Catalyzed Synthesis and Characterization of a Novel Lignin-Based Curing Agent for the Curing of High-Performance Epoxy Resin
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
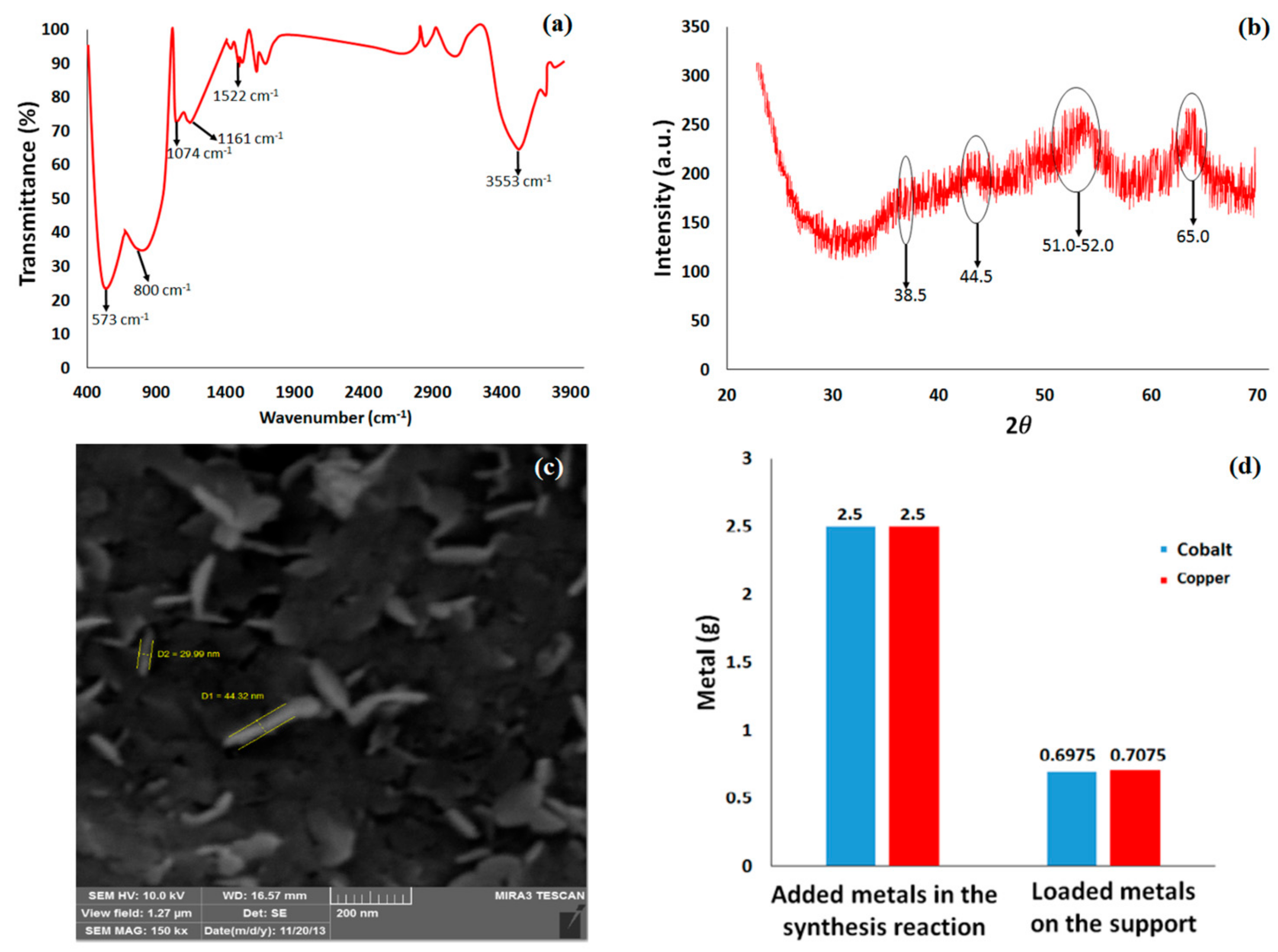
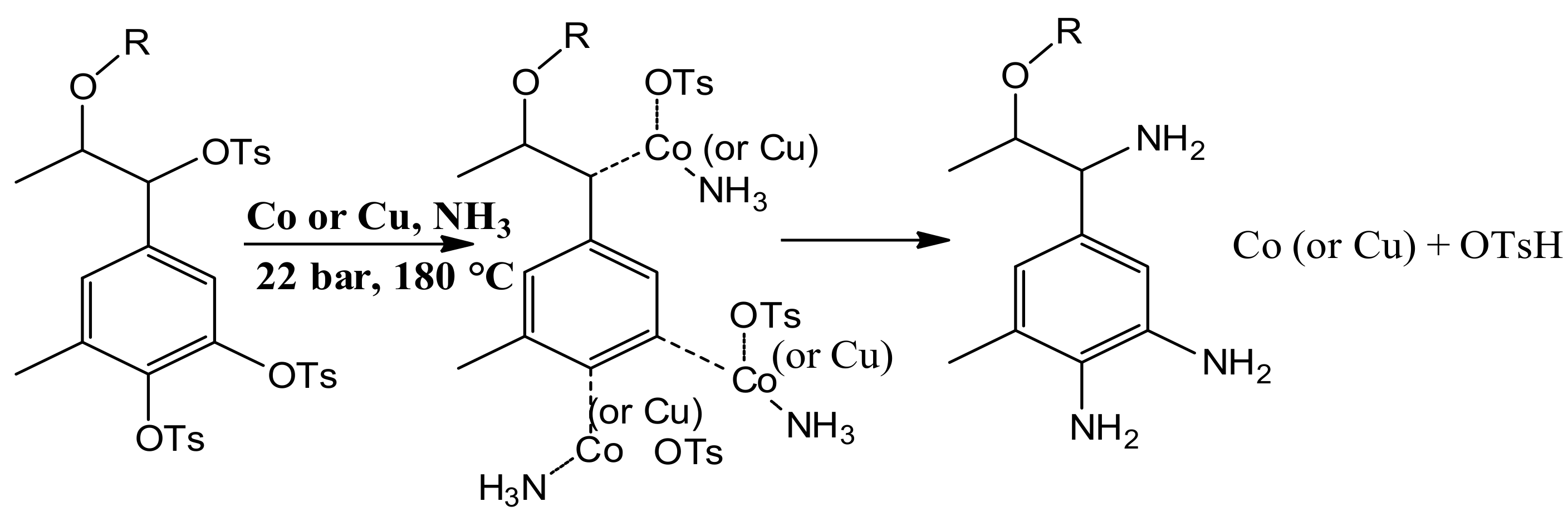
2.2.1. Synthesis of Nanocatalyst for Amination Reaction
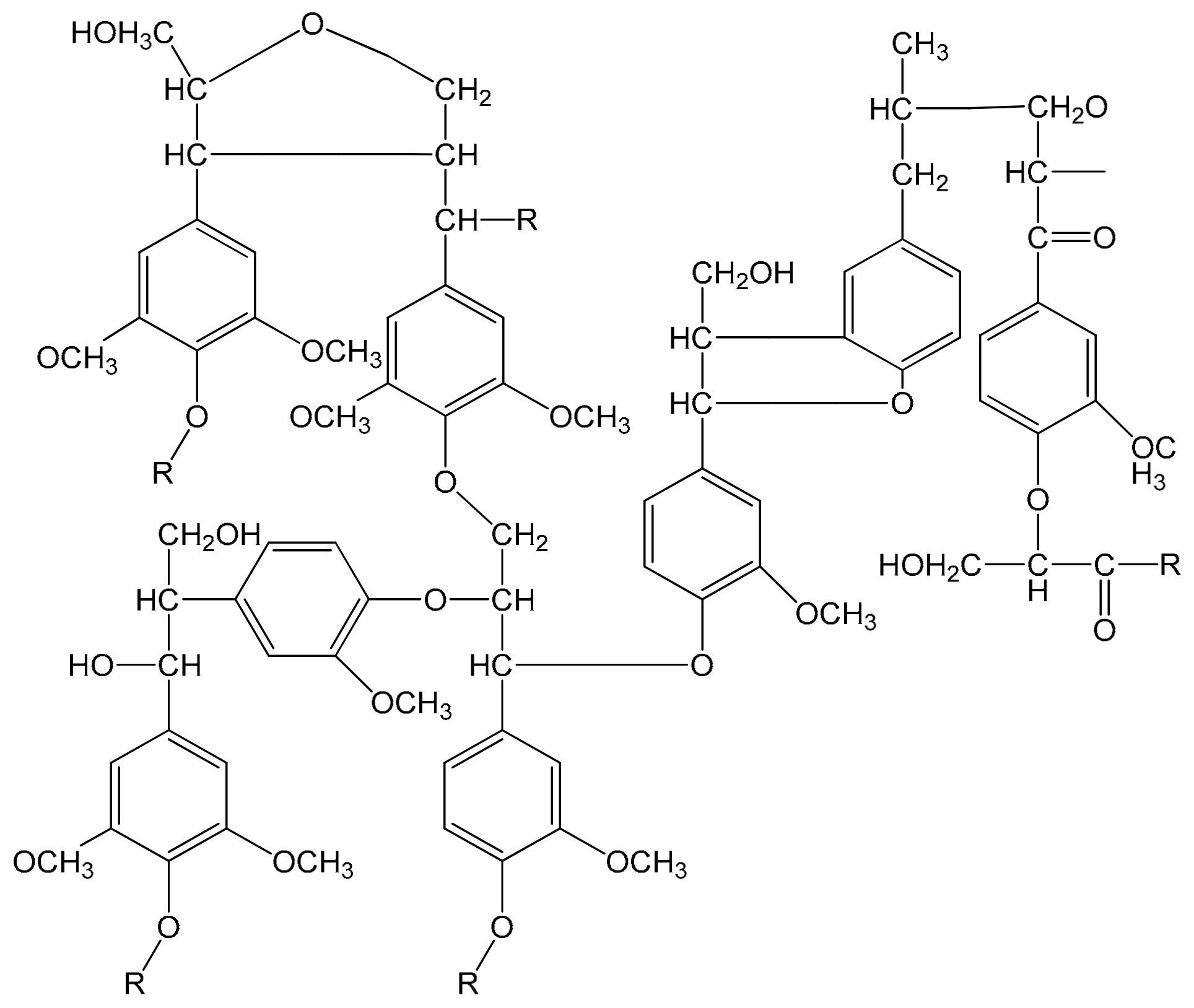
2.2.2. Separation Lignin from Black Liquor
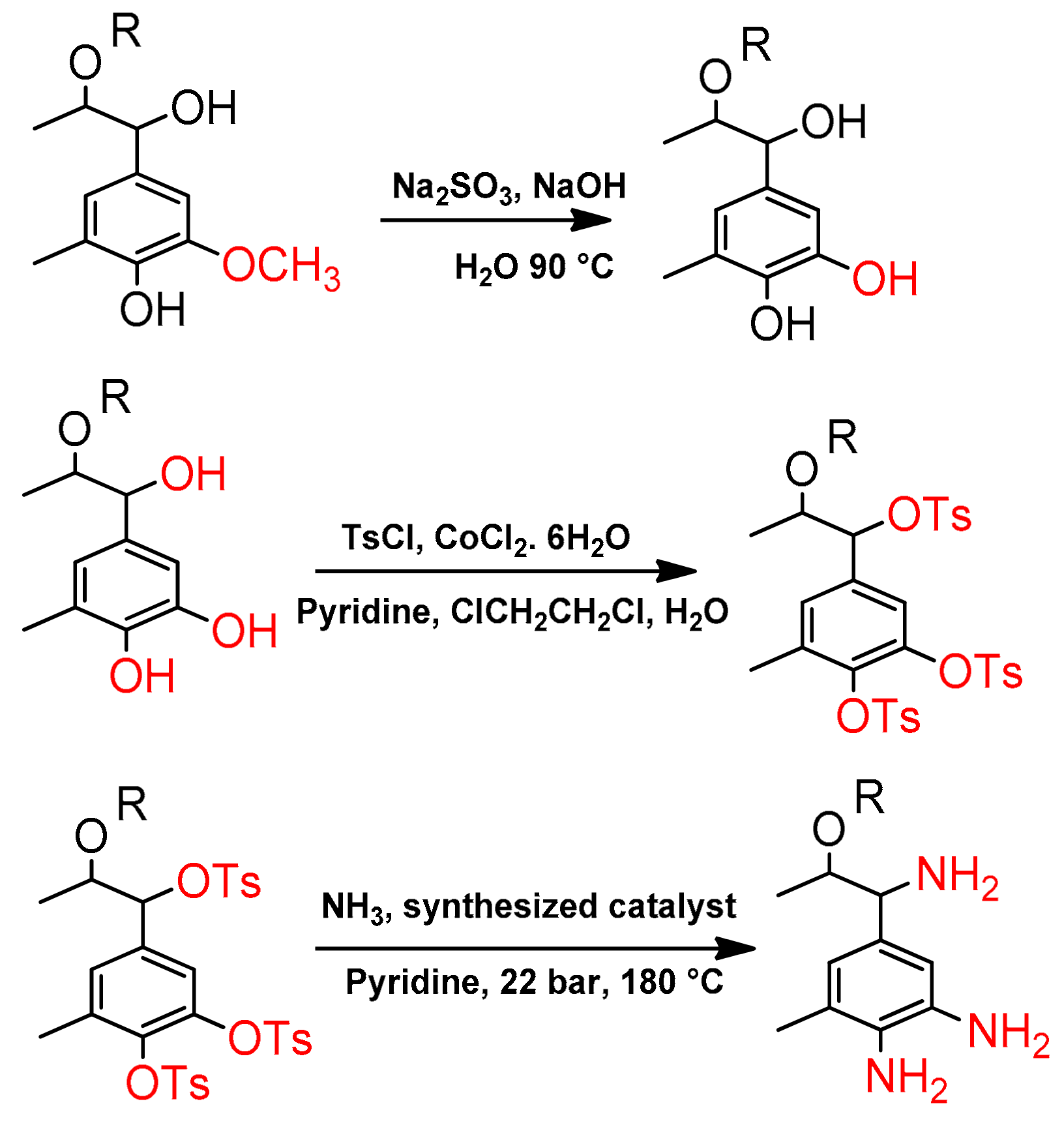
2.2.3. Synthesis of Lignin-Based Curing Agent
2.2.4. Epoxy Systems Preparation
2.3. Characterizations
3. Results and Discussion
3.1. Nanocatalyst Characterization
3.2. Lignin-Based Curing Agent Characterization
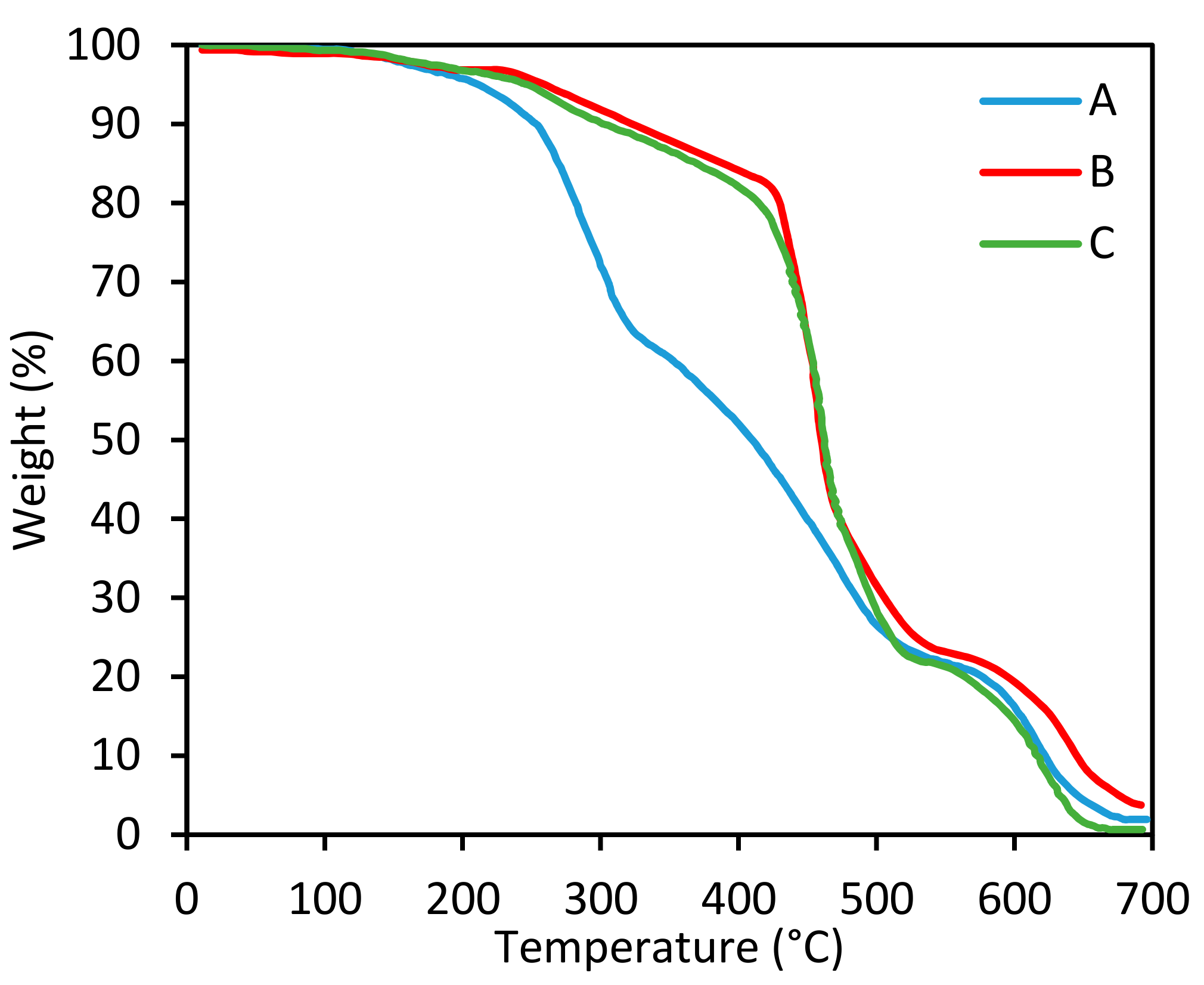
3.3. Thermal Properties of Epoxy Systems
3.4. Mechanical Performance of Epoxy Systems
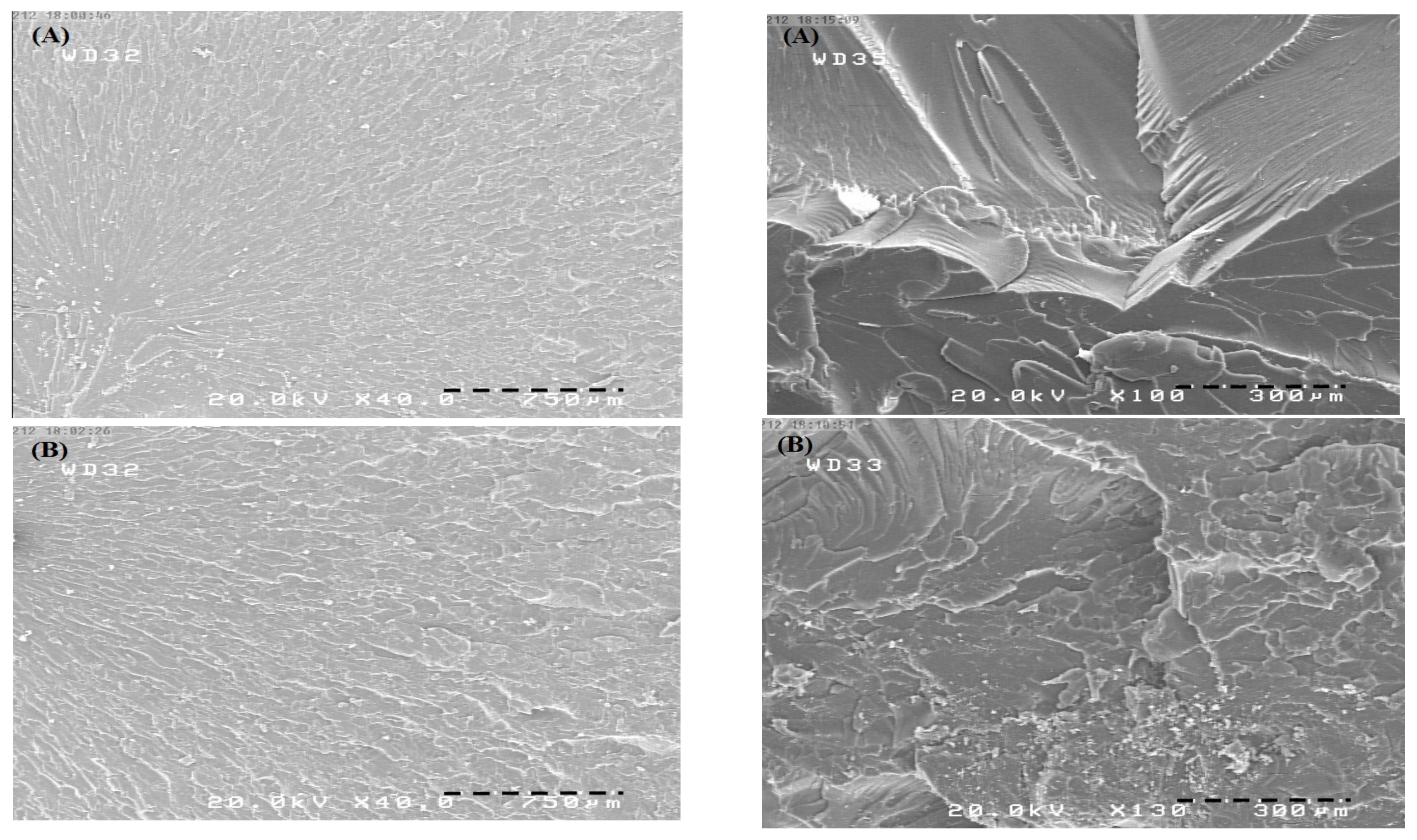
3.5. Morphological Observations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, S.; Liu, X.; Jiang, Y.; Tang, Z.; Zhang, C.; Zhu, J. Bio-based epoxy resin from itaconic acid and its thermosets cured with anhydride and comonomers. Green Chem. 2013, 15, 245–254. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with dgeba. RSC Adv. 2017, 7, 8694–8701. [Google Scholar] [CrossRef]
- Oroumei, A.; Fox, B.; Naebe, M. Thermal and rheological characteristics of biobased carbon fiber precursor derived from low molecular weight organosolv lignin. ACS Sustain. Chem. Eng. 2015, 3, 758–769. [Google Scholar] [CrossRef]
- Zhao, R.; Torley, P.; Halley, P.J. Emerging biodegradable materials: Starch- and protein-based bio-nanocomposites. J. Mater. Sci. 2008, 43, 3058–3071. [Google Scholar] [CrossRef]
- Avella, M.; Buzarovska, A.; Errico, M.E.; Gentile, G.; Grozdanov, A. Eco-challenges of bio-based polymer composites. Materials 2009, 2, 911–925. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-based bio-and nanocomposites: A review. Int. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Xiong, X.; Ren, R.; Liu, S.; Lu, S.; Chen, P. The curing kinetics and thermal properties of epoxy resins cured by aromatic diamine with hetero-cyclic side chain structure. Thermochim. Acta 2014, 595, 22–27. [Google Scholar] [CrossRef]
