The Unexploited Potential of Nutrient Analysis in Potato Tissues at the Onset of Tuberization for Tuber Yield Prediction
Abstract
1. Introduction
2. Materials and Methods
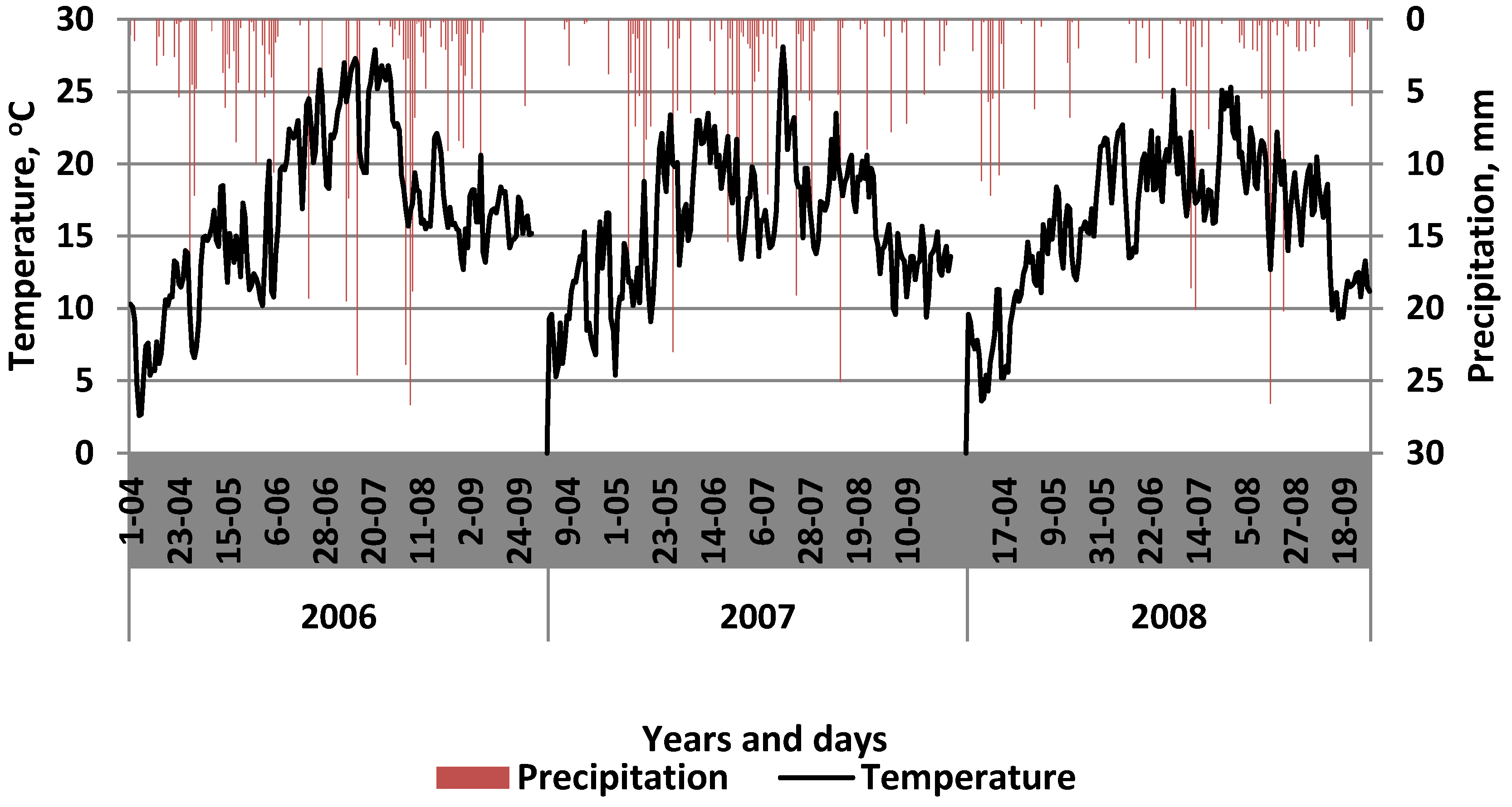
2.1. Site Description
2.2. Experimental Design
- (1)
- N rate (acronym N): 60 and 120 kg N ha−1;
- (2)
- Nitrogen fertilizer type (F): Urea (U) and urea stabilized with urease inhibitor, i.e., NBPT [N-(n-butyl) thiophosphoric triamide] (Agrotain, SU);
- (3)
- Sulfur: without S (S0), with sulfur (S50).
