High-Temperature Hay Biochar Application into Soil Increases N2O Fluxes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Biochar
2.3. Fertilizers
2.4. Cover Crop
2.5. Flux Measurements
2.6. Fourier Transformed Infrared Spectrophotometer (FTIR) Analysis
2.7. Statistical Analysis
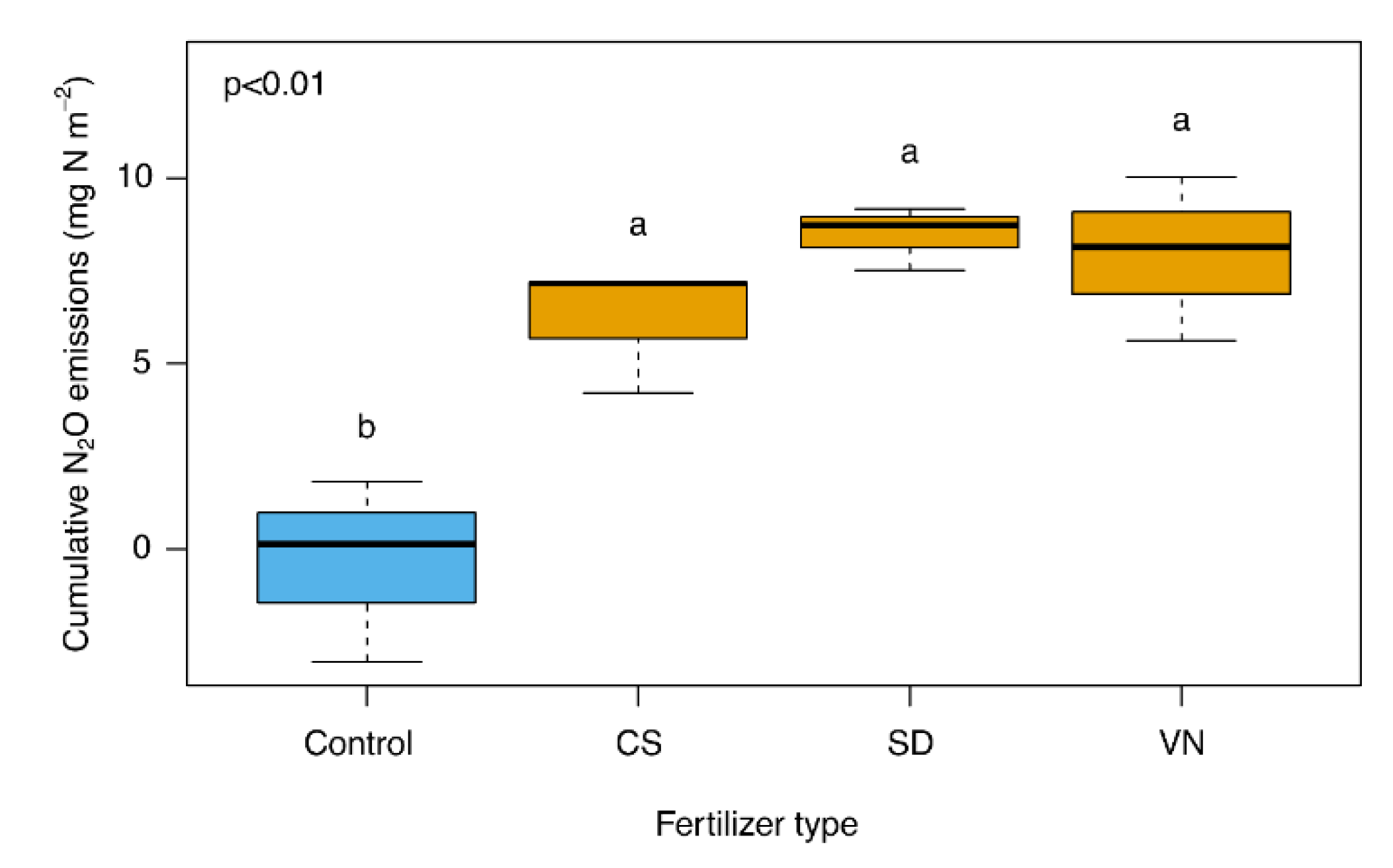
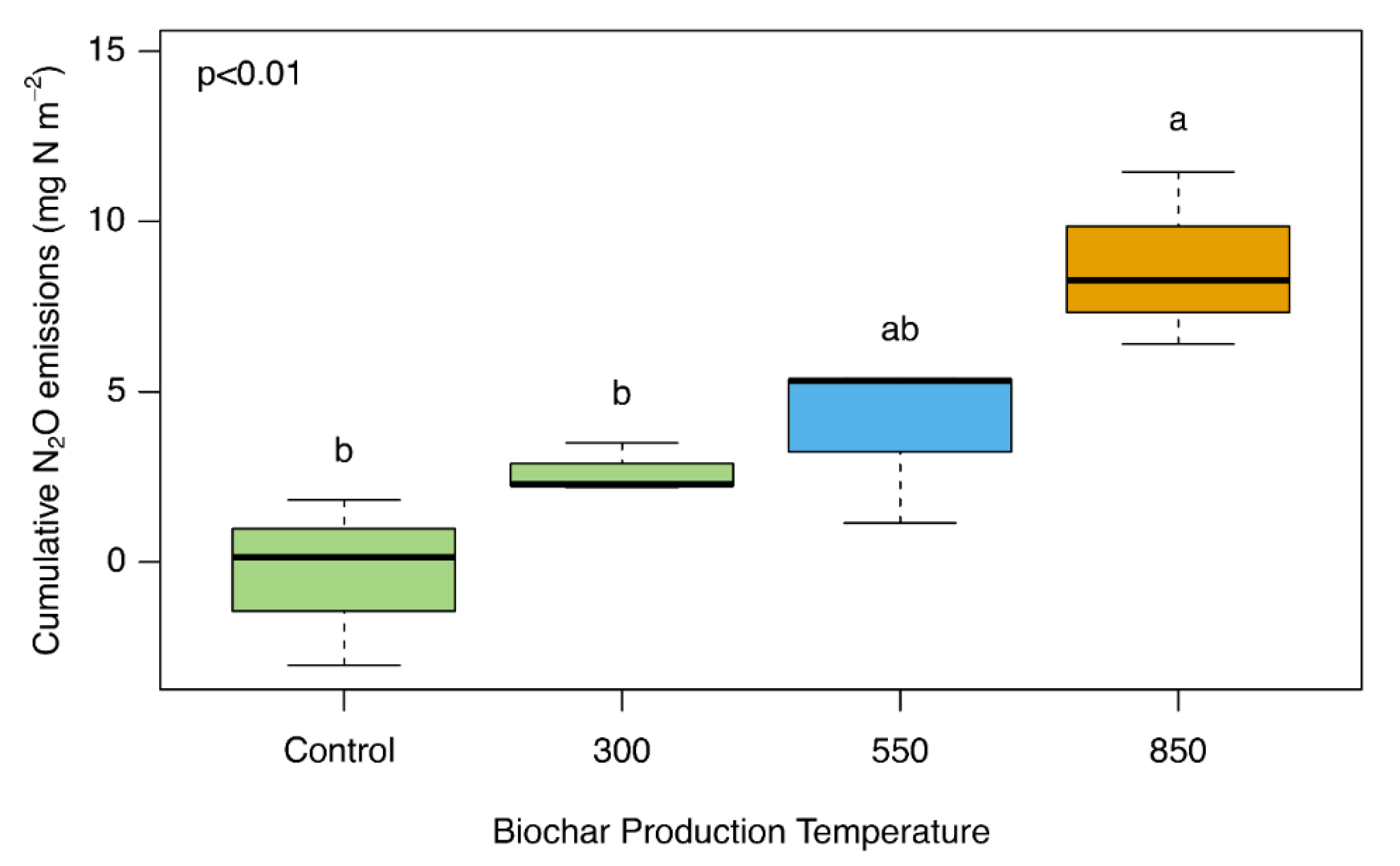
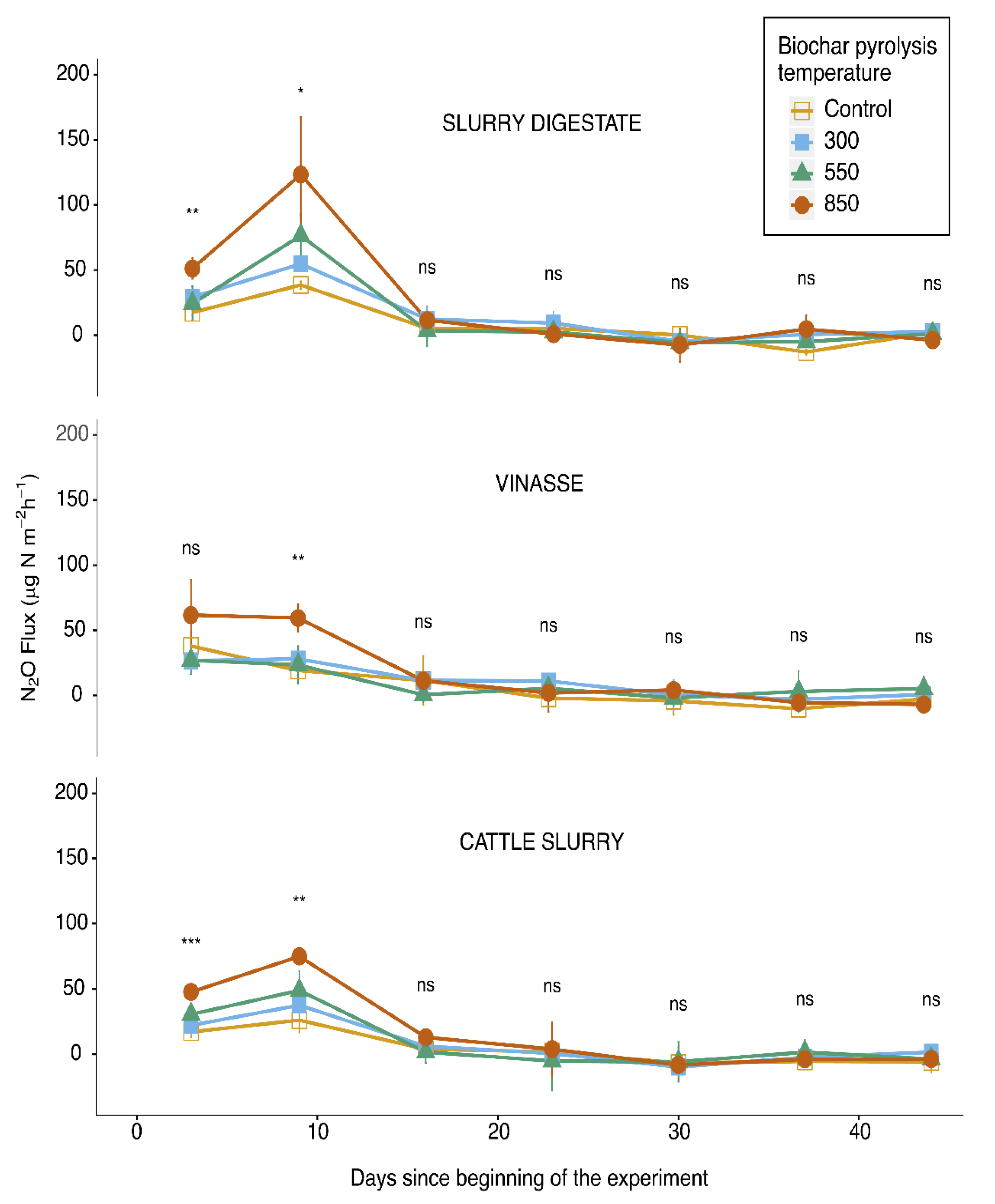
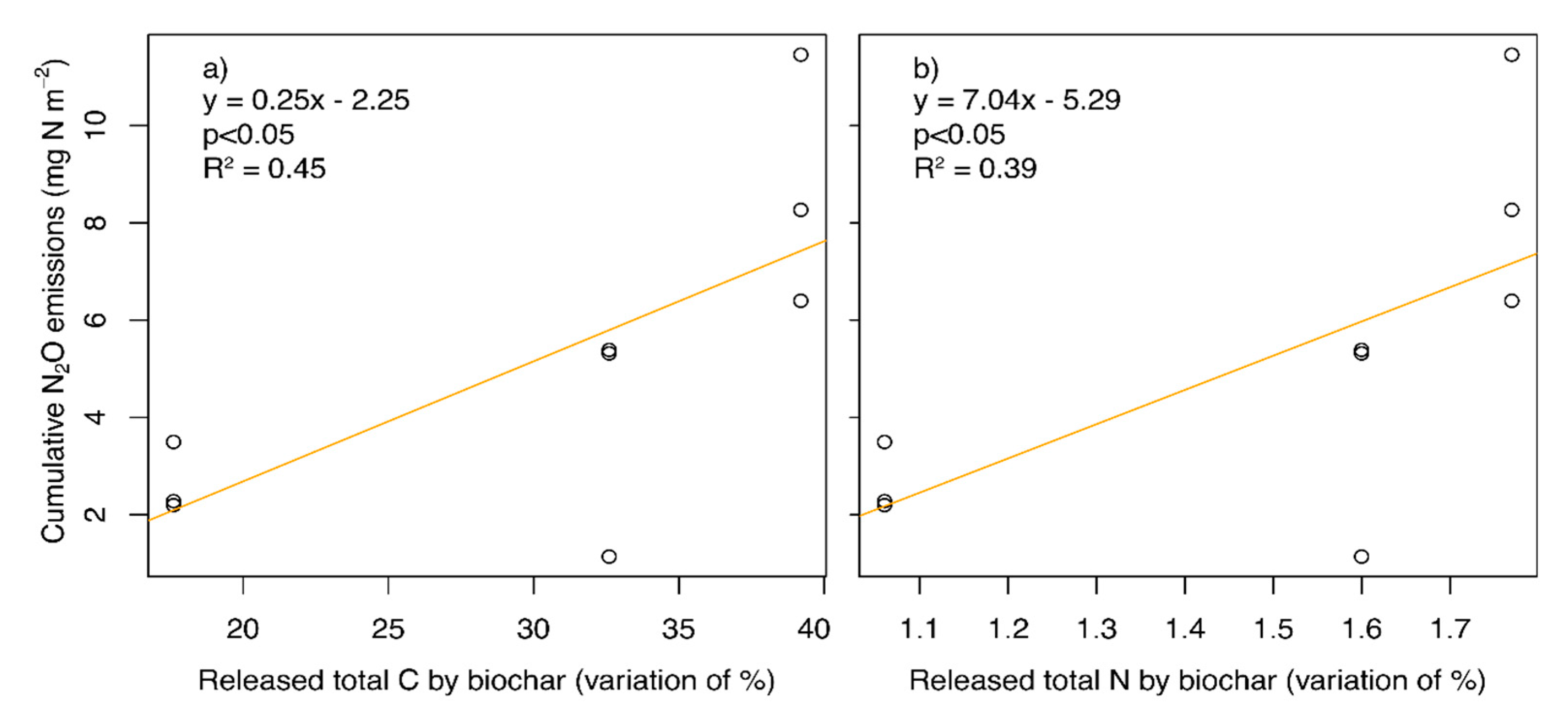
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNFCCC. Adoption of the Paris Agreement. FCCC/CP/2015/L.9/Rev.1. In Proceedings of the United Nations Framework Convention on Climate Change: 21st Conference of the Parties, Paris, France, 30 November–11 December 2015. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Meyer, L.A., Pachauri, R.K., Eds.; Core Writing Team: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Ussiri, D.; Lal, R. Soil Emission of Nitrous Oxide and Its Mitigation; Springer: Dordrecht, The Netherlands, 2013; Volume XVIII, p. 378. [Google Scholar]
- Park, S.; Croteau, P.; Boering, K.A.; Etheridge, D.M.; Ferretti, D.; Fraser, P.J.; Kim, K.R.; Krummel, P.B.; Langenfelds, R.L.; van Ommen, T.D.; et al. Trends and seasonal cycles in the isotopic composition of nitrous oxide since 1940. Nat. Geosci. 2012, 5, 261–265. [Google Scholar] [CrossRef]
- Firestone, M.K.; Davidson, E.A. Microbiological Basis of NO and N2O Production and Consumption in Soils. In Exchange of Trace Gases between Terrestrial Ecosystems and the Atmosphere; Andreae, M.O., Schimel, D.S., Eds.; John Willey and Sons: New York, NY, USA, 1989; pp. 7–21. [Google Scholar]
- Braker, G.; Conrad, R. Diversity, Structure, and Size of N2O-Producing Microbial Communities in Soils—What Matters for Their Functioning? In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2011; Volume 75, pp. 33–70. [Google Scholar]
- Shen, J.; Treu, R.; Wang, J.; Nicholson, F.; Bhogal, A.; Thorman, R. Modeling nitrous oxide emissions from digestate and slurry applied to three agricultural soils in the United Kingdom: Fluxes and emission factors. Environ. Pollut. 2018, 243, 1952–1965. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.K.; Rees, R.M.; Skiba, U.M.; Ball, B.C. Influence of organic and mineral N fertiliser on N2O fluxes from a temperate grassland. Agric. Ecosyst. Environ. 2007, 121, 74–83. [Google Scholar] [CrossRef]
- van Groenigen, J.W.; Kasper, G.J.; Velthof, G.L.; van den Pol-van Dasselaar, A.; Kuikman, P.J. Nitrous oxide emissions from silage maize fields under different mineral nitrogen fertilizer and slurry applications. Plant Soil 2004, 263, 101–111. [Google Scholar] [CrossRef]
- Marchaim, U. Biogas Processes for Sustainable Development; FAO: Rome, Italy, 1992. [Google Scholar]
- Ciborowski, P. Anaerobic Digestion of Livestock Manure for Pollution Control and Energy Production: A Feasibility Assessment; Minnesota Pollution Control Agency: St. Paul, MN, USA, 2001; p. 233.