- Ren, R.; Xiong, X.; Ma, X.; Liu, S.; Wang, J.; Chen, P.; Zeng, Y. Isothermal curing kinetics and mechanism of dgeba epoxy resin with phthalide-containing aromatic diamine. Thermochim. Acta 2016, 623, 15–21. [Google Scholar] [CrossRef]
- Zabihi, O.; Khayyam, H.; Fox, B.L.; Naebe, M. Enhanced thermal stability and lifetime of epoxy nanocomposites using covalently functionalized clay: Experimental and modelling. New J. Chem. 2015, 39, 2269–2278. [Google Scholar] [CrossRef]
- Zabihi, O. Preparation and characterization of toughened composites of epoxy/poly(3,4-ethylenedioxythiophene) nanotube: Thermal, mechanical and electrical properties. Compos. B Eng. 2013, 45, 1480–1485. [Google Scholar] [CrossRef]
- Mirmohseni-Namin, A.; Nikafshar, S.; Mirmohseni, F. Increasing toughness and tensile strength of an epoxy-diamine system using an inorganic ultra-accelerator. RSC Adv. 2015, 5, 53025–53035. [Google Scholar] [CrossRef]
- Petrie, E. Epoxy Adhesive Formulations; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Tsantzalis, S.; Karapappas, P.; Vavouliotis, A.; Tsotra, P.; Paipetis, A.; Kostopoulos, V.; Friedrich, K. Enhancement of the mechanical performance of an epoxy resin and fiber reinforced epoxy resin composites by the introduction of cnf and pzt particles at the microscale. Compos. A Appl. Sci. Manuf. 2007, 38, 1076–1081. [Google Scholar] [CrossRef]
- Plastics Europe Epoxy Resins Committee. Epoxy Resins and Curing Agents: Toxicology, Health, Safety and Environmental Aspects; Plastics Europe Epoxy Resins Committee: Brussels, Belgium, 2006. [Google Scholar]
- Le, N.-T.; Byun, A.; Han, Y.; Lee, K.-I.; Kim, H. Preparation of 2, 5-bis (aminomethyl) furan by direct reductive amination of 2, 5-diformylfuran over nickel-raney catalysts. Green Sustain. Chem. 2015, 5, 115. [Google Scholar] [CrossRef]
- Hu, F.; Yadav, S.K.; La Scala, J.J.; Sadler, J.M.; Palmese, G.R. Preparation and characterization of fully furan-based renewable thermosetting epoxy-amine systems. Macromol. Chem. Phys. 2015, 216, 1441–1446. [Google Scholar] [CrossRef]