2.3. Experimental Measurements and Chemical Analysis
2.4. Nutrient Standardized Range Calculation
- (1)
- Linear:Yn = a × MTY + b
- (2)
- Quadratic:Yn = a × MTY2 + b × MTY + c
Ynmax = c − b2/4a
2.5. Statistical Analysis
3. Results
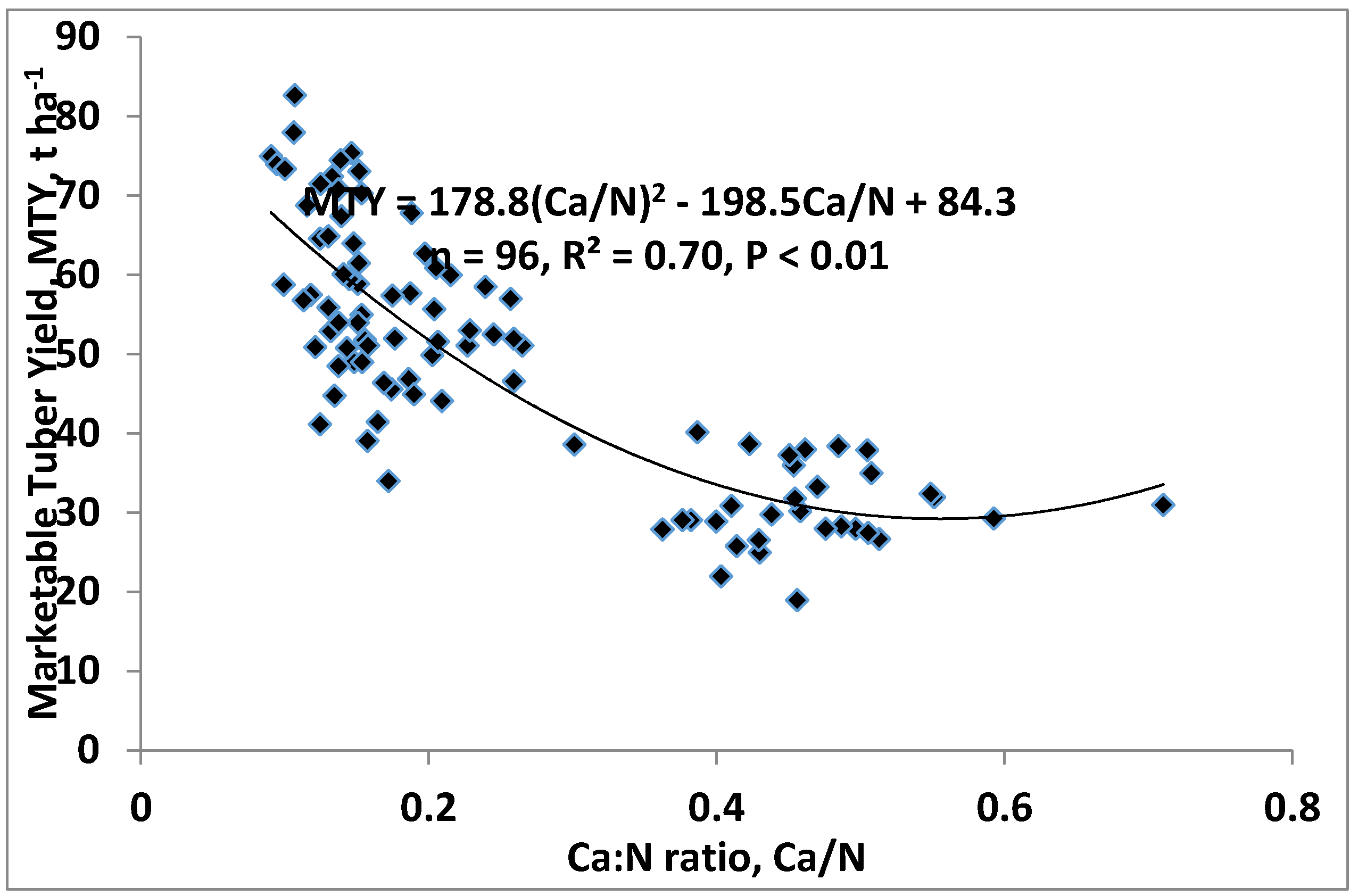
3.1. Tuber Yield
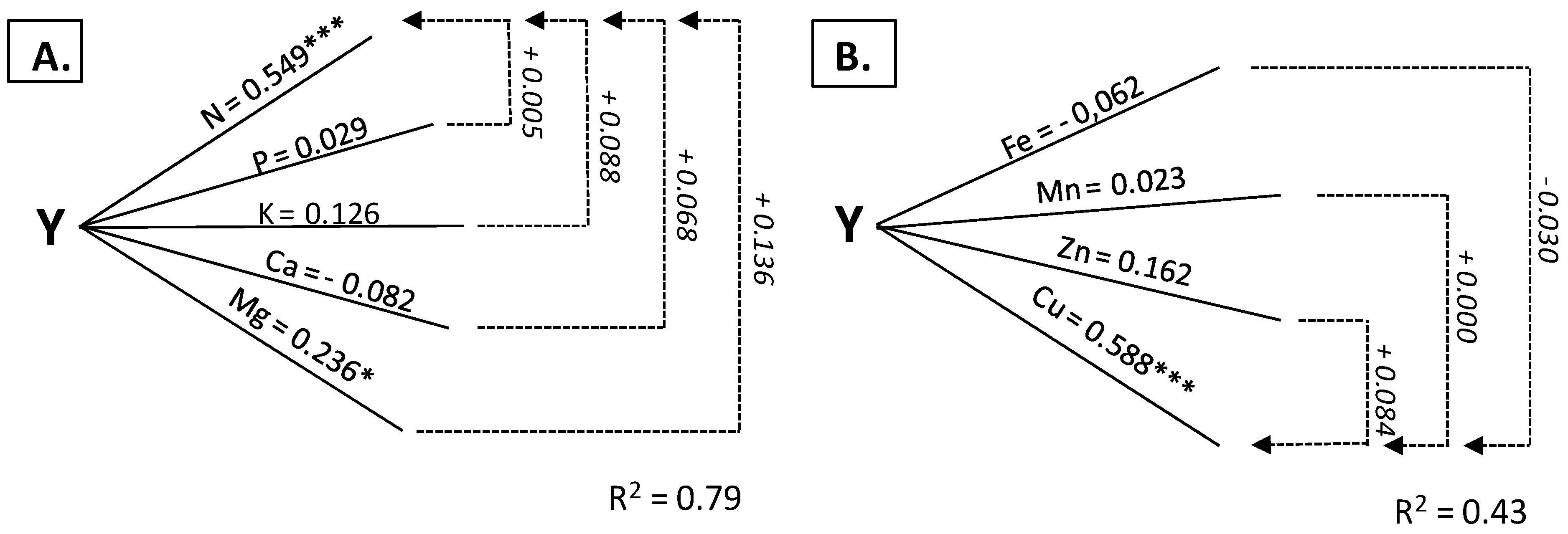
3.2. Leaves as Diagnostic Tissue
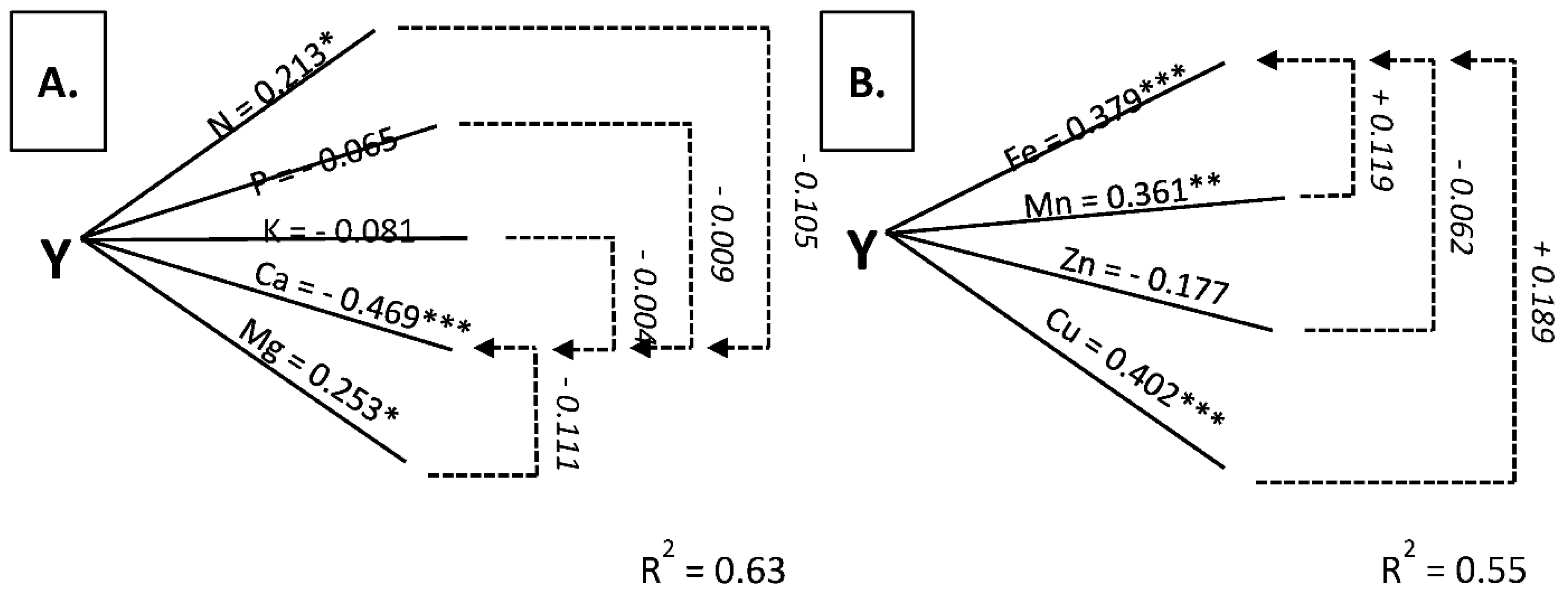
3.3. Stems as Diagnostic Tissue
3.4. Stolons + Roots as Diagnostic Tissue
for n = 96; R2 = 0.66.
4. Discussion
4.1. Potato Nutritional Status—Indicatory Nutrients
4.2. Calcium as a Controller of Nutrient Content
4.3. Phosphorus–An Apparent Excess
4.4. Approximate Ranges of the Optimal Content of Nutrients in the Stems
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Interactions | MTY | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| t ha−1 | g kg−1 DW | mg kg−1 DW | ||||||||
| Y × N | 11.9 *** | 2.5 | 1.7 | 1.7 | 70.0 *** | 7.4 ** | 6.2 ** | 10.6 *** | 7.2 ** | 13.2 *** |
| Y × F | 8.8 *** | 1.6 | 2.3 | 5.0 ** | 11.0 *** | 9.1 *** | 12.6 *** | 18.0 *** | 0.3 | 2.1 |
| N × F | 36.2 *** | 1.4 | 0.9 | 5.9 * | 15.0 *** | 8.5 ** | 65.5 *** | 0.2 | 0.0 | 0.0 |
| Y × S | 2.9 | 0.8 | 2.4 | 6.5 *** | 14.7 *** | 0.6 | 10.4 *** | 0.1 | 2.1 | 8.0 *** |
| N × S | 1.4 | 0.4 | 1.0 | 6.1 * | 37.1 *** | 5.2 * | 1.6 | 1.3 | 5.6 * | 3.5 |
| F × S | 4.3 * | 0.3 | 12.1 *** | 0.2 | 37.0 *** | 0.2 | 23.3 *** | 0.0 | 1.2 | 22.6 *** |
| Y × N × F | 45.5 *** | 1.3 | 2.2 | 2.2 | 37.0 *** | 0.7 | 28.5 *** | 12.0 *** | 5.6 ** | 0.1 |
| Y × N × S | 8.7 *** | 2.1 | 0.2 | 0.1 | 9.5 *** | 8.6 *** | 8.5 *** | 2.3 | 2.6 | 13.0 *** |
| Y × F × S | 3.8 * | 3.8 * | 5.2 ** | 0.5 | 7.6 *** | 12.6 *** | 2.8 | 3.4 * | 0.7 | 8.2 *** |
| N × F × S | 2.9 | 1.4 | 14.4 *** | 0.0 | 5.6 * | 14.9 *** | 14.8 *** | 0.1 | 7.0 * | 34.6 *** |
| Y × N × F × S | 0.3 | 1.0 | 9.9 *** | 0.8 | 12.8 *** | 11.9 *** | 5.4 ** | 0.9 | 2.8 | 6.3 ** |
| Traits | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| MTY 1 | 0.58 *** | −0.69 *** | −0.29 ** | −0.54 ** | −0.24 * | 0.49 *** | 0.05 | 0.71 *** | 0.62 *** |