- Petersen, S.O. Greenhouse gas emissions from liquid dairy manure: Prediction and mitigation. J. Dairy Sci. 2018, 101, 6642–6654. [Google Scholar] [CrossRef]
- Holly, M.A.; Larson, R.A.; Powell, J.M.; Ruark, M.D.; Aguirre-Villegas, H. Greenhouse gas and ammonia emissions from digested and separated dairy manure during storage and after land application. Agric. Ecosyst. Environ. 2017, 239, 410–419. [Google Scholar] [CrossRef]
- Lourenco, K.S.; Rossetto, R.; Vitti, A.C.; Montezano, Z.F.; Soares, J.R.; Sousa, R.M.; do Carmo, J.B.; Kuramae, E.E.; Cantarella, H. Strategies to mitigate the nitrous oxide emissions from nitrogen fertilizer applied with organic fertilizers in sugarcane. Sci. Total Environ. 2019, 650, 1476–1486. [Google Scholar] [CrossRef]
- Carmo, J.B.D.; Filoso, S.; Zotelli, L.C.; de Sousa Neto, E.R.; Pitombo, L.M.; Duarte-Neto, P.J.; Vargas, V.P.; Andrade, C.A.; Gava, G.J.C.; Rossetto, R.; et al. Infield greenhouse gas emissions from sugarcane soils in Brazil: Effects from synthetic and organic fertilizer application and crop trash accumulation. GCB Bioenergy 2013, 5, 267–280. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009. [Google Scholar]
- Sohi, S.P. Carbon storage with benefits. Science 2012, 338, 1034–1035. [Google Scholar] [CrossRef]
- Crane-Droesch, A.; Abiven, S.; Jeffery, S.; Torn, M.S. Heterogeneous global crop yield response to biochar: A meta-regression analysis. Environ. Res. Lett. 2013, 8. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Monnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Liu, B.; Amonette, J.E.; Lin, Z.; Liu, G.; Ambus, P.; Xie, Z. How does biochar influence soil N cycle? A meta-analysis. Plant Soil 2018, 426, 211–225. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Scheer, C.; Grace, P.R.; Rowlings, D.W.; Kimber, S.; Van Zwieten, L. Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant Soil 2011, 345, 47–58. [Google Scholar] [CrossRef]
- Suddick, E.C.; Six, J. An estimation of annual nitrous oxide emissions and soil quality following the amendment of high temperature walnut shell biochar and compost to a small scale vegetable crop rotation. Sci. Total Environ. 2013, 465, 298–307. [Google Scholar] [CrossRef]
- Rittl, T.F.; Butterbach-Bahl, K.; Basile, C.M.; Pereira, L.A.; Alms, V.; Dannenmann, M.; Couto, E.G.; Cerri, C.E.P. Greenhouse gas emissions from soil amended with agricultural residue biochars: Effects of feedstock type, production temperature and soil moisture. Biomass Bioenergy 2018, 117, 1–9. [Google Scholar] [CrossRef]
- Buchkina, N.P.; Hüppi, R.; Leifeld, J. Biochar and short-term N2O and CO2 emission from plant residue-amended soil with different fertilisation history. Zemdirb. Agric. 2019, 106, 99–106. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Singh, B.; Joseph, S.; Kimber, S.; Cowie, A.; Chan, K.Y. Biochar and Emissions of Non-CO2 Greenhouse Gases from Soil. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2009. [Google Scholar] [CrossRef]
- Clough, T.J.; Bertram, J.E.; Ray, J.L.; Condron, L.M.; O’Callaghan, M.; Sherlock, R.R.; Wells, N.S. Unweathered Wood Biochar Impact on Nitrous Oxide Emissions from a Bovine-Urine-Amended Pasture Soil. Soil Sci. Soc. Am. J. 2010, 74, 852–860. [Google Scholar] [CrossRef]
- Verhoeven, E.; Six, J. Biochar does not mitigate field-scale N2O emissions in a Northern California vineyard: An assessment across two years. Agric. Ecosyst. Environ. 2014, 191, 27–38. [Google Scholar] [CrossRef]
- Spokas, K.A.; Reicosky, D.C. Impacts of Sixteen Different Biochars on Soil Greenhouse Gas Production. Ann. Environ. Sci. 2009, 3, 179–193. [Google Scholar]
- Malghani, S.; Gleixner, G.; Trumbore, S.E. Chars produced by slow pyrolysis and hydrothermal carbonization vary in carbon sequestration potential and greenhouse gases emissions. Soil Biol. Biochem. 2013, 62, 137–146. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Roig, A.; Sánchez-Monedero, M.A.; Cayuela, M.L. Biochar increases soil N2O emissions produced by nitrification-mediated pathways. Front. Environ. Sci. 2014, 2, 25. [Google Scholar] [CrossRef]
- Christel, W.; Zhu, K.; Hoefer, C.; Kreuzeder, A.; Santner, J.; Bruun, S.; Magid, J.; Jensen, L.S. Spatiotemporal dynamics of phosphorus release, oxygen consumption and greenhouse gas emissions after localised soil amendment with organic fertilisers. Sci. Total Environ. 2016, 554–555, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, H.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef] [PubMed]
- Harter, J.; Krause, H.M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Hüppi, R.; Felber, R.; Neftel, A.; Six, J.; Leifeld, J. Effect of biochar and liming on soil nitrous oxide emissions from a temperate maize cropping system. Soil 2015, 1, 707–717. [Google Scholar] [CrossRef]
- Obia, A.; Cornelissen, G.; Mulder, J.; Dorsch, P. Effect of Soil pH Increase by Biochar on NO, N2O and N2 Production during Denitrification in Acid Soils. PLoS ONE 2015, 10, e0138781. [Google Scholar] [CrossRef]
- Bird, M.I.; Wynn, J.G.; Saiz, G.; Wurster, C.M.; McBeath, A. The Pyrogenic Carbon Cycle. Annu. Rev. Earth Planet. Sci. 2015, 43, 273–298. [Google Scholar] [CrossRef]
- Santin, C.; Doerr, S.H.; Merino, A.; Bucheli, T.D.; Bryant, R.; Ascough, P.; Gao, X.; Masiello, C.A. Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars. Sci. Rep. 2017, 7, 11233. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Novak, J.M.; Busscher, W.J.; Ahmedna, M.; Rehrah, D.; Watts, D.W. Switchgrass biochar affects two aridisols. J. Environ. Qual. 2012, 41, 1123–1130. [Google Scholar] [CrossRef]
- Clough, T.; Condron, L.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Feng, Z.; Zhu, L. Impact of biochar on soil N2O emissions under different biochar-carbon/fertilizer-nitrogen ratios at a constant moisture condition on a silt loam soil. Sci. Total Environ. 2017, 584–585, 776–782. [Google Scholar] [CrossRef]
- Tybirk, K.; Luostarinen, S.; Hamelin, L.; Rodhe, L.; Haneklaus, S.; Poulsen, H.D.; Jensen, A.L.S. Sustainable Manure Management in the Baltic Sea Region; Jokioinen, Finland, 2013; Available online: http://eu.baltic.net/Project_Database.5308.html?contentid=58&contentaction=single (accessed on 1 October 2019).