- Fache, M.; Montérémal, C.; Boutevin, B.; Caillol, S. Amine hardeners and epoxy cross-linker from aromatic renewable resources. Eur. Polym. J. 2015, 73, 344–362. [Google Scholar] [CrossRef]
- Ding, C.; Matharu, A.S. Recent developments on biobased curing agents: A review of their preparation and use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; LeBaron, P.C.; Park, I.; Pinnavaia, T.J. Epoxy−clay fabric film composites with unprecedented oxygen-barrier properties. Chem. Mater. 2006, 18, 4393–4398. [Google Scholar] [CrossRef]
- Metkar, P.S.; Scialdone, M.A.; Moloy, K.G. Lysinol: A renewably resourced alternative to petrochemical polyamines and aminoalcohols. Green Chem. 2014, 16, 4575–4586. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, F.; Wong, C. Novel, environmentally friendly crosslinking system of an epoxy using an amino acid: Tryptophan-cured diglycidyl ether of bisphenol a epoxy. J. Polym. Sci. A Polym. Chem. 2007, 45, 181–190. [Google Scholar] [CrossRef]
- Motahari, A.; Rostami, A.A.; Omrani, A.; Ehsani, M. On the thermal degradation of a novel epoxy-based nanocomposite cured with tryptophan as an environment-friendly curing agent. J. Macromol. Sci. B 2015, 54, 517–532. [Google Scholar] [CrossRef]
- Takahashi, T.; Hirayama, K.I.; Teramoto, N.; Shibata, M. Biocomposites composed of epoxidized soybean oil cured with terpene-based acid anhydride and cellulose fibers. J. Appl. Polym. Sci. 2008, 108, 1596–1602. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Liu, B.; Zhang, J.; Xian, M. Synthesis of rosin-based flexible anhydride-type curing agents and properties of the cured epoxy. Polym. Int. 2009, 58, 1435–1441. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhang, P.; Wolcott, M.; Zhang, J. Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym. Int. 2014, 63, 760–765. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Makino, K. Preparation and properties of biocomposites composed of epoxidized soybean oil, tannic acid, and microfibrillated cellulose. J. Appl. Polym. Sci. 2011, 120, 273–278. [Google Scholar] [CrossRef]