| N | 1.00 | −0.50 *** | −0.65 *** | −0.52 ** | −0.40 *** | 0.14 | −0.11 | 0.82 *** | 0.58 *** |
| P | 1.00 | 0.29 ** | 0.46 * | 0.42 *** | −0.48 *** | −0.10 | −0.62 *** | −0.29 ** | |
| K | 1.00 | 0.44 ** | 0.35 * | 0.15 | 0.13 | −0.58 ** | −0.22 * | ||
| Mg | 1.00 | 0.21 * | −0.26 * | −0.08 | −0.54 *** | −0.43 *** | |||
| Ca | 1.00 | −0.05 | 0.02 | −0.41 *** | −0.09 | ||||
| Fe | 1.00 | 0.25 * | 0.26 * | 0.29 ** | |||||
| Mn | 1.00 | −0.02 | 0.16 | ||||||
| Zn | 1.00 | 0.65 *** |
| Interactions | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 DW | mg kg−1 DW | ||||||||
| Y × N | 1.2 | 7.7 *** | 0.3 | 1.3 | 52.4 *** | 0.2 | 9.3 *** | 16.3 *** | 6.5 ** |
| Y × F | 9.3 *** | 7.1 *** | 1.9 | 2.4 | 39.0 *** | 10.5 *** | 2.0 | 61.1 *** | 4.7 * |
| N × F | 4.4 * | 4.1 * | 2.1 | 45.2 *** | 2.8 | 0.4 | 2.7 | 0.0 | 0.0 |
| Y × S | 1.6 | 16.7 *** | 16.6 *** | 11.0 *** | 12.5 *** | 0.5 | 5.2 ** | 52.9 *** | 4.8 * |
| N × S | 2.9 | 13.3 *** | 0.0 | 10.0 ** | 0.6 | 7.3 ** | 0.0 | 0.0 | 1.0 |
| F × S | 0.3 | 1.1 | 4.2 * | 5.3 ** | 0.2 | 1.1 | 8.2 ** | 3.9 | 0.7 |
| Y × N × F | 10.3 *** | 4.3 * | 1.1 | 1.6 | 3.4 * | 10.9 *** | 5.9 ** | 3.6 * | 4.7 * |
| Y × N × S | 0.1 | 0.0 | 3.9 * | 3.4 * | 1.8 | 6.7 ** | 6.1 ** | 74.1 *** | 0.9 |
| Y × F × S | 0.8 | 18.2 *** | 0.0 | 2.8 | 2.2 | 1.3 | 0.1 | 17.1 *** | 6.1 * |
| N × F × S | 0.0 | 0.4 | 0.1 | 0.4 | 7.1 ** | 52.7 *** | 0.0 | 2.6 | 22.3 *** |
| Y × N × F × S | 1.8 | 0.3 | 2.2 | 3.8 ** | 7.7 *** | 0.8 | 6.4 ** | 48.8 *** | 1.3 |
| Traits | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| MTY 1 | 0.85 *** | 0.03 | 0.77 *** | 0.70 *** | −0.76 *** | 0.28 ** | 0.01 | 0.45 ** | 0.64 *** |
| N | 1.00 | 0.18 | 0.70 *** | 0.57 *** | −0.83 *** | 0.27 ** | −0.10 | 0.56 *** | 0.75 *** |
| P | 1.00 | −0.21 * | −0.31 ** | −0.06 | −0.22 * | −0.33 ** | 0.27 ** | 0.15 | |
| K | 1.00 | 0.88 *** | −0.70 *** | 0.61 *** | 0.20 | 0.43 *** | 0.63 *** | ||
| Mg | 1.00 | −0.56 ** | 0.56 ** | 0.21 * | 0.34 ** | 0.47 *** | |||
| Ca | 1.00 | −0.36 *** | 0.02 | −0.49 *** | −0.79 *** | ||||
| Fe | 1.00 | 0.29 ** | 0.30 ** | 0.49 *** | |||||
| Mn | 1.00 | 0.04 | 0.04 | ||||||
| Zn | 1.00 | 0.52 *** |
