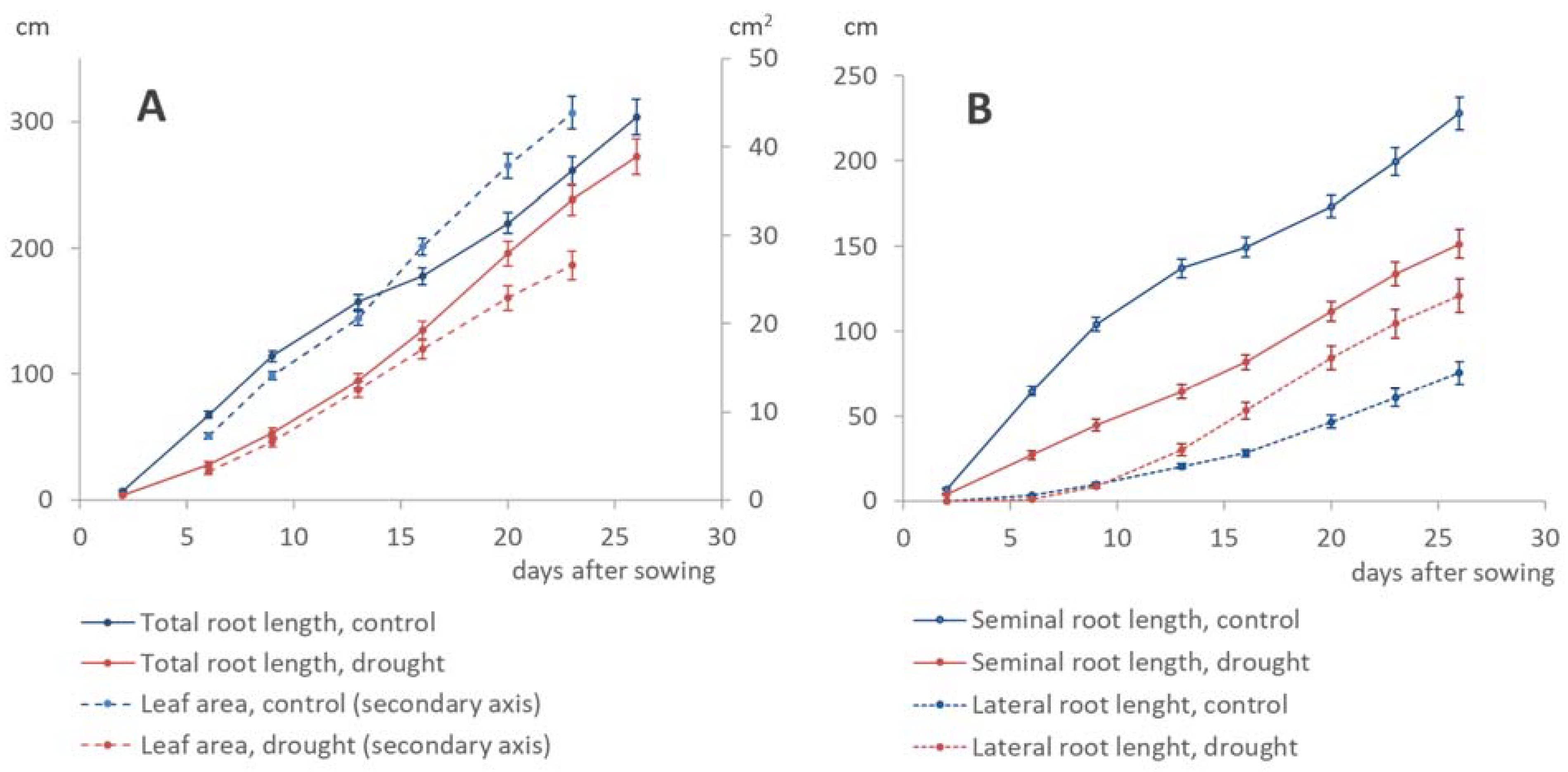
3.1. Control vs Drought
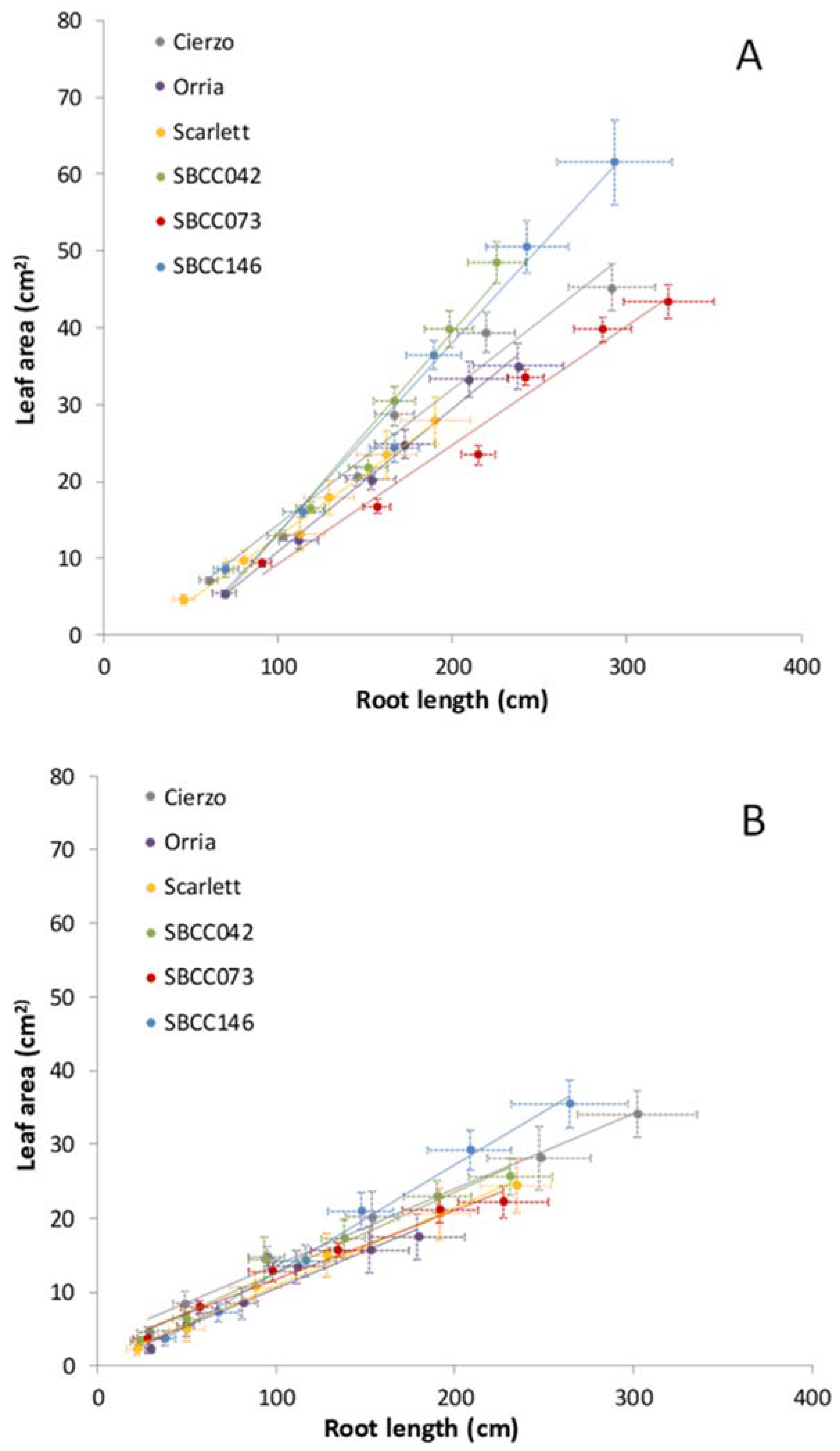
3.2. Genotypic Differences
3.3. Dynamics of Shoot–Root Partitioning
4. Discussion
4.1. Drought Effect on Barley Genotypes
4.2. Low Water Availability Causes Changes in Root System Architecture
4.3. Shoot vs. Root Growth
4.4. Usefulness of Physiological Traits
4.5. Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jana, S.; Wilen, R.W. Breeding for abiotic stress tolerance in barley. In Abiotic Stresses Plant Resistance through Breeding and Molecular Approaches; Ashraf, M., Harris, P.J.C., Eds.; Haworth Press: New York, NY, USA, 2005; pp. 491–511. [Google Scholar]
- Ray, D.K.; West, P.C.; Clark, M.; Gerber, J.S.; Prishchepov, A.V.; Chatterjee, S. Climate change has likely already affected global food production. PLoS ONE 2019, 14, e0217148. [Google Scholar] [CrossRef]
- van Oosterom, E.J.; Ceccarelli, S. Indirect selection for grain yield of barley in harsh Mediterranean environments. Crop Sci. 1993, 33, 1127–1131. [Google Scholar] [CrossRef]
- van Oosterom, E.J.; Kleijn, D.; Ceccarelli, S.; Nachit, M.M. Genotype-by-environment interactions of barley in the Mediterranean region. Crop Sci. 1993, 33, 669–674. [Google Scholar] [CrossRef]
- Voltas, J.; Romagosa, I.; Lafarga, A.; Armesto, A.P.; Sombrero, A.; Araus, J.L. Genotype by environment interaction for grain yield and carbon isotope discrimination of barley in Mediterranean Spain. Aust. J. Agric. Res. 1999, 50, 1263–1271. [Google Scholar] [CrossRef]
- Reynolds, M.; Tuberosa, R. Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 2008, 11, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Grando, S.; Tutwiler, R.; Baha, J.; Martini, A.M.; Salahieh, H.; Goodchild, A.; Michael, M. A methodological study on participatory barley breeding I. Selection phase. Euphytica 2000, 111, 91–104. [Google Scholar] [CrossRef]
- Ceccarelli, S. Positive interpretation of genotype by environment interactions in Relation to sustainability and biodiversity. In Plant Adaptation and Crop Improvement; Cooper, M., Hammer, G.L., Eds.; CABI Publishing: Wallingford, UK, 1996; pp. 467–486. [Google Scholar]
- Yahiaoui, S.; Cuesta-Marcos, A.; Gracia, M.P.; Medina, B.; Lasa, J.M.; Casas, A.M.; Ciudad, F.J.; Montoya, J.L.; Moralejo, M.; Molina-Cano, J.L.; et al. Spanish barley landraces outperform modern cultivars at low productivity sites. Plant Breed. 2014, 133, 218–226. [Google Scholar] [CrossRef]
- Teulat, B.; Zoumarou-Wallis, N.; Rotter, B.; Salem, M.B.; Bahri, H.; This, D. QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theor. Appl. Genet. 2003, 108, 181–188. [Google Scholar] [CrossRef]