- Stemmelen, M.; Lapinte, V.; Habas, J.-P.; Robin, J.-J. Plant oil-based epoxy resins from fatty diamines and epoxidized vegetable oil. Eur. Polym. J. 2015, 68, 536–545. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, Y.; Li, M.; Lian, J.; Yang, X.; Xia, J. Preparation of a light color cardanol-based curing agent and epoxy resin composite: Cure-induced phase separation and its effect on properties. Prog. Org. Coat. 2012, 74, 240–247. [Google Scholar] [CrossRef]
- Pathak, S.K.; Rao, B. Structural effect of phenalkamines on adhesive viscoelastic and thermal properties of epoxy networks. J. Appl. Polym. Sci. 2006, 102, 4741–4748. [Google Scholar] [CrossRef]
- Darroman, E.; Bonnot, L.; Auvergne, R.; Boutevin, B.; Caillol, S. New cardanol-based aromatic amines for the synthesis of biobased epoxy networks. Eur. J. Lipid Sci. Technol. 2014, 117, 178–189. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Hao, C.; Dai, X.; Zhou, Z.; Si, N. Ultrasonic-assisted synthesis of aminated lignin by a mannich reaction and its decolorizing properties for anionic azo-dyes. RSC Adv. 2014, 4, 28156–28164. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.-F.; Deng, L.; Liao, B.; Guo, Q.-X. Improving aging resistance and mechanical properties of waterborne polyurethanes modified by lignin amines. J. Appl. Polym. Sci. 2013, 130, 1736–1742. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, H.; Qin, C.; Zhou, J.; Zhao, J.R.; Wang, S. Adsorption of heavy metal ion from aqueous single metal solution by aminated epoxy-lignin. BioResources 2013, 8, 2257–2269. [Google Scholar] [CrossRef]
- Ge, Y.; Song, Q.; Li, Z. A mannich base biosorbent derived from alkaline lignin for lead removal from aqueous solution. J. Ind. Eng. Chem. 2015, 23, 228–234. [Google Scholar] [CrossRef]
- Dilling, P.; Falkehag, S. Process for Producing Cationic Lignin Amines. U.S. Patent 3,718,639, 27 Feberury 1973. [Google Scholar]