| Interactions | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 DW | mg kg−1 DW | ||||||||
| Y × N | 1.2 | 7.7 *** | 0.3 | 1.3 | 52.4 *** | 0.2 | 9.3 *** | 16.3 *** | 6.5 ** |
| Y × F | 9.3 *** | 7.1 *** | 1.9 | 2.4 | 39.0 *** | 10.5 *** | 2.0 | 61.1 *** | 4.7 * |
| N × F | 4.4 * | 4.1 * | 2.1 | 45.2 *** | 2.8 | 0.4 | 2.7 | 0.0 | 0.0 |
| Y × S | 1.6 | 16.7 *** | 16.6 *** | 11.0 *** | 12.5 *** | 0.5 | 5.2 ** | 52.9 *** | 4.8 * |
| N × S | 2.9 | 13.3 *** | 0.0 | 10.0 ** | 0.6 | 7.3 ** | 0.0 | 0.0 | 1.0 |
| F × S | 0.3 | 1.1 | 4.2 * | 5.3 ** | 0.2 | 1.1 | 8.2 ** | 3.9 | 0.7 |
| Y × N × F | 10.3 *** | 4.3 * | 1.1 | 1.6 | 3.4 * | 10.9 *** | 5.9 ** | 3.6 * | 4.7 * |
| Y × N × S | 0.1 | 0.0 | 3.9 * | 3.4 * | 1.8 | 6.7 ** | 6.1 ** | 74.1 *** | 0.9 |
| Y × F × S | 0.8 | 18.2 *** | 0.0 | 2.8 | 2.2 | 1.3 | 0.1 | 17.1 *** | 6.1 * |
| N × F × S | 0.0 | 0.4 | 0.1 | 0.4 | 7.1 ** | 52.7 *** | 0.0 | 2.6 | 22.3 *** |
| Y × N × F × S | 1.8 | 0.3 | 2.2 | 3.8 ** | 7.7 *** | 0.8 | 6.4 ** | 48.8 *** | 1.3 |
| Traits | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| MTY 1 | 0.45 *** | −0.19 | −0.49 *** | 0.53 *** | −0.74 *** | 0.63 *** | 0.45 *** | 0.24 * | 0.50 *** |
| N | 1.00 | 0.20 | −0.06 | 0.05 | −0.50 *** | 0.41 *** | 0.12 | 0.41 *** | 0.63 *** |
| P | 1.00 | 0.20 | −0.34 ** | 0.13 | 0.16 | −0.20 * | 0.08 | 0.11 | |
| K | 1.00 | −0.50 *** | 0.62 ** | −0.55 *** | -0.22 * | −0.14 | −0.17 | ||
| Mg | 1.00 | −0.44 *** | 0.41 *** | 0.29 ** | 0.32 ** | 0.15 | |||
| Ca | 1.00 | −0.77 *** | −0.39 *** | −0.38 ** | −0.59 *** | ||||
| Fe | 1.00 | 0.33 ** | 0.35 ** | 0.47 *** | |||||
| Mn | 1.00 | 0.27 ** | 0.03 | ||||||
| Zn | 1.00 | 0.47 *** |
References
- Andre, C.M.; Legay, S.; Iammarino, C.; Ziebel, J.; Guignard, C.; Larondelle, Y.; Hausman, J.-F.; Evers, D.; Miranda, L.M. The potato in the human diet: A complex matrix with potential health benefits. Potato Res. 2014, 57, 201–214. [Google Scholar] [CrossRef]
- De Jong, H. Impact of potato on society. Am. J. Potato Res. 2016, 93, 415–429. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://faostat.fao.org/site/567/default.aspx#ancor (accessed on 25 September 2019).