- Comadran, J.; Russell, J.R.; Van Eeuwij, F.A.; Ceccarelli, S.; Grando, S.; Baum, M.; Stanca, A.M.; Pecchioni, N.; Mastrangelo, A.M.; Akar, T.; et al. Mapping adaptation of barley to droughted environments. Euphytica 2008, 161, 35–45. [Google Scholar] [CrossRef]
- von Korff, M.; Grando, S.; Del Greco, A.; This, D.; Baum, M.; Ceccarelli, S. Quantitative trait loci associated with adaptation to Mediterranean dryland conditions in barley. Theor. Appl. Genet. 2008, 117, 653–669. [Google Scholar] [CrossRef]
- Li, H.B.; Zhou, M.X.; Liu, C.J. A major QTL conferring crown rot resistance in barley and its association with plant height. Theor. Appl. Genet. 2009, 118, 903–910. [Google Scholar] [CrossRef]
- Silvar, C.; Casas, A.M.; Igartua, E.; Ponce-Molina, L.J.; Gracia, M.P.; Schweizer, G.; Herz, M.; Flath, K.; Waugh, R.; Kopahnke, D.; et al. Resistance to powdery mildew in Spanish barley landraces is controlled by different sets of quantitative trait loci. Theor. Appl. Genet. 2011, 123, 1019–1028. [Google Scholar] [CrossRef]
- Boudiar, R.; Casas, A.M.; Cantalapiedra, C.P.; Gracia, M.P.; Igartua, E. Identification of quantitative trait loci for agronomic traits contributed by a barley (Hordeum vulgare) Mediterranean landrace. Crop Pasture Sci. 2016, 67, 37–46. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Mujeeb-Kazi, A.; Sawkins, M. Prospects for utilising plant-adaptive mechanisms to improve wheat and other crops in drought-and salinity-prone environments. Ann. Appl. Biol. 2005, 146, 239–259. [Google Scholar] [CrossRef]
- López-Castañeda, C.; Richards, R.A.; Farquhar, G.D. Variation in early vigour between barley and wheat. Crop Sci. 1995, 35, 472–479. [Google Scholar] [CrossRef]
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G.; Van Herwaarden, A.F. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci. 2002, 42, 111–121. [Google Scholar] [CrossRef]
- Wang, Y.; Thorup-Kristensen, K.; Jensen, L.S.; Magid, J. Vigorous root growth is a better indicator of early nutrient uptake than root hair traits in spring wheat grown under low fertility. Front. Plant Sci. 2016, 7, 865. [Google Scholar] [CrossRef]
- Lilley, J.M.; Kirkegaard, J.A. Benefits of increased soil exploration by wheat roots. Field Crops Res. 2011, 122, 118–130. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Palta, J.A.; Chen, X.; Milroy, S.P.; Rebetzke, G.J.; Dreccer, M.F.; Watt, M. Large root systems: Are they useful in adapting wheat to dry environments? Funct. Plant Biol. 2011, 38, 347–354. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef]