- Naae, D.G.; Whittington, L.E.; Ledoux, W.A.; DeBons, F.E. Surfactants from Lignin. U.S. Patent 4,739,040, 19 April 1988. [Google Scholar]
- Schilling, P. Sulfomethylated Lignin Amines. U.S. Patent 4,859,362, 8 October 1988. [Google Scholar]
- Wang, M.; Sjöholm, E.; Li, J. Fast and reliable quantification of lignin reactivity via reaction with dimethylamine and formaldehyde (mannich reaction). Holzforschung 2017, 71, 27–34. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Dorrestijn, E.; Laarhoven, L.J.; Arends, I.W.; Mulder, P. The occurrence and reactivity of phenoxyl linkages in lignin and low rank coal. J. Anal. Appl. Pyrolysis 2000, 54, 153–192. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C.C. Synthesis of lignin-based epoxy resins: Optimization of reaction parameters using response surface methodology. RSC Adv. 2014, 4, 31745–31753. [Google Scholar] [CrossRef]
- Sasaki, C.; Wanaka, M.; Takagi, H.; Tamura, S.; Asada, C.; Nakamura, Y. Evaluation of epoxy resins synthesized from steam-exploded bamboo lignin. Ind. Crops Prod. 2013, 43, 757–761. [Google Scholar] [CrossRef]
- Jafari, H.; Afshar, S.; Zabihi, O.; Naebe, M. Enhanced photocatalytic activities of tio2–sio2 nanohybrids immobilized on cement-based materials for dye degradation. Res. Chem. Intermed. 2016, 42, 2963–2978. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Pan, H.; Sun, G.; Zhao, T. Synthesis and characterization of aminated lignin. Int. J. Biol. Macromol. 2013, 59, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Sun, G.; Zhao, T.; Wang, G. Thermal properties of epoxy resins crosslinked by an aminated lignin. Polym. Eng. Sci. 2015, 55, 924–932. [Google Scholar] [CrossRef]
- Adler, E. Lignin chemistry—Past, present and future. Wood Sci. Technol. 1977, 11, 169–218. [Google Scholar] [CrossRef]
- Ward, J.; Leach, B. Applied Industrial Catalysis; Leach, B.E., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 3, pp. 271–392. [Google Scholar]
- Wefers, K. Alumina Chemicals: Science and Technology Handbook; The American Ceramic Society: Westerville, OH, USA, 1990. [Google Scholar]