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Biomass torrefaction: Modeling of volatile and solid product evolution kinetics. Bioresour. Technol. 2012, 124, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.; Bucheli, T. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef]
- Raave, H.; Escuer-Gatius, J.; Kauer, K.; Shanskiy, M.; Tõnutare, T.; Astover, A. Pyrolysis temperature influence on nutrient release from hay biochar and on the biochar impact on perennial ryegrass nutrient uptake and yield. Manuscript submitted for publication.
- Hutchinson, G.L.; Livingston, G.P. Use of chamber systems to measure trace gas fluxes. In Agricultural Ecosystem Effects on Trace Gases and Global Climate Change; Harper, L.A., Mosier, A.R., Duxbury, J.M., Rolston, D.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1993; pp. 63–78. [Google Scholar] [CrossRef]
- Järveoja, J.; Peichl, M.; Maddison, M.; Soosaar, K.; Vellak, K.; Karofeld, E.; Teemusk, A.; Mander, Ü. Impact of water table level on annual carbon and greenhouse gas balances of a restored peat extraction area. Biogeosciences 2016, 13, 2637–2651. [Google Scholar] [CrossRef]
- Loftfield, N.; Flessa, H.; Augustin, J.; Beese, F. Automated Gas Chromatographic System for Rapid Analysis of the Atmospheric Trace Gases Methane, Carbon Dioxide, and Nitrous Oxide. J. Environ. Qual. 1997, 26, 560–564. [Google Scholar] [CrossRef]
- Livingston, G.P.; Hutchinson, G.L. Enclosure-based measurement of trace gas exchange: Applications and sources of error. In Biogenic Trace Gases: Measuring Emissions from Soil and Water; Matson, P.A., Harriss, R.C., Eds.; Blackwell Publishing: Oxford, UK, 1995; pp. 14–51. [Google Scholar]
- Parkin, T.B.; Venterea, R.T.; Follett, R.F. Chapter 3. Chamber-Based Trace Gas Flux Measurements; USDA-ARS: Beltsville, MD, USA, 2010.
- Vinzent, B.; Fuß, R.; Maidl, F.-X.; Hülsbergen, K.-J. N2O emissions and nitrogen dynamics of winter rapeseed fertilized with different N forms and a nitrification inhibitor. Agric. Ecosyst. Environ. 2018, 259, 86–97. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Mendiburu, F.D. Agricolae: Statistical Procedures for Agricultural Research; R Package Version 1.2-3; The Comprehensive R Archive Network: Vienna, Austria, 2015. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Publishing Company: Reading, MA, USA, 1977. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Song, K.; Zhang, H.; Wu, Q.; Zhang, Z.; Zhou, C.; Zhang, Q.; Lei, T. Structure and thermal properties of tar from gasification of agricultural crop residue. J. Therm. Anal. Calorim. 2014, 119, 27–35. [Google Scholar] [CrossRef]
- Horikawa, Y.; Hirano, S.; Mihashi, A.; Kobayashi, Y.; Zhai, S.; Sugiyama, J. Prediction of Lignin Contents from Infrared Spectroscopy: Chemical Digestion and Lignin/Biomass Ratios of Cryptomeria japonica. Appl. Biochem. Biotechnol. 2019, 188, 1066–1076. [Google Scholar] [CrossRef]
- Firestone, M.K.; Tiedje, J.M. Temporal change in nitrous oxide and dinitrogen from denitrification following onset of anaerobiosis. Appl. Environ. Microbiol. 1979, 38, 673–679. [Google Scholar] [CrossRef]
- Clemens, J.; Huschka, A. The effect of biological oxygen demand of cattle slurry and soil moisture on nitrous oxide emissions. Nutr. Cycl. Agroecosystems 2001, 59, 193–198. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Jørgensen, S.E. Process Chemistry and Biochemistry of Nitrification. In Studies in Environmental Science; Halling-Sørensen, B., Jørgensen, S.E., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1993; Volume 54, pp. 55–118. [Google Scholar]