- Chen, Y.; Palta, J.A.; Wu, P.; Siddique, K.H.M. Crop root systems and rhizosphere interactions. Plant Soil 2019, 439, 1–5. [Google Scholar] [CrossRef]
- Pswarayi, A.; Van Eeuwijk, F.A.; Ceccarelli, S.; Grando, S.; Comadran, J.; Russell, J.R.; Francia, E.; Pecchioni, N.; Li Destri, O.; Akar, T.; et al. Barley adaptation and improvement in the Mediterranean basin. Plant Breed. 2008, 127, 554–560. [Google Scholar] [CrossRef]
- Palta, J.A.; Turner, N.C. Crop root system traits cannot be seen as a silver bullet delivering drought resistance. Plant Soil 2019, 439, 31–43. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Zarebanadkouki, M.; Meunier, F.; Javaux, M.; Kaestner, A.; Carminati, A. Root type matters: Measurement of water uptake by seminal, crown, and lateral roots in maize. J. Exp. Bot. 2018, 69, 1199–1206. [Google Scholar] [CrossRef]
- Gracia, M.P.; Mansour, E.; Casas, A.M.; Lasa, J.M.; Medina, B.; Molina-Cano, J.L.; Moralejo, M.A.; López, A.; López-Fuster, P.; Escribano, J.; et al. Progress in the Spanish National Barley Breeding Program. Span. J. Agric. Res. 2012, 10, 741–751. [Google Scholar] [CrossRef]
- Nagel, K.A.; Putz, A.; Gilmer, F.; Heinz, K.; Fischbach, A.; Pfeifer, J.; Faget, M.; Blossfeld, S.; Ernst, E.; Dimaki, C.; et al. GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Funct. Plant Biol. 2012, 39, 891–904. [Google Scholar] [CrossRef]
- Belachew, K.Y.; Nagel, K.A.; Fiorani, F.; Stoddard, F.L. Diversity in root growth responses to moisture deficit in young faba bean (Vicia faba L.) plants. PeerJ 2018, 6, e4401. [Google Scholar] [CrossRef]
- Nagel, K.A.; Kastenholz, B.; Jahnke, S.; van Dusschoten, D.; Aach, T.; Mühlich, M.; Truhn, D.; Scharr, H.; Terjung, S.; Walter, A.; et al. Temperature responses of roots: Impact on growth, root system architecture and implications for phenotyping. Funct. Plant Biol. 2009, 36, 947–959. [Google Scholar] [CrossRef]
- Fowler, C.W.; Rasmusson, D.C. Leaf area relationships and inheritance in Barley. Crop Sci. 1969, 9, 729–731. [Google Scholar] [CrossRef]
- Payne, R.W.; Murray, D.A.; Harding, S.A.; Baird, D.B.; Soutar, D.M. GenStat for Windows Introduction, 12th ed.; VSN International: Hemel Hempstead, UK, 2009. [Google Scholar]
- Tyagi, K.; Park, M.R.; Lee, H.J.; Lee, C.A.; Rehman, S.; Steffenson, B.; Yun, S.J. Fertile crescent region as source of drought tolerance at early stage of plant growth of wild barley (Hordeum vulgare L. ssp. spontaneum). Pak. J. Bot. 2011, 43, 475–486. [Google Scholar]