- Trueba, M.; Trasatti, S.P. Γ-alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.S.; Park, N.-K.; Lee, T.J.; Kang, M. Low temperature synthesis of α-alumina from aluminum hydroxide hydrothermally synthesized using [al(c2o4)x(oh)y] complexes. Chem. Eng. J. 2013, 230, 351–360. [Google Scholar] [CrossRef]
- Parida, K.M.; Pradhan, A.C.; Das, J.; Sahu, N. Synthesis and characterization of nano-sized porous gamma-alumina by control precipitation method. Mater. Chem. Phys. 2009, 113, 244–248. [Google Scholar] [CrossRef]
- El-Naggar, A.Y. Characterization of modified and polymer coated alumina surfaces by infrared spectroscopy. J. Spectrosc. 2013, 2013, 706960. [Google Scholar] [CrossRef]
- Rozita, Y.; Brydson, R.; Scott, A. An investigation of commercial gamma-Al2O3 nanoparticles. J. Phys. 2010, 241, 012096. [Google Scholar]
- Salavati-Niasari, M.; Davar, F.; Mazaheri, M.; Shaterian, M. Preparation of cobalt nanoparticles from [bis(salicylidene)cobalt(ii)]–oleylamine complex by thermal decomposition. J. Magn. Magn. Mater. 2008, 320, 575–578. [Google Scholar] [CrossRef]
- Sperotto, E.; van Klink, G.P.; van Koten, G.; de Vries, J.G. The mechanism of the modified ullmann reaction. Dalton Trans. 2010, 39, 10338–10351. [Google Scholar] [CrossRef] [PubMed]
- Aubin, Y.; Fischmeister, C.; Thomas, C.M.; Renaud, J.-L. Direct amination of aryl halides with ammonia. Chem. Soc. Rev. 2010, 39, 4130–4145. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, J.L.; Hartwig, J.F. Catalytic organometallic reactions of ammonia. Angew. Chem. Int. Ed. 2011, 50, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Vo, G.D.; Hartwig, J.F. Palladium-catalyzed coupling of ammonia with aryl chlorides, bromides, iodides and sulfonates: A general method for the preparation of primary arylamines. J. Am. Chem. Soc. 2009, 131, 11049. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K. A study of chemical structure of soft and hardwood and wood polymers by ftir spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2008. [Google Scholar]