- Simek, M.; Cooper, J.E. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Zhang, S.; Feng, K.; Yan, F.; Schubert, S. Effect of pH on denitrification losses from different arable soils. Plant Nutr. 2001, 962–963. [Google Scholar] [CrossRef]
- Anderson, C.R.; Peterson, M.E.; Frampton, R.A.; Bulman, S.R.; Keenan, S.; Curtin, D. Rapid increases in soil pH solubilise organic matter, dramatically increase denitrification potential and strongly stimulate microorganisms from the Firmicutes phylum. PeerJ 2018, 6, e6090. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.S.; Parsons, L.L. Persistence of Denitrifying Enzyme Activity in Dried Soils. Appl. Environ. Microbiol. 1985, 49, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the Dynamics of Dissolved Organic Matter in Soils: A Review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Lundquist, E.J.; Jackson, L.E.; Scow, K.M.; Hsu, C. Changes in microbial biomass and community composition, and soil carbon and nitrogen pools after incorporation of rye into three California agricultural soils. Soil Biol. Biochem. 1999, 31, 221–236. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Petersen, S.O. Nitrous Oxide Emissions from Manure and Inorganic Fertilizers Applied to Spring Barley. J. Environ. Qual. 1999, 28, 1610–1618. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, W.; Luo, Y.; Yu, H.; Xu, Y.; Müller, C.; Xu, X.; Zhu, T. Nitrous oxide emissions from cultivated black soil: A case study in Northeast China and global estimates using empirical model. Glob. Biogeochem. Cycles 2014, 28, 1311–1326. [Google Scholar] [CrossRef]
- Yamulki, S.; Harrison, R.M.; Goulding, K.W.T.; Webster, C.P. N2O, NO and NO2 fluxes from a grassland: Effect of soil pH. Soil Biol. Biochem. 1997, 29, 1199–1208. [Google Scholar] [CrossRef]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J.; Hardjono, A. Effects of phosphorus addition on N2O and NO emissions from soils of an Acacia mangium plantation. Soil Sci. Plant Nutr. 2010, 56, 782–788. [Google Scholar] [CrossRef]
- Dancer, W.S.; Peterson, L.A.; Chesters, G. Ammonification and Nitrification of N as Influenced by Soil pH and Previous N Treatments1. Soil Sci. Soc. Am. J. 1973, 37, 67–69. [Google Scholar] [CrossRef]
- Haynes, R.J.; Swift, R.S. Effect of rewetting air-dried soils on pH and accumulation of mineral nitrogen. J. Soil Sci. 1989, 40, 341–347. [Google Scholar] [CrossRef]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars. Environ. Sci. Pollut. Res. Int. 2018, 25, 25638–25647. [Google Scholar] [CrossRef] [PubMed]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 17627. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lehmann, J.; Hanley, K.; Hestrin, R.; Enders, A. Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere 2015, 138, 120–126. [Google Scholar] [CrossRef]
- Wang, B.; Lehmann, J.; Hanley, K.; Hestrin, R.; Enders, A. Ammonium retention by oxidized biochars produced at different pyrolysis temperatures and residence times. RSC Adv. 2016, 6, 41907–41913. [Google Scholar] [CrossRef]

| Treatment | 0–10 cm Depth |
|---|---|
| Soil (S) | Soil without fertilizer or BC |
| S + CS | Soil + CS (2736 g m−2) |
| S + SD | Soil + SD (2736 g m−2) |
| S + VN | Soil + VN (178.9 g m−2) |
| S + BC300 | Soil + BC300 (915.8 g m−2) |
| S + BC300 + CS | Soil + BC300 (915.8 g m−2) + CS (2736 g m−2) |
| S + BC300 + SD | Soil + BC300 (915.8 g m−2) + SD (2736 g m−2) |