- Wu, Y.; Cosgrove, D.J. Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J. Exp. Bot. 2000, 51, 1543–1553. [Google Scholar] [CrossRef]
- Sharp, R.E.; Davies, W.J. Root growth and water uptake by maize plants in drying soil. J. Exp. Bot. 1985, 36, 1441–1456. [Google Scholar] [CrossRef]
- Thornley, J.M. Modelling shoot [ratio] root relations: The only way forward? Ann. Bot. 1998, 81, 165–171. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants. Anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef]
- Polania, J.; Poschenrieder, C.; Rao, I.; Beehe, S. Root traits and their potential links to plant ideotypes to improve drought resistance in common bean. Theor. Exp. Plant Physiol. 2017, 29, 143–154. [Google Scholar] [CrossRef]
- Grando, S.; Ceccarelli, S. Seminal root morphology and coleoptile length in wild (Hordeum vulgare ssp. spontaneum) and cultivated (Hordeum vulgare ssp. vulgare) barley. Euphytica 1995, 86, 73–80. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance and its improvement. In Plant Breeding for Water-Limited Environments; Springer: New York, NY, USA, 2011; pp. 53–152. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; García-Pereira, M.J.; Gracia, M.P.; Igartua, E.; Casas, A.M.; Contreras-Moreira, B. Large differences in gene expression responses to drought and heat stress between elite barley cultivar Scarlett and a Spanish landrace. Front. Plant Sci. 2017, 8, 647. [Google Scholar] [CrossRef]
- van Oosterom, E.J.; Acevedo, E. Adaptation of barley (Hordeum vulgare L.) to harsh Mediterranean environments. Euphytica 1992, 62, 1–14. [Google Scholar] [CrossRef]
- Peleg, Z.; Fahima, T.; Abbo, S.; Krugman, T.; Nevo, E.; Yakir, D.; Sarange, Y. Genetic diversity for drought resistance in wild emmer wheat and its ecogeographical associations. Plant Cell Environ. 2005, 28, 176–191. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Nakhforoosh, A.; Bodewein, T.; Fiorani, F.; Bodner, G. Identification of water use strategies at early growth stages in durum wheat from shoot phenotyping and physiological measurements. Front. Plant Sci. 2016, 7, 1155. [Google Scholar] [CrossRef]
- Jin, Y.; He, J.; Turner, N.C.; Du, Y.L.; Li, F.M. Water-conserving and biomass-allocation traits are associated with higher yields in modern cultivars compared to landraces of soybean [Glycine max (L.) Merr.] in rainfed water-limited environments. Environ. Exp. Bot. 2019, 168, 103883. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Kuhlmann, H.; Weir, A.H. The effects of prolonged drought and nitrogen fertilizer on root and shoot growth and water uptake by winter wheat. J. Agron. Crop Sci. 1989, 163, 352–360. [Google Scholar] [CrossRef]