- Zabihi, O. Modeling of phenomenological mechanisms during thermal formation and degradation of an epoxy-based nanocomposite. Thermochim. Acta 2012, 543, 239–245. [Google Scholar] [CrossRef]
- Zabihi, O.; Khodabandeh, A.; Ghasemlou, S. Investigation of mechanical properties and cure behavior of dgeba/nano-fe2o3 with polyamine dendrimer. Polym. Degrad. Stab. 2012, 97, 1730–1736. [Google Scholar] [CrossRef]
- Zabihi, O.; Aghaie, M.; Zare, K. Study on a novel thermoset nanocomposite form dgeba–cycloaliphatic diamine and metal nanoparticles. J. Therm. Anal. Calorim. 2013, 111, 703–710. [Google Scholar] [CrossRef]
- Tant, M.R.; Hill, A.J. Structure and Properties of Glassy Polymers; American Chemical Society; Distributed by Oxford University Press: Washington, DC, USA, 1998. [Google Scholar]
- Fox, T.G., Jr.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Podgorski, L. Analysis of the wood coating ageing and prediction of the durability through calorimetric investigations. Proc. Wood Coat. Syst. Wood-Cost E18 2004, 12, 88–92. [Google Scholar]
- Chung, H.; Washburn, N.R. Improved lignin polyurethane properties with lewis acid treatment. ACS Appl. Mater. Interfaces 2012, 4, 2840–2846. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.A.; Sperling, L.H.; Kim, S.L. Influence of Crosslinking on the Mechanical Properties of High t (g) Polymers; DTIC: Bethlehem, PA, USA, 1977. [Google Scholar]
- Gupta, V.; Drzal, L.; Lee, C.C.; Rich, M. The temperature-dependence of some mechanical properties of a cured epoxy resin system. Polym. Eng. Sci. 1985, 25, 812–823. [Google Scholar] [CrossRef]
- Fu, J.F.; Shi, L.Y.; Zhong, Q.D.; Chen, Y.; Chen, L.Y. Thermally conductive and electrically insulative nanocomposites based on hyperbranched epoxy and nano-Al2O3 particles modified epoxy resin. Polym. Adv. Technol. 2011, 22, 1032–1041. [Google Scholar] [CrossRef]
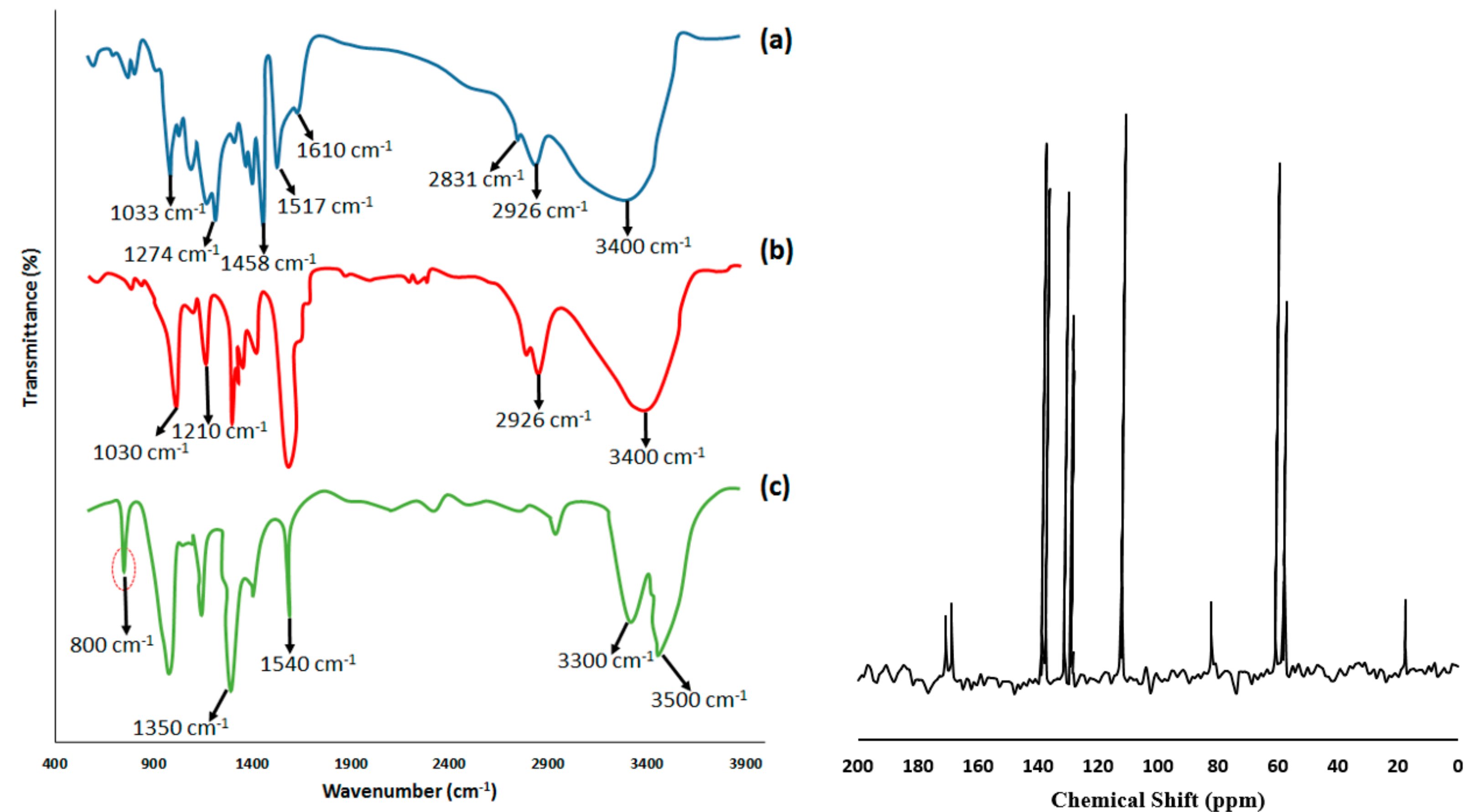


| Type of Lignin | Carbon (%) | Hydrogen (%) | Oxygen (%) | Nitrogen (%) | Sulfur (%) |
|---|---|---|---|---|---|
