Structure of Composite Based on Polyheteroarylene Matrix and ZrO2 Nanostars Investigated by Quantitative Nanomechanical Mapping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZrO2 Nanostars
2.3. Preparation of Composites
2.4. Characterization Methods
2.4.1. Microscopic Investigation
2.4.2. Wide-Angle X-ray Diffraction Study
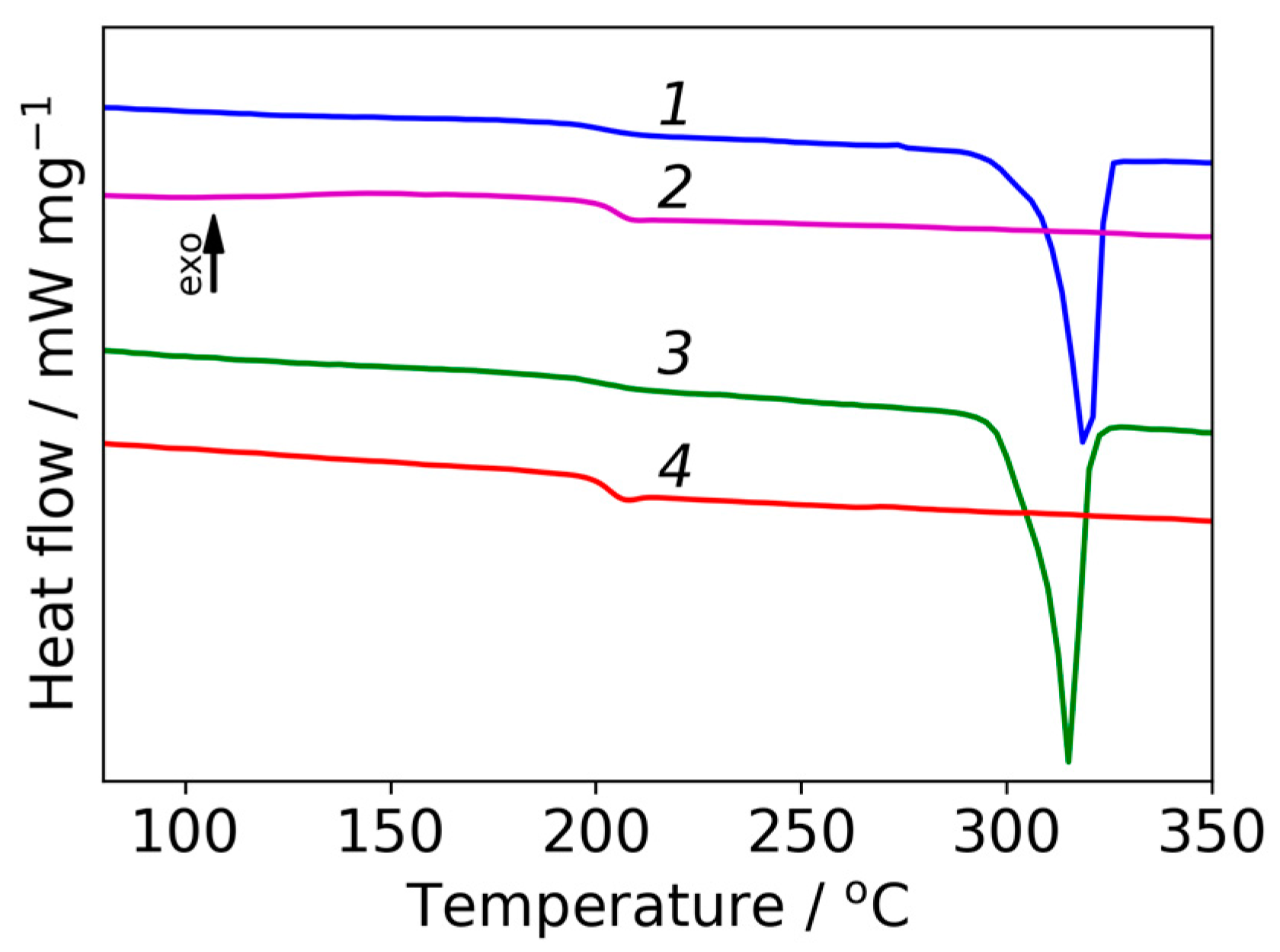
2.4.3. Analysis of Thermal Properties
2.4.4. FTIR Spectroscopy Investigation
3. Results and Discussion
3.1. WAXD Data
3.2. Investigation of Morphology with Electron Microscopy
3.3. FT-IR Spectroscopy
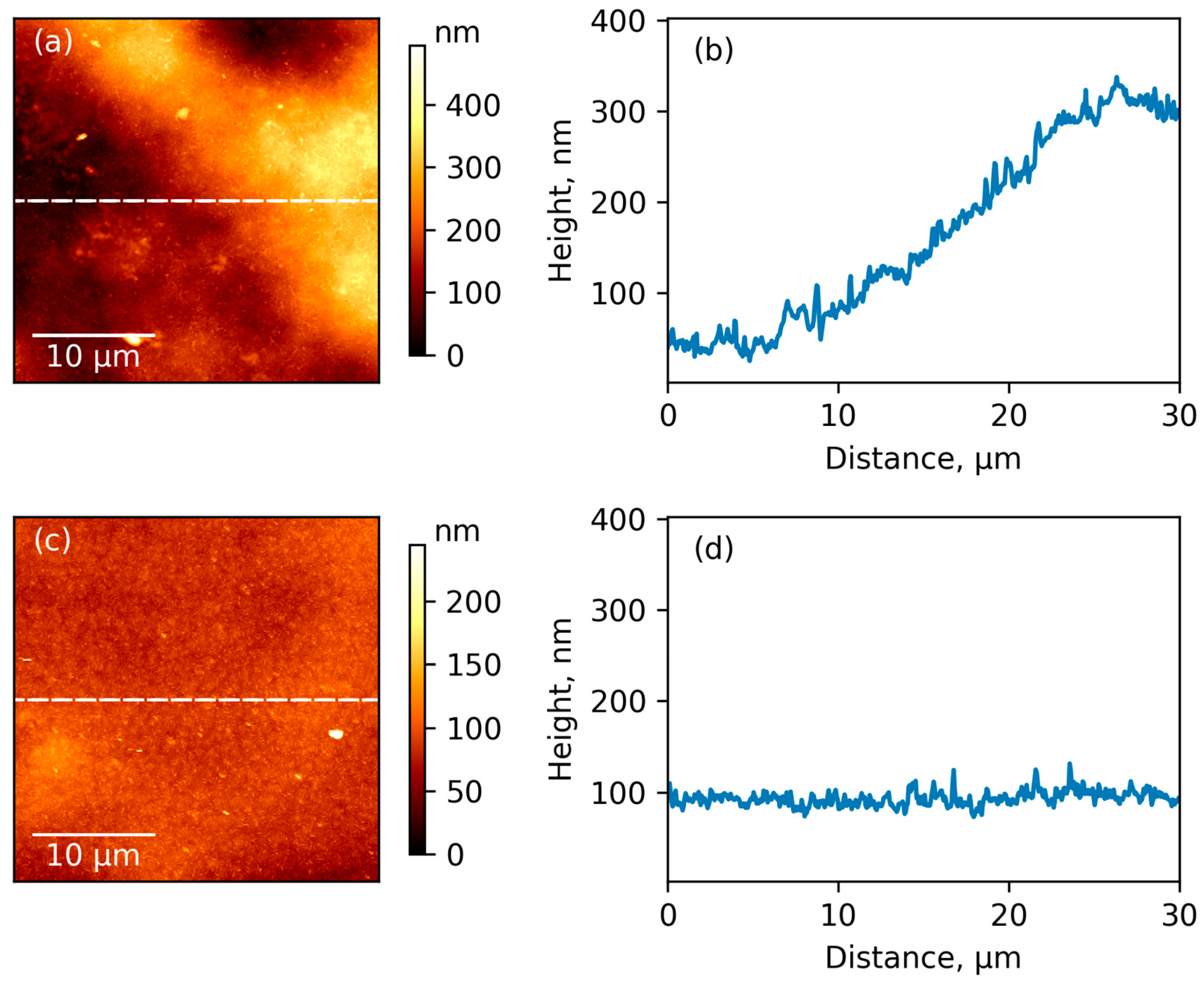
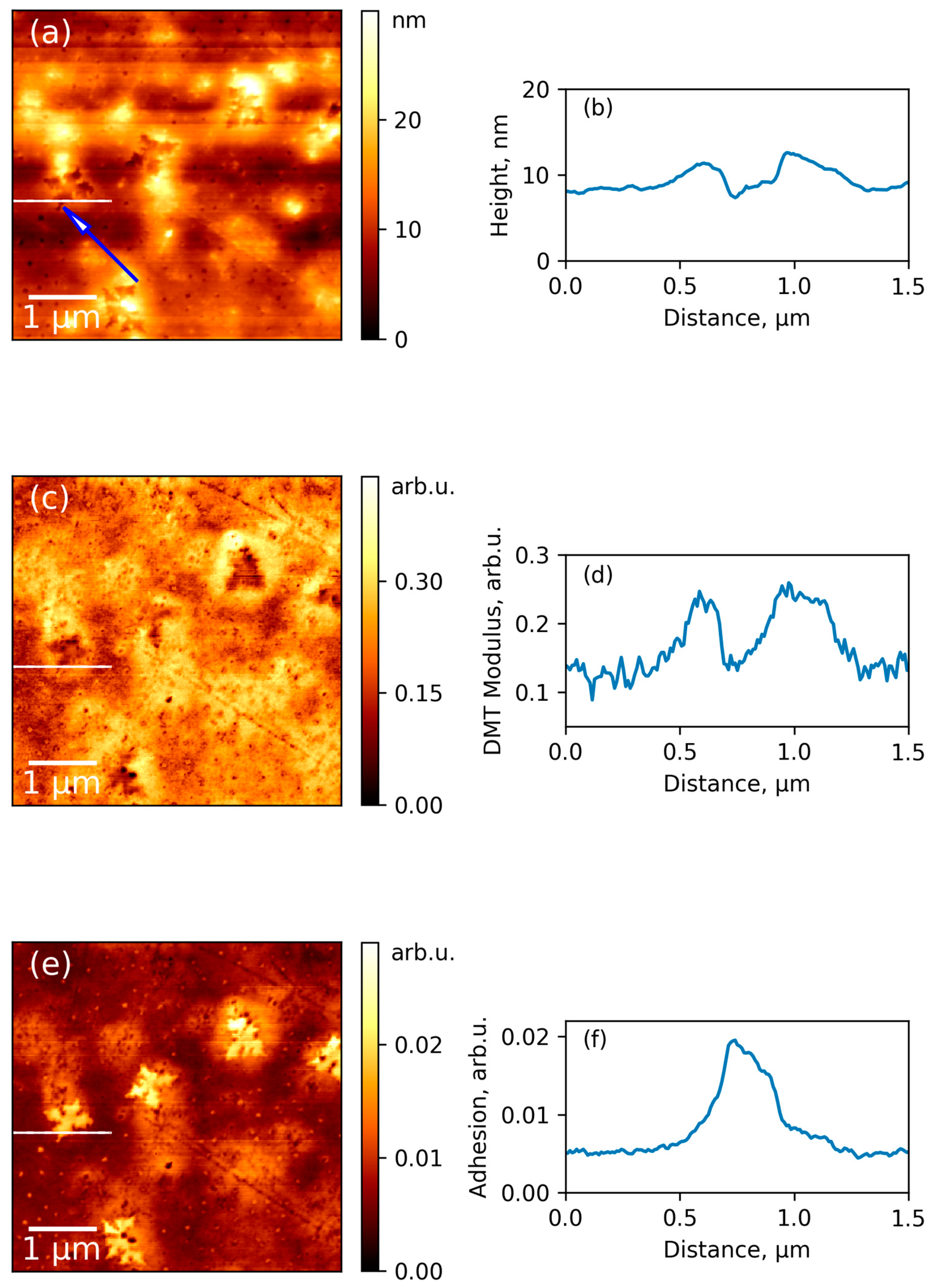
3.4. AFM Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced Polyimide Materials: Syntheses, Physical Properties and Applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y. Novel Thermally Cross-Linked Polyimide Membranes for Ethanol Dehydration via Pervaporation. J. Membr. Sci. 2015, 496, 142–155. [Google Scholar] [CrossRef]
- Pulyalina, A.Y.; Polotskaya, G.A.; Toikka, A.M. Membrane Materials Based on Polyheteroarylenes and Their Application for Pervaporation. Russ. Chem. Rev. 2016, 85, 81–98. [Google Scholar] [CrossRef]
- Cornelius, C.J.; Marand, E. Hybrid Silica-Polyimide Composite Membranes: Gas Transport Properties. J. Membr. Sci. 2002, 202, 97–118. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.J.; Pinnau, I.; Song, J.; Du, N.; Robertson, G.P.; Guiver, M.D. Gas Transport Behavior of Mixed-Matrix Membranes Composed of Silica Nanoparticles in a Polymer of Intrinsic Microporosity (PIM-1). J. Membr. Sci. 2010, 346, 280–287. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A.; Kaszuwara, W.; Awietjan, S.; Molak, R.; Sysel, P.; Grzywna, Z.J. The Magnetic Inorganic-Organic Hybrid Membranes Based on Polyimide Matrices for Gas Separation. Compos. Part B Eng. 2017, 110, 161–170. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A Review on Colorless and Optically Transparent Polyimide Films: Chemistry, Process and Engineering Applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly Transparent Polyimide Hybrids for Optoelectronic Applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Chen, X.; Huang, H.; Shu, X.; Liu, S.; Zhao, J. Preparation and Properties of a Novel Graphene Fluoroxide/polyimide Nanocomposite Film with a Low Dielectric Constant. RSC Adv. 2017, 7, 1956–1965. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Fabrication of Polyimide/titania Nanocomposites Containing Benzimidazole Side Groups via Sol-Gel Process. Prog. Org. Coat. 2012, 75, 373–378. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Feng, C.; Zeng, Q. Improved Dielectric Properties of Surface Modified BaTiO3/polyimide Composite Films. Microelectron. Eng. 2016, 154, 17–21. [Google Scholar] [CrossRef]
- Dinari, M.; Ahmadizadegan, H. Preparation, Characterization and Gas Separation Properties of Nanocomposite Materials Based on Novel Silane Functionalizing Polyimide Bearing Pendent Naphthyl Units and ZnO Nanoparticles. RSC Adv. 2015, 5, 8630–8639. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Geydt, P.; Bugrov, A.N.; Ovaska, S.S.; Lahderanta, E.; Toikka, A.M. Structure and Transport Properties of Mixed-Matrix Membranes Based on Polyimides with ZrO2 Nanostars. Polymers 2016, 8, 403. [Google Scholar] [CrossRef]
- Yudin, V.E.; Bugrov, A.N.; Didenko, A.L.; Smirnova, V.E.; Gofman, I.V.; Kononova, S.V.; Kremnev, R.V.; Popova, E.N.; Svetlichnyi, V.M.; Kudryavtsev, V.V. Composites of Multiblock (Segmented) Aliphatic Poly(ester Imide) with Zirconia Nanoparticles: Synthesis, Mechanical Properties, and Pervaporation Behavior. Polym. Sci. Ser. B 2014, 56, 919–926. [Google Scholar] [CrossRef]