- Birch, P.R.J.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Van Niekerk, C.; Schönfeldt, H.; Hall, N.; Pretorius, B. The role of biodiversity in food security and nutrition: A potato cultivar case study. Food Nutr. Sci. 2016, 7, 371–382. [Google Scholar] [CrossRef]
- Sardans, J.; Peňuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 262–275. [Google Scholar] [CrossRef]
- Westermann, D.T. Nutritional requirements of potatoes. Am. J. Potato Res. 2005, 82, 301–307. [Google Scholar] [CrossRef]
- Da Silva Oliveira, C.A. Potato crop growth as affected by nitrogen and plant density. Pesqui. Agropecu. Bras. 2000, 35, 939–950. [Google Scholar]
- Jackson, S.D. Multiple signaling pathway control tuber induction in potato. Plant Physiol. 1999, 119, 1–8. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Allen, E.J.; Firman, D.M. A review of some studies into tuber initiation in potato (Solanum tuberosum) crops. J. Agric. Sci. Camb. 1998, 130, 251–270. [Google Scholar] [CrossRef]
- Katoh, A.; Ashida, H.; Kasajima, I.; Shigeoka, S.; Yokota, A. Potato yield enhancement through intensification of sink and source performances. Breed. Sci. 2015, 65, 77–85. [Google Scholar] [CrossRef][Green Version]
- Grzebisz, W.; Potarzycki, J.; Biber, M. The early prognosis of tuber yield based on nitrogen status in potato. tops. Plant Soil Environ. 2018, 64, 539–545. [Google Scholar] [CrossRef]
- Vos, J.; van der Putten, P.E.L. Effect of nitrogen supply on leaf growth, leaf nitrogen economy and photosynthetic capacity in potato. Field Crop. Res. 1998, 59, 63–72. [Google Scholar] [CrossRef]
- Gayler, S.; Wang, E.; Priesack, E.; Schaaf, T.; Maidl, F.X. Modelling biomass growth, N-uptake and phenological development of potato crop. Geoderma 2002, 105, 367–383. [Google Scholar] [CrossRef]
- Walworth, J.L.; Muniz, J.E. A compendium of tissue nutrient concentrations for field-grown potatoes. Am. J. Potato Res. 1993, 70, 579–597. [Google Scholar] [CrossRef]
- Mackay, D.C.; Carefoot, J.M.; Entz, T. Evaluation of the DRIS procedure for assessing the nutritional status of potato (Solanum Tuberosum L.). Commun. Soil Sci. Plant Anal. 1987, 18, 1331–1353. [Google Scholar] [CrossRef]
- Baxter, I. Should we treat the ionome as a combination of individual elements, or should we be deriving novel combined traits? J. Exp. Bot. 2015, 66, 2127–2131. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Salt, D.E. Plant ionomics: From elemental profiling to environmental adaption. Mol. Plant 2016, 9, 787–797. [Google Scholar] [CrossRef]
- Rosen, C. Tissue analysis as a nutrient management for potatoes. Minnesota. Veg. IPM Newsl. 2000, 3, 9. [Google Scholar]
- Musilová, L.; Lošák, T.; Hlušek, J.; Vitězová, M.; Jůzl, M.; Elzner, P.; Filipčík, R.; Jůzl, M.; Bennewitz, E. The effect of urea with urease inhibitor on the content of macronutrients in tubers and tops of potatoes (Solanum tuberosum L.). Agric. Et Silvic. Mendel. Brun. 2012, LX, 167–172. [Google Scholar]
- Gao, Y.; Jia, L.; Hu, B.; Alva, A.; Fan, M. Potato stolon and tuber growth influenced by nitrogen form. Plant Prod. Sci. 2014, 17, 138–143. [Google Scholar] [CrossRef]
- Lahlou, O.; Ledent, J.-F. Root mass and depth, stolons and roots formed on stolons in four cultivars of potato under water stress. Eur. J. Agron. 2005, 22, 159–173. [Google Scholar] [CrossRef]
- Reis, R., Jr.; Monnerat, P.H. Nutrient concentration in potato stem, petiole and leaflet in response to potassium fertilizer. Sci. Agric. 2000, 57, 251–255. [Google Scholar] [CrossRef]
- Alva, A.K.; Ren, H.; Moore, A.D. Water and nitrogen management effects on biomass accumulation and partitioning in two potato cultivars. Am. J. Plant Sci. 2012, 3, 164–170. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Comm. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Konys, L.; Wiśniewski, P. Path analysis in cause and effect relationships. Rocz. AR W Pozn. 1984, CLIII, 37–54. (In Polish) [Google Scholar]