- Friedli, C.N.; Abiven, S.; Fossati, D.; Hund, A. Modern wheat semi-dwarfs root deep on demand: Response of rooting depth to drought in a set of Swiss era wheats covering 100 years of breeding. Euphytica 2019, 215, 85. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Pei, D.; Chen, S.Y. Root growth and soil water utilization of winter wheat in the North China Plain. Hydrol. Process. 2004, 18, 2275–2287. [Google Scholar] [CrossRef]
- Li, F.M.; Liu, X.L.; Li, S.Q. Effects of early soil water distribution on the dry matter partition between roots and shoots of winter wheat. Agric. Water Manag. 2001, 49, 163–171. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Fisher, M.C.; Eissenstat, D.M.; Lynch, J.P. Lack of evidence for programmed root senescence in common bean (Phaseolus vulgaris) grown at different levels of phosphorus supply. New Phytol. 2002, 153, 63–71. [Google Scholar] [CrossRef]
- El Hassouni, K.; Alahmad, S.; Belkadi, B.; Filali-Maltouf, A.; Hickey, L.T.; Bassi, F.M. Root system architecture and its association with yield under different water regimes in durum wheat. Crop Sci. 2018, 58, 2331–2346. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Belford, R.K.; Tennant, D. Root:Shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant Soil 1990, 121, 89–98. [Google Scholar] [CrossRef]
- Fang, Y.; Du, Y.; Wang, J.; Wu, A.; Qiao, S.; Xu, B.; Chen, Y. Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 2017, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Mathew, I.; Shimelis, H.; Mutema, M.; Clulow, A.; Zengeni, R.; Mbava, N.; Chaplot, V. Selection of wheat genotypes for biomass allocation to improve drought tolerance and carbon sequestration into soils. J. Agron. Crop Sci. 2019, 295, 385–400. [Google Scholar] [CrossRef]
- Monteagudo, A.; Casas, A.M.; Cantalapiedra, C.P.; Contreras-Moreira, B.; Gracia, M.P.; Igartua, E. Harnessing novel diversity from landraces to improve an elite barley variety. Front. Plant Sci. 2019, 10, 434. [Google Scholar] [CrossRef]
- Meier, I.C.; Knutzen, F.; Eder, L.M.; Müller-Haubold, H.; Goebel, M.O.; Bachmann, J.; Dietrich, H.; Leuschner, C. The deep root system of Fagus sylvatica on sandy soil: Structure and variation across a precipitation gradient. Ecosystems 2018, 21, 280–296. [Google Scholar] [CrossRef]