| S + BC300 + VN | Soil + BC300 (915.8 g m−2) + VN (178.9 g m−2) |
| S + BC550 | Soil + BC550 (915.8 g m−2) |
| S + BC550 + CS | Soil + BC550 (915.8 g m−2) + CS (2736 g m−2) |
| S + BC550 + SD | Soil + BC550 (915.8 g m−2) + SD (2736 g m−2) |
| S + BC550 + VN | Soil + BC550 (915.8 g m−2) + VN (178.9 g m−2) |
| S + BC850 | Soil + BC850 (915.8 g m−2) |
| S + BC850 + CS | Soil + BC850 (915.8 g m−2) + CS (2736 g m−2) |
| S + BC850 + SD | Soil + BC850 (915.8 g m−2) + SD (2736 g m−2) |
| S + BC850 + VN | Soil + BC850 (915.8 g m−2) + VN (178.9 g m−2) |
| Parameter | BC300 | BC550 | BC850 |
|---|---|---|---|
| pH | 6.8 | 10.1 | 11.8 |
| Ash content (%) | 10.4 | 20.3 | 23.0 |
| Neutralization ability, CaCO3 (%) | 4.35 | 8.15 | 8.11 |
| Surface area (BET) 1 (m2 g−1) | 0.99 | 3.91 | 6.17 |
| Cumulative pore volume (DFT) 2 (cm3 g−1) | 0.002 | 0.018 | 0.008 |
| Ntot (%) | 2.82 | 2.92 | 2.56 |
| Ctot (%) | 53.5 | 65.9 | 68.1 |
| C/N | 19.0 | 22.6 | 26.6 |
| Ptot (%) | 0.24 | 0.54 | 0.60 |
| Ktot (%) | 2.28 | 4.62 | 5.10 |
| Catot (%) | 0.93 | 2.11 | 2.44 |
| Mgtot (%) | 0.47 | 0.93 | 1.03 |
| Cattle Slurry | Slurry Digestate | Vinasse | |
|---|---|---|---|
| Dry Matter (%) | 10.7 | 7.1 | 67.0 |
| pH | 7.5 | 7.9 | 4.5 |
| NH4+-N (%)1 | 0.214 | 0.257 | 0.27 |
| NO3−-N (%) 1 | <md | <md | nd |
| P (%) 2 | 0.112 | 0.102 | 0.101 |
| K (%) 2 | 0.362 | 0.302 | 13.25 |
| Ca (%) 2 | 0.182 | 0.162 | 2.061 |
| Mg (%) 2 | 0.066 | 0.041 | 0.161 |
| Df | Sum of Squares | Mean Square | F Value | Pr(>F) | Significance † | ω2 | |
|---|---|---|---|---|---|---|---|
| BC | 3 | 7057 | 2352 | 25.11 | 4.82 × 10−14 | *** | 0.039 |
| FERT | 3 | 6696 | 2232 | 23.824 | 2.02 × 10−13 | *** | 0.037 |
| TIME | 6 | 92,000 | 15,333 | 163.674 | <2 × 10−16 | *** | 0.529 |
| BC:FERT | 9 | 602 | 67 | 0.714 | 0.6957 | ns | 0.000 |
| BC:TIME | 18 | 17,973 | 999 | 10.658 | <2 × 10−16 | *** | 0.094 |
| FERT:TIME | 18 | 20,224 | 1124 | 11.993 | <2 × 10−16 | *** | 0.107 |
| BC:FERT:TIME | 54 | 7223 | 134 | 1.428 | 0.0392 | * | 0.012 |
| Residuals | 224 | 20,985 | 94 | 0.182 |
| Biochar | |||||
|---|---|---|---|---|---|
| no-bc | 300 | 550 | 850 | ||
| Fertilizer | no-fert | 5.15 | 5.44 | 5.82 | 5.77 |
| CS | 5.20 | 5.67 | 6.09 | 6.05 | |
| VN | 5.20 | 5.56 | 5.91 | 5.87 | |
| SD | 5.08 | 5.42 | 5.95 | 5.88 | |
| Biochar | Ntot | Ctot | |
|---|---|---|---|
| Initial (%) | 300 | 2.82 | 53.5 |
| 550 | 2.92 | 65.9 | |
| 850 | 2.56 | 68.1 | |
| Final (%) | 300 | 1.76 | 35.9 |
| 550 | 1.32 | 33.3 | |
| 850 | 0.79 | 28.9 | |
| Released (difference %, w:w) | 300 | 1.06 | 17.6 |
| 550 | 1.60 | 32.6 | |
| 850 | 1.77 | 39.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escuer-Gatius, J.; Shanskiy, M.; Soosaar, K.; Astover, A.; Raave, H. High-Temperature Hay Biochar Application into Soil Increases N2O Fluxes. Agronomy 2020, 10, 109. https://doi.org/10.3390/agronomy10010109
Escuer-Gatius J, Shanskiy M, Soosaar K, Astover A, Raave H. High-Temperature Hay Biochar Application into Soil Increases N2O Fluxes. Agronomy. 2020; 10(1):109. https://doi.org/10.3390/agronomy10010109
Chicago/Turabian StyleEscuer-Gatius, Jordi, Merrit Shanskiy, Kaido Soosaar, Alar Astover, and Henn Raave. 2020. "High-Temperature Hay Biochar Application into Soil Increases N2O Fluxes" Agronomy 10, no. 1: 109. https://doi.org/10.3390/agronomy10010109
APA StyleEscuer-Gatius, J., Shanskiy, M., Soosaar, K., Astover, A., & Raave, H. (2020). High-Temperature Hay Biochar Application into Soil Increases N2O Fluxes. Agronomy, 10(1), 109. https://doi.org/10.3390/agronomy10010109