- Chang, D.C.; Jin, Y.K.; Nam, J.H.; Cheon, C.G.; Cho, J.H.; Kim, S.J. Early drought effect on canopy development under growth of potato cultivars with different maturities. Field Crop. Res. 2018, 215, 156–162. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; van de Geijn, S. Root, shoot and soil parameters required for process-oriented models of crop growth limited by water or nutrients. Plant Soil 1996, 183, 1–25. [Google Scholar] [CrossRef]
- Li, H.; Parent, L.E.; Karam, A. Simulation modeling of soil and plant nitrogen use in potato cropping system in the humid and cool environment. Agric. Ecosyst. Environ. 2006, 115, 248–260. [Google Scholar] [CrossRef]
- Li, W.; Xiong, B.; Wang, S.; Deng, X.; Yin, L.; Li, H. Regulation effects of water and nitrogen on the source-sink relationship in potato during the tuber bulking stage. PLoS ONE 2016, 11, e0146877. [Google Scholar] [CrossRef]
- Khan, M.; Yin, X.; van der Putten, P.E.I.; Struik, P.C. An eco-physiological model analysis of yield differences within a set of contrasting cultivars and f1 segregating population of potato (Solanum tuberosum L.) grown under diverse environment. Ecol. Model. 2014, 290, 146–154. [Google Scholar] [CrossRef]
- Grzebisz, W.; Potarzycki, J. The in-season nitrogen concentration in the potato tuber as the yield driver. Agron. J. 2020, in press. [Google Scholar] [CrossRef]
- Busse, J.S.; Palta, J.P. Investigating the in vivo calcium transport path to developing potato tuber using 45Ca: A new concept in potato tuber calcium nutrition. Physiol. Plant. 2006, 128, 313–323. [Google Scholar] [CrossRef]
- Gupta, U.C.; Kening, W.; Siyuan, L. Micronutrients in soils, crops and livestock. Earth Sci. Front. 2008, 15, 110–125. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef]
- Forde, B.; Lorenzo, H. The nutritional control of root development. Plant Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Marschner, H.; Kirkby, E.A.; Cakmak, I. Effect of mineral nutrition status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- White, P.J.; Bradshaw, J.E.; Brown, L.K.; Finlay, M.; Dale, B.; Dupuy, L.X.; George, T.S.; Hammond, J.P.; Subramanian, N.K.; Thompson, J.A.; et al. Juvenile root vigour improves phosphorus use efficiency of potato. Plant Soil 2018, 432, 45–63. [Google Scholar] [CrossRef]
- Rosen, C.J.; Birman, P.M. Potato yield and tuber set as affected by phosphorus fertilization. Am. J. Potato Res. 2008, 85, 110–120. [Google Scholar] [CrossRef]
- Fixen, P.E.; Bruulsema, T.W. Potato management challenges created by phosphorus chemistry and plant roots. Am. J. Potato Res. 2014, 91, 121–131. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Franke, A.C.; Steyn, J.M.; Pronk, A.A.; Caldiz, D.O.; Kooman, P.L. A robust potato model: LINTUL-POTATO-DSS. Potato Res. 2015, 58, 313–327. [Google Scholar] [CrossRef]
- Jarrel, W.M.; Beverly, R.B. The dilution effect in plant nutrition studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar] [CrossRef]

| Year | Layer cm | Corg 1 | pH 2 | K 3 | Ca 3 | Mg 3 | P 3 | Nmin 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD 5 | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| g kg−1 | kg ha−1 | mg kg−1 | |||||||||||||
| 2006 | 0–30 | 33 | 0.9 | 6.6 | 0.5 | 253 | 117 | 1605 | 137 | 117 | 14 | 305 | 71 | 33 | 6 |
| 30–60 | 15 | 0.4 | 7.6 | 0.2 | 88 | 26 | 1105 | 725 | 85 | 19 | 199 | 71 | 27 | 5 | |
| 60–90 | 8 | 0.2 | 7.7 | 0.1 | 55 | 20 | 243 | 63 | 56 | 8 | 137 | 81 | 32 | 8 | |
| 2007 | 0–30 | 27 | 0.1 | 7.0 | 0.1 | 160 | 13 | 1678 | 619 | 106 | 39 | 267 | 28 | 41 | 10 |
| 30–60 | 18 | 0.3 | 7.2 | 0.4 | 180 | 82 | 1259 | 966 | 91 | 44 | 277 | 58 | 42 | 11 | |
| 60–90 | 9 | 0.4 | 7.2 | 0.2 | 81 | 32 | 165 | 68 | 54 | 18 | 221 | 90 | 51 | 17 | |