- Bugrov, A.N.; Vlasova, E.N.; Mokeev, M.V.; Popova, E.N.; Ivan’kova, E.M.; Al’myasheva, O.V.; Svetlichnyi, V.M. Distribution of Zirconia Nanoparticles in the Matrix of poly(4,4′-Oxydiphenylenepyromellitimide). Polym. Sci. Ser. B 2012, 54, 486–495. [Google Scholar] [CrossRef]
- Li, Y.; Guan, H.M.; Chung, T.S.; Kulprathipanja, S. Effects of Novel Silane Modification of Zeolite Surface on Polymer Chain Rigidification and Partial Pore Blockage in Polyethersulfone (PES)-Zeolite A Mixed Matrix Membranes. J. Membr. Sci. 2006, 275, 17–28. [Google Scholar] [CrossRef]
- Penkova, A.V.; Acquah, S.F.A.; Dmitrenko, M.E.; Sokolova, M.P.; Mikhailova, M.Y.; Polyakov, E.S.; Ermakov, S.S.; Markelov, D.A.; Roizard, D. Improvement of Pervaporation PVA Membranes by the Controlled Incorporation of Fullerenol Nanoparticles. Mater. Des. 2016, 96, 416–423. [Google Scholar] [CrossRef]
- Zhang, B.; Lee, M.H.; Chakoli, A.N.; Zang, W.; Zhang, K.; Zhang, Y.; Song, G.; Chen, C.; Li, X.; Li, Y. Carbon Nanotube-Induced Morphological Transformation for Toughening of Benzoxazole-Containing Semi-Crystalline Polyimide. RSC Adv. 2014, 4, 14024. [Google Scholar] [CrossRef]
- Zhang, B.; Chakoli, A.N.; Zang, W.; Tian, Y.; Zhang, K.; Chen, C.; Li, Y. A Comparative Study on Effect of Aromatic Polyimide Chain Conformation on Reinforcement of Carbon Nanotube/polyimide Nanocomposites. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Larin, S.V.; Falkovich, S.G.; Nazarychev, V.M.; Gurtovenko, A.A.; Lyulin, A.V.; Lyulin, S.V. Molecular-Dynamics Simulation of Polyimide Matrix Pre-Crystallization near the Surface of a Single-Walled Carbon Nanotube. RSC Adv. 2014, 4, 830–844. [Google Scholar] [CrossRef]