- Ruggiero, C.; Angelino, G. Changes of root hydraulic conductivity and root/shoot ratio of durum wheat and barley in relation to nitrogen availability and mercury exposure. Ital. J. Agron. 2007, 3, 281–290. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Ehdaie, B.; Mohammadi, S.A.; Nouraein, M. QTLs for root traits at mid-tillering and for root and shoot traits at maturity in a RIL population of spring bread wheat grown under well-watered conditions. Euphytica 2016, 211, 17–38. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Y.; Gruber, B.D.; Neumann, K.; Kilian, B.; Graner, A.; Von Wirén, N. Genetic dissection of root system architectural traits in spring barley. Front. Plant Sci. 2019, 10, 400. [Google Scholar] [CrossRef]
- Abdel-Ghani, A.H.; Sharma, R.; Wabila, C.; Dhanagond, S.; Owais, S.J.; Duwayri, M.A.; Al-Dalain, S.; Klukas, C.; Chen, D.; Lübberstedt, T.; et al. Genome-wide association mapping in a diverse spring barley collection reveals the presence of QTL hotspots and candidate genes for root and shoot architecture traits at seedling stage. BMC Plant Biol. 2019, 19, 216. [Google Scholar] [CrossRef]
- Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 2010, 129, 630–636. [Google Scholar] [CrossRef]
- Ramireddy, E.; Hosseini, S.A.; Eggert, K.; Gillandt, S.; Gnad, H.; von Wirén, N.; Schmülling, T. Root engineering in barley: Increasing cytokinin degradation produces a larger root system, mineral enrichment in the shoot and improved drought tolerance. Plant Physiol. 2018, 177, 1078–1095. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Baum, M.; Varshney, R.K.; Graner, A.; Grando, S.; Ceccarelli, S. QTLs for chlorophyll and chlorophyll fluorescence parameters in barley under post-flowering drought. Euphytica 2008, 163, 203–214. [Google Scholar] [CrossRef]
- del Pozo, A.; Castillo, D.; Inostroza, L.; Matus, I.; Méndez, A.M.; Morcuende, R. Physiological and yield responses of recombinant chromosome substitution lines of barley to terminal drought in a Mediterranean-type environment. Ann. Appl. Biol. 2012, 160, 157–167. [Google Scholar] [CrossRef]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; von Korff, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Honsdorf, N.; March, T.J.; Hecht, A.; Eglinton, J.; Pillen, K. Evaluation of juvenile drought stress tolerance and genotyping by sequencing with wild barley introgression lines. Mol Breed. 2014, 34, 1475–1495. [Google Scholar] [CrossRef]
- Araus, J.L.; Amaro, T.; Voltas, J.; Nakkoul, H.; Nachit, M.M. Chlorophyll fluorescence as a selection criterion for grain yield in durum wheat under Mediterranean conditions. Field Crops Res. 1998, 55, 209–223. [Google Scholar] [CrossRef]