| 2008 | 0–30 | 55 | 0.4 | 7.0 | 0.1 | 225 | 47 | 2190 | 625 | 139 | 41 | 344 | 35 | 37 | 9 |
| 30–60 | 46 | 0.3 | 7.2 | 0.1 | 149 | 142 | 2748 | 388 | 166 | 25 | 228 | 69 | 42 | 12 | |
| 60–90 | 44 | 0.9 | 7.3 | 0.2 | 74 | 50 | 2220 | 655 | 134 | 10 | 168 | 37 | 43 | 11 | |
| Factor | Level of Factor | MTY | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| t ha−1 | g kg−1 DW | mg kg−1 DW | |||||||||
| Year (Y) | 2006 | 59.3 c | 50.2 c | 3.5 a | 24.4 a | 1.3 a | 13.1 a | 314.3 b | 47.6 a | 64.0 c | 9.6 c |
| 2007 | 55.8 b | 36.6 b | 3.6 a | 30.1 b | 1.5 b | 15.7 b | 424.3 c | 51.6 b | 44.6 b | 8.4 c | |
| 2008 | 31.3 a | 29.7 a | 4.8 b | 30.3 b | 1.7 b | 15.6 b | 234.0 a | 46.9 a | 29.3 a | 6.5 a | |
| Nitrogen (N), kg ha−1 | 60 | 48.7 | 38.7 | 3.9 | 27.8 | 1.4 a | 15.1 | 336.9 b | 47.1 a | 47.1 b | 8.0 a |
| 120 | 48.9 | 39.1 | 4.0 | 28.7 | 1.5 b | 14.5 | 311.5 a | 50.3 b | 44.8 a | 8.3 b | |
| N Fertilizer (F) | U | 45.6 a | 38.5 | 4.0 | 28.2 | 1.5 b | 14.6 | 317.7 | 48.5 | 46.8 b | 8.1 |
| SU | 52.0 b | 39.2 | 4.0 | 28.3 | 1.4 a | 14.9 | 330.7 | 48.9 | 45.1 a | 8.2 | |
| Sulfur Rate (S) kg ha−1 | 0 | 45.4 a | 39.6 | 3.9 | 27.3 a | 1.4 a | 14.9 | 313.0 a | 47.8 | 45.1 a | 7.9 a |
| 50 | 52.3 b | 38.1 | 4.0 | 29.2 a | 1.5 b | 14.6 | 335.4 b | 49.6 | 46.8 b | 8.4 b | |
| Source of Variation | |||||||||||
| Year (Y) | 467.8 *** | 173.1 *** | 105.9 *** | 63.1 *** | 176.2 *** | 29.0 *** | 184.6 *** | 6.4 ** | 648.9 *** | 115.1 *** | |
| Nitrogen Rate (N) | 0.1 | 0.2 | 2.8 | 3.0 | 31.1 *** | 2.7 | 9.7 *** | 7.8 ** | 8.9 ** | 5.0 * | |
| Nitrogen Fertilizer (F) | 51.7 *** | 0.6 | 0.0 | 0.0 | 7.8 ** | 1.0 | 2.6 | 0.2 | 4.9 * | 1.1 | |
| Sulfur (S) | 59.8 *** | 2.7 | 0.9 | 14.6 *** | 132.8 *** | 0.8 | 7.6 ** | 2.5 | 4.9 * | 10.3 *** | |
| Traits | Leaves | Stems | Stolons + Roots | |||
|---|---|---|---|---|---|---|
| Correlation | Path | Correlation | Path | Correlation | Path | |
| Nitrogen | 0.584 *** | 0.375 *** | 0.846 *** | 0.549 *** | 0.0448 *** | 0.213 * |
| Phosphorus | −0.686 *** | −0.491 *** | 0.039 | 0.029 | −0.187 | −0.065 |
| Potassium | −0.286 ** | 0.152 | 0.769 *** | 0.126 | −0.492 *** | −0.081 |
| Magnesium | −0.545 *** | −0.209 * | 0.700 *** | 0.236 * | 0.531 *** | 0.253 * |
| Calcium | −0.246 *** | 0.107 | −0.761 *** | −0.082 | −0.738 *** | −0.469 *** |
| Traits | Leaves | Stems | Stolons + Roots | |||
|---|---|---|---|---|---|---|
| Correlation | Path | Correlation | Path | Correlation | Path | |
| Iron | 0.494 *** | 0.319 ** | 0.281 ** | −0.062 | 0.625 *** | 0.379 *** |
| Manganese | 0.046 | −0.061 | 0.014 | 0.023 | 0.446 *** | 0.361 ** |
| Zinc | 0.712 *** | 0.480 *** | 0.450 *** | 0.162 | 0.2418 | −0.177 |
| Copper | 0.624 *** | 0.229 * | 0.642 *** | 0.588 *** | 0.303 ** | 0.402 *** |
| Factor | Level of Factor | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 DW | mg kg−1 DW | |||||||||
| Year (Y) | 2006 | 32.6 c | 2.8 c | 50.2 b | 2.1 b | 4.3 a | 82.7 b | 22.5 a | 62.3 c | 6.0 a |
| 2007 | 26.8 b | 1.3 a | 56.3 c | 2.7 c | 5.1 b | 94.3 c | 28.3 b | 48.8 b | 5.2 a | |
| 2008 | 17.3 a | 2.2 b | 35.6 a | 1.3 a | 7.8 c | 72.6 a | 24.4 a | 37.9 a | 3.7 a | |
| Nitrogen (N), kg ha−1 | 60 | 25.6 | 2.1 | 47.3 | 2.0 | 5.6 a | 80.7 a | 25.1 | 51.1 b | 4.9 |
| 120 | 25.6 | 2.1 | 47.5 | 2.1 | 5.8b | 85.7 b | 25.1 | 48.3 a | 5.1 | |
| N Fertilizer (F) | U | 25.0 a | 2.0 a | 46.8 | 2.0 | 5.6 a | 82.2 | 25.7 | 48.3 a | 5.0 |
| SU | 26.1 b | 2.2 b | 48.0 | 2.0 | 5.8 b | 84.2 | 24.5 | 51.1 b | 4.9 | |
| Sulfur Rate (S) kg ha−1 | 0 | 25.1 | 1.9 a | 45.7 a | 1.9 a | 5.5 a | 83.0 | 24.0 a | 49.5 | 4.9 |
| 50 | 26.0 | 2.3 b | 49.1 b | 2.1 b | 5.9 b | 83.4 | 26.2 b | 49.9 | 5.0 | |
| Source of Variation | ||||||||||
| Year (Y) | 337.7 *** | 168.8 *** | 592.5 *** | 260.5 *** | 929.0 *** | 78.1 *** | 20.1 *** | 195.0 *** | 171.2 *** | |