| Separated lignin | 57.16 | 4.61 | 34.17 | 1.68 | 1.56 |
| Aminated lignin | 55.87 | 7.58 | 29.82 | 4.63 | 1.39 |
| Sample Code | Curing Agent (Phr) | Ti (°C) | Tp (°C) | ∆H (J/g) | Tg (°C) |
|---|---|---|---|---|---|
| A | 9.9 | 114.1 | 124.8 | 317.21 | 97.4 |
| B | 12.9 | 102.6 | 129.4 | 628.79 | 158.3 |
| C | 15.9 | 93.9 | 121.2 | 684.36 | 171.8 |
| Sample Code | Curing Agent (Phr) | Tonset (°C) | T5% (°C) | Tmax (°C) |
|---|---|---|---|---|
| A | 9.9 | 172.7 | 257.7 | 286.6 |
| B | 12.9 | 243.2 | 309.8 | 456.2 |
| C | 15.9 | 239.4 | 297.1 | 457.9 |
| Sample Code | Curing Agent (Phr) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Elongation at Break (%) | Izod Impact Strengths (kJ/m2) |
|---|---|---|---|---|---|
| A | 9.9 | 43.47 ± 2.6 | 4104.71 ± 38.5 | 4.46 ± 0.37 | 27.11 |
| B | 12.9 | 86.91 ± 4.3 | 3029.64 ± 54.6 | 3.23 ± 0.62 | 35.73 |
| C | 15.9 | 79.12 ± 4.7 | 3467.28 ± 45.8 | 2.17 ± 0.22 | 34.29 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikafshar, S.; Zabihi, O.; Moradi, Y.; Ahmadi, M.; Amiri, S.; Naebe, M. Catalyzed Synthesis and Characterization of a Novel Lignin-Based Curing Agent for the Curing of High-Performance Epoxy Resin. Polymers 2017, 9, 266. https://doi.org/10.3390/polym9070266
Nikafshar S, Zabihi O, Moradi Y, Ahmadi M, Amiri S, Naebe M. Catalyzed Synthesis and Characterization of a Novel Lignin-Based Curing Agent for the Curing of High-Performance Epoxy Resin. Polymers. 2017; 9(7):266. https://doi.org/10.3390/polym9070266
Chicago/Turabian StyleNikafshar, Saeid, Omid Zabihi, Yousef Moradi, Mojtaba Ahmadi, Saba Amiri, and Minoo Naebe. 2017. "Catalyzed Synthesis and Characterization of a Novel Lignin-Based Curing Agent for the Curing of High-Performance Epoxy Resin" Polymers 9, no. 7: 266. https://doi.org/10.3390/polym9070266
APA StyleNikafshar, S., Zabihi, O., Moradi, Y., Ahmadi, M., Amiri, S., & Naebe, M. (2017). Catalyzed Synthesis and Characterization of a Novel Lignin-Based Curing Agent for the Curing of High-Performance Epoxy Resin. Polymers, 9(7), 266. https://doi.org/10.3390/polym9070266