- Filek, M.; Łabanowska, M.; Kościelniak, J.; Biesaga-Kościelniak, J.; Kurdziel, M.; Szarejko, I.; Hartikainen, H. Characterization of barley leaf tolerance to drought stress by chlorophyll fluorescence and electron paramagnetic resonance studies. J. Agron. Crop Sci. 2015, 201, 228–240. [Google Scholar] [CrossRef]

| Source of Variation | df | TN | LN | LA | F0 | Fv | Fm | Fv/Fm | SPAD | SFW |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | 1 | ** | *** | ** | * | ** | * | *** | ns | *** |
| Genotypes | 5 | *** | ** | *** | ns | ns | ns | ** | *** | *** |
| Type (cultivars vs landraces) | 1 | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Genotype × Treatment | 5 | ns | ns | * | ns | ns | ns | ns | ns | ns |
| Type × Treatment | 1 | ns | *** | * | ns | ** | ** | * | ns | ns |
| df | SDW | RDW | S/R | RSD | RSW | TRL | SRL | LRL | ||
| Treatments | 1 | *** | ns | ** | *** | * | ns | * | * | |
| Genotypes | 5 | *** | *** | *** | *** | ** | *** | *** | ** | |
| Type (cultivars vs landraces) | 1 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Genotype × Treatment | 5 | * | * | ns | ns | ns | ns | ns | ns | |
| Type × Treatment | 1 | * | ns | ns | ns | ns | ns | ns | ns |
| TN | LN | LA | F0 | Fv | Fm | Fv/Fm | SPAD | SFW | SDW | RDW | S/R | RSD | RSW | TRL | SRL | LRL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||||||||||
| Cierzo | 2.09a | 5.74bc | 45.1b | 321.1a | 853.9a | 1175a | 0.727b | 37.68bc | 2.027b | 0.232a | 0.064bc | 3.64bc | 51.04ab | 15.11a | 354.7a | 250.3ab | 107.0 |
| Orria | 1.17b | 5.43c | 34.9cd | 286.8a | 833.6a | 1124a | 0.742b | 39.30b | 1.432cd | 0.166b | 0.052c | 3.16cd | 43.33b | 11.79a | 263.0b | 228.2ab | 35.4c |
| Scarlett | 1.96a | 6.07bc | 27.9d | 313.4a | 838.5a | 1152a | 0.728b | 37.74bc | 1.177d | 0.149b | 0.035d | 4.39a | 46.41b | 14.41a | 214.0b | 147.6c | 65.4abc |
| SBCC042 | 2.57a | 7.62a | 48.5b | 316.6a | 870.8a | 1187a | 0.734b | 37.78bc | 1.922b | 0.242a | 0.065bc | 3.70bc | 45.85b | 12.26a | 263.1b | 201.9b | 59.6bc |
| SBCC073 | 2.29a | 6.68ab | 43.7bc | 288.1a | 989.8a | 1277a | 0.775a | 44.38a | 1.667bc | 0.249a | 0.091a | 2.85d | 47.89b | 12.98a | 364.8a | 270.2a | 95.2ab |
| SBCC146 | 2.25a | 6.86ab | 61.4a | 306.4a | 853.5a | 1160a | 0.736b | 33.50c | 2.496a | 0.281a | 0.068b | 4.18ab | 58.61a | 14.34a | 351.9a | 266.1a | 83.9ab |
| Cultivars | 1.74A | 5.75B | 36.0A+ | 307.1A | 842.0A+ | 1150A+ | 0.732A+ | 38.24A | 1.545A | 0.182B | 0.050B | 3.73A | 46.93A | 13.77A | 277.2A | 208.7A | 69.3A |
| Landraces | 2.37A | 7.05A | 51.2A+ | 303.7A | 904.7A+ | 1208A+ | 0.748A+ | 38.55A | 2.028A | 0.257A | 0.075A | 3.58A | 50.78A | 13.19A | 326.6A | 246.1A | 79.6A |