| Nitrogen Rate (N) | 0.0 | 0.0 | 0.2 | 2.9 | 4.5 * | 12.5 *** | 0.0 | 7.4 ** | 3.3 | |
| N Fertilizer (F) | 4.7 * | 6.4 * | 5.4 *** | 0.3 | 11.3 ** | 2.1 | 2.5 | 7.6 ** | 0.2 | |
| Sulfure Rate (S) | 3.5 | 42.8 *** | 45.2 *** | 14.7 *** | 26.8 *** | 0.1 | 8.3 ** | 0.2 | 0.1 | |
| Factor | Level of Factor | N | P | K | Mg | Ca | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1DW | mg kg−1 DW | |||||||||
| Year (Y) | 2006 | 25.4 c | 3.2 b | 15.7 b | 1.8 b | 4.3 a | 457.2 c | 38.5 b | 53.2 c | 5.7 c |
| 2007 | 17.6 b | 2.5 a | 14.9 a | 2.7 c | 5.1 b | 404.3 b | 40.5 b | 50.4 b | 4.5 b | |
| 2008 | 15.5 a | 3.1 b | 17.4 c | 1.3 a | 7.7 c | 305.2 a | 31.1 a | 38.4 a | 3.7 a | |
| Nitrogen (N), kg ha−1 | 60 | 19.9 | 2.9 | 16.4 b | 1.8 a | 5.6 a | 374.5 a | 34.9 a | 48.1 | 4.8 b |
| 120 | 19.1 | 3.0 | 15.6 a | 2.0 b | 5.8 b | 403.2 b | 38.5 b | 46.6 | 4.4 a | |
| N Fertilizer (F) | U | 19.0 | 3.1 b | 16.2 | 1.9 | 5.6 a | 400.7 b | 38.1 b | 52.7 b | 4.6 a |
| SU | 20.0 | 2.8 a | 15.9 | 1.9 | 5.8 b | 377.1 a | 35.3 a | 42.0 a | 4.7 b | |
| Sulfur Rate (S) kg ha−1 | 0 | 20.2 b | 2.9 | 15.8 a | 1.9 a | 5.5 a | 402.2 b | 36.0 | 47.0 | 4.5 a |
| 50 | 18.8 a | 3.0 | 16.3 b | 2.0 b | 5.9 b | 375.6 a | 37.4 | 47.7 | 4.8 b | |
| Source of Variation | ||||||||||
| Year (Y) | 101.9 *** | 32.8 *** | 79.0 *** | 186.3 *** | 593.7 *** | 207.2 *** | 46.6 *** | 135.3 *** | 238.9 *** | |
| Nitrogen Rate (N) | 1.5 | 0.5 | 21.6 *** | 10.6 ** | 5.0 * | 21.5 *** | 18.1 *** | 3.6 | 37.0 *** | |
| N Fertilizer (F) | 2.7 | 16.2 *** | 2.4 | 0.1 | 8.0 ** | 14.4 *** | 10.9 *** | 188.3 *** | 4.2 * | |
| Sulfur Rate (S) | 5.8 * | 2.9 | 7.9 ** | 5.4 * | 23.3 *** | 18.4 *** | 2.9 | 0.9 | 21.7 *** | |
| Nutrient | Equation | Optimum Yield MTYop, t ha−1 | Maximum Nutrient Content for MTYop | Approximate Nutrient Range for Y → 70–80 t ha−1 |
|---|---|---|---|---|
| Stems | ||||
| Nitrogen (N), g kg−1 | N = 0.38MTY + 7.1, for R2 = 0.71, p ≤ 0.01 | - | - | 28–38 |
| Phosphorus (P), g kg−1 | - | - | - | 1.3–2.9 |
| Potassium (K), g kg−1 | K = −0.015MTY2 + 1.86MTY – 6.36 for R2 = 0.71, p ≤ 0.01 | 62 | 51.3 | 50–60 * |
| Calcium (Ca), g kg−1 | Ca = −0.0014MTY2 + 0.222MTY + 12.8 for R2 = 0.63, p ≤ 0.01 | 79.3 | 4.0 | 3.0–5.0 ** |
| Magnesium (Mg), g kg−1 | Mg = −0.0005MTY2 + 0.082MTY − 0.64 for R2 = 0.52, p ≤ 0.01 | 82.0 | 2.7 | 1.3–4.1 ** |
| Leaves | ||||
| Iron (Fe), mg kg−1 | Fe = −0.118MTY2 + 15.18MTY + 107.6 for p = 0.31, p ≤ 0.05 | 64.3 | 375 | 300–400 * |
| Manganese (Mn), mg kg−1 | - | - | - | 33–65 |
| Zinc (Zn), mg kg−1 | Zn = 0.69MTY + 12.2, for R2 = 0.51, p ≤ 0.01 | - | - | 31–65 |
| Copper (Cu), mg kg−1 | Cu = −0.0011MTY2 + 0.186MTY + 2.03 for R2 = 0.41, p ≤ 0.05 | 84.5 | 9.9 | 7.5–12.3 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzebisz, W.; Frąckowiak, K.; Potarzycki, J.; Diatta, J.; Szczepaniak, W. The Unexploited Potential of Nutrient Analysis in Potato Tissues at the Onset of Tuberization for Tuber Yield Prediction. Agronomy 2020, 10, 103. https://doi.org/10.3390/agronomy10010103
Grzebisz W, Frąckowiak K, Potarzycki J, Diatta J, Szczepaniak W. The Unexploited Potential of Nutrient Analysis in Potato Tissues at the Onset of Tuberization for Tuber Yield Prediction. Agronomy. 2020; 10(1):103. https://doi.org/10.3390/agronomy10010103
Chicago/Turabian StyleGrzebisz, Witold, Karolina Frąckowiak, Jarosław Potarzycki, Jean Diatta, and Witold Szczepaniak. 2020. "The Unexploited Potential of Nutrient Analysis in Potato Tissues at the Onset of Tuberization for Tuber Yield Prediction" Agronomy 10, no. 1: 103. https://doi.org/10.3390/agronomy10010103
APA StyleGrzebisz, W., Frąckowiak, K., Potarzycki, J., Diatta, J., & Szczepaniak, W. (2020). The Unexploited Potential of Nutrient Analysis in Potato Tissues at the Onset of Tuberization for Tuber Yield Prediction. Agronomy, 10(1), 103. https://doi.org/10.3390/agronomy10010103