- Fumagalli, L.; Esteban-Ferrer, D.; Cuervo, A.; Carrascosa, J.L.; Gomila, G. Label-Free Identification of Single Dielectric Nanoparticles and Viruses with Ultraweak Polarization Forces. Nat. Mater. 2012, 11, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, M.A.; Sokolova, M.P.; Geydt, P.; Smirnov, N.N.; Bobrova, N.V.; Toikka, A.M.; Lahderanta, E. Dual Doped Electroactive Hydrogelic Fibrous Mat with High Areal Capacitance. Mater. Lett. 2017, 199, 192–195. [Google Scholar] [CrossRef]
- Dokukin, M.E.; Sokolov, I. Quantitative Mapping of the Elastic Modulus of Soft Materials with HarmoniX and PeakForce QNM AFM Modes. Langmuir 2012, 28, 16060–16071. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, Z.; Sun, J.; Li, Z. Nano-Mechanical Characterization of Disassembling Amyloid Fibrils Using the Peak Force QNM Method. Biopolymers 2017, 107, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, D.; Russell, T.P.; Zhang, L. Nanomechanical Mapping of a Deformed Elastomer: Visualizing a Self-Reinforcement Mechanism. ACS Macro Lett. 2016, 5, 839–843. [Google Scholar] [CrossRef]
- Smolyakov, G.; Pruvost, S.; Cardoso, L.; Alonso, B.; Belamie, E.; Duchet-Rumeau, J. AFM PeakForce QNM Mode: Evidencing Nanometre-Scale Mechanical Properties of Chitin-Silica Hybrid Nanocomposites. Carbohydr. Polym. 2016, 151, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Smolyakov, G.; Pruvost, S.; Cardoso, L.; Alonso, B.; Belamie, E.; Duchet-Rumeau, J. PeakForce QNM AFM Study of Chitin-Silica Hybrid Films. Carbohydr. Polym. 2017, 166, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Megevand, B.; Pruvost, S.; Lins, L.C.; Livi, S.; Gérard, J.F.; Duchet-Rumeau, J. Probing Nanomechanical Properties with AFM to Understand the Structure and Behavior of Polymer Blends Compatibilized with Ionic Liquids. RSC Adv. 2016, 6, 96421–96430. [Google Scholar] [CrossRef]
- Shu, Z.; Jiao, X.; Chen, D. Hydrothermal Synthesis and Selective Photocatalytic Properties of Tetragonal Star-like ZrO2 Nanostructures. CrystEngComm 2013, 15, 4288. [Google Scholar] [CrossRef]