| Drought | |||||||||||||||||
| Cierzo | 1.14abc | 3.96a | 34.0ab | 286.5a | 986.2a | 1270a | 0.777ab | 34.84bc | 1.116a | 0.155a | 0.054ab | 2.70ab | 27.25a | 14.90a | 336.3a | 176.2a | 161.8a |
| Orria | 0.39c | 3.49a | 17.7c | 270.5a | 941.5a | 1212a | 0.777ab | 39.18ab | 0.546a | 0.083b | 0.033c | 2.44ab | 19.95a | 08.83c | 202.1b | 126.9a | 74.4c |
| Scarlett | 0.89bc | 4.26a | 24.3abc | 282.1a | 1006.0 a | 1284a | 0.783b | 41.94a | 0.893a | 0.125ab | 0.040bc | 3.12a | 28.12a | 14.16ab | 283.5ab | 151.3a | 129.4ab |
| SBCC042 | 1.95a | 4.29a | 25.8abc | 252.4a | 900.8a | 1154a | 0.781ab | 40.68a | 0.951a | 0.123ab | 0.054ab | 2.37b | 21.22a | 10.38bc | 269.9ab | 136.1a | 134.3ab |
| SBCC073 | 0.65bc | 3.03a | 21.2bc | 268.6a | 999.6a | 1261a | 0.793a | 43.59a | 0.732a | 0.118ab | 0.052abc | 2.29b | 18.20a | 09.94bc | 236.7b | 146.1a | 91.6bc |
| SBCC146 | 1.27ab | 3.76a | 35.6a | 300.4a | 880.4a | 1180a | 0.746b | 32.63c | 1.056a | 0.144a | 0.057a | 2.59ab | 33.09a | 12.78abc | 293.7ab | 174.1a | 123.0abc |
| Cultivars | 0.81A | 3.90A | 25.3A+ | 279.7A | 977.9A+ | 1255A+ | 0.779A+ | 38.65A | 0.852A | 0.121A | 0.042A | 3.12A | 25.11A | 12.63A | 2740A | 151.5A | 121.9A |
| Landraces | 1.29A | 3.69A | 27.5A+ | 273.8A | 926.9A+ | 1198A+ | 0.773A+ | 38.97A | 0.913A | 0.128A | 0.054A | 2.42A | 24.17A | 11.03A | 266.8A | 152.1A | 116.3A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudiar, R.; Casas, A.M.; Gioia, T.; Fiorani, F.; Nagel, K.A.; Igartua, E. Effects of Low Water Availability on Root Placement and Shoot Development in Landraces and Modern Barley Cultivars. Agronomy 2020, 10, 134. https://doi.org/10.3390/agronomy10010134
Boudiar R, Casas AM, Gioia T, Fiorani F, Nagel KA, Igartua E. Effects of Low Water Availability on Root Placement and Shoot Development in Landraces and Modern Barley Cultivars. Agronomy. 2020; 10(1):134. https://doi.org/10.3390/agronomy10010134
Chicago/Turabian StyleBoudiar, Ridha, Ana M. Casas, Tania Gioia, Fabio Fiorani, Kerstin A. Nagel, and Ernesto Igartua. 2020. "Effects of Low Water Availability on Root Placement and Shoot Development in Landraces and Modern Barley Cultivars" Agronomy 10, no. 1: 134. https://doi.org/10.3390/agronomy10010134
APA StyleBoudiar, R., Casas, A. M., Gioia, T., Fiorani, F., Nagel, K. A., & Igartua, E. (2020). Effects of Low Water Availability on Root Placement and Shoot Development in Landraces and Modern Barley Cultivars. Agronomy, 10(1), 134. https://doi.org/10.3390/agronomy10010134