- Kudryavtsev, V.V.; Sukhanova, T.E.; Didenko, A.L.; Gubanova, G.N.; Svetlichnyi, V.M.; Yudin, V.E.; Marom, G.; Ratner, S. Semicrystalline Polyimide Matrices for Composites: Crystallization and Properties. J. Appl. Polym. Sci. 2002, 83, 2873–2882. [Google Scholar]
- Yudin, V.E.; Svetlichnyi, V.M. Effect of the Structure and Shape of Filler Nanoparticles on the Physical Properties of Polyimide Composites. Russ. J. Gen. Chem. 2010, 80, 2157–2169. [Google Scholar] [CrossRef]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolova, M.P.; Smirnov, M.A.; Bugrov, A.N.; Geydt, P.; Popova, E.N.; Lahderanta, E.; Svetlichnyi, V.M.; Toikka, A.M. Structure of Composite Based on Polyheteroarylene Matrix and ZrO2 Nanostars Investigated by Quantitative Nanomechanical Mapping. Polymers 2017, 9, 268. https://doi.org/10.3390/polym9070268
Sokolova MP, Smirnov MA, Bugrov AN, Geydt P, Popova EN, Lahderanta E, Svetlichnyi VM, Toikka AM. Structure of Composite Based on Polyheteroarylene Matrix and ZrO2 Nanostars Investigated by Quantitative Nanomechanical Mapping. Polymers. 2017; 9(7):268. https://doi.org/10.3390/polym9070268
Chicago/Turabian StyleSokolova, Maria P., Michael A. Smirnov, Alexander N. Bugrov, Pavel Geydt, Elena N. Popova, Erkki Lahderanta, Valentin M. Svetlichnyi, and Alexander M. Toikka. 2017. "Structure of Composite Based on Polyheteroarylene Matrix and ZrO2 Nanostars Investigated by Quantitative Nanomechanical Mapping" Polymers 9, no. 7: 268. https://doi.org/10.3390/polym9070268
APA StyleSokolova, M. P., Smirnov, M. A., Bugrov, A. N., Geydt, P., Popova, E. N., Lahderanta, E., Svetlichnyi, V. M., & Toikka, A. M. (2017). Structure of Composite Based on Polyheteroarylene Matrix and ZrO2 Nanostars Investigated by Quantitative Nanomechanical Mapping. Polymers, 9(7), 268. https://doi.org/10.3390/polym9070268










