RETRACTED: Controlled Light Cross-Linking Technique to Prepare Healable Materials
Abstract
:1. Introduction
2. Materials and Methods
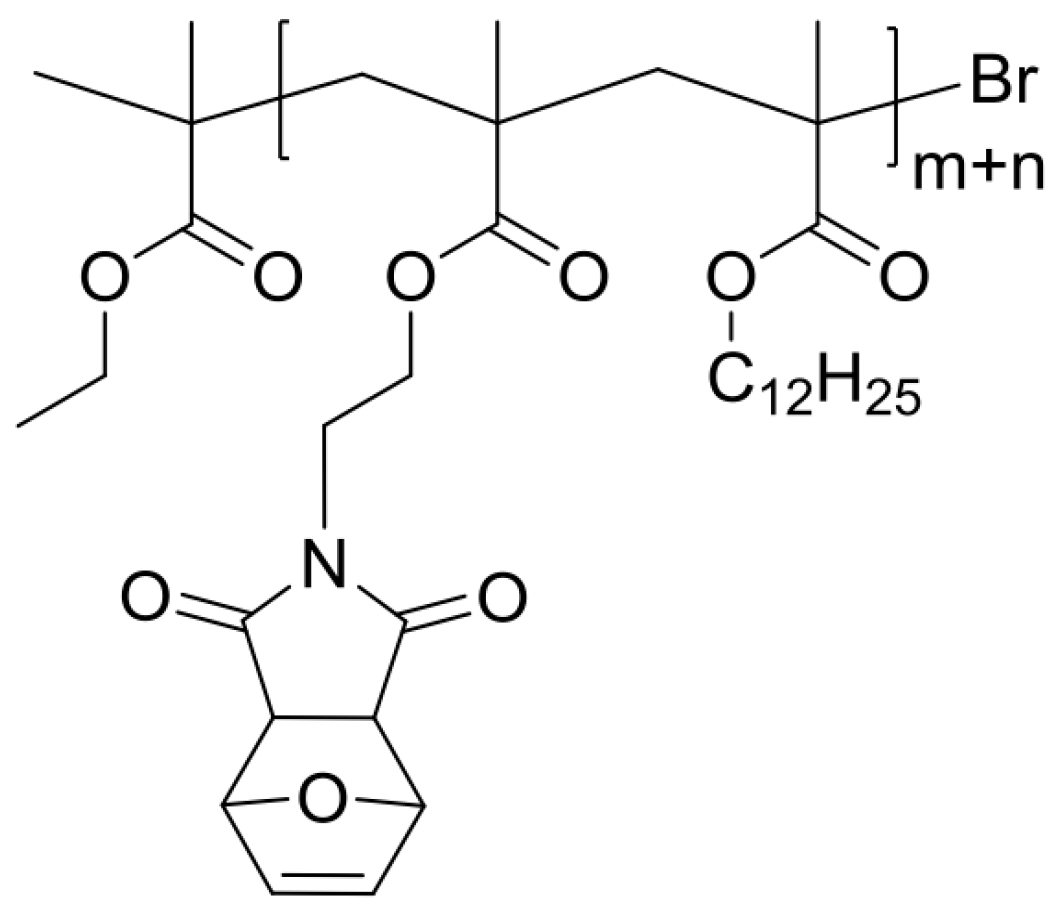
2.1. General Procedure for Preparation of Cross Linkers (X#PON and X#POFF)
- In argon ambient: We dissolved co-polymer P (1.0 equivalent maleimide side-chains) and cross-linker XON (0.7 equivalent furan termini) in a minimum amount of dry tetra-hydro-furan (THF) in a 2.5 mL vial.
- Then, we drop-casted onto a glass slide (GS) positioned in a Schlenk flask.
- We pursuit this by immediate evacuation of Schlenk flask.
- In order to get rid of residual solvent and to anneal the polymer mixture, we heated the evacuated-Schlenk flask (ESF) at 130 °C for 1.5 h.
- In ESF, at room temperature, we carried out thermal cross-linking for about 16 h.
- Then, we perform drop casting on to steel plates, for rheology.
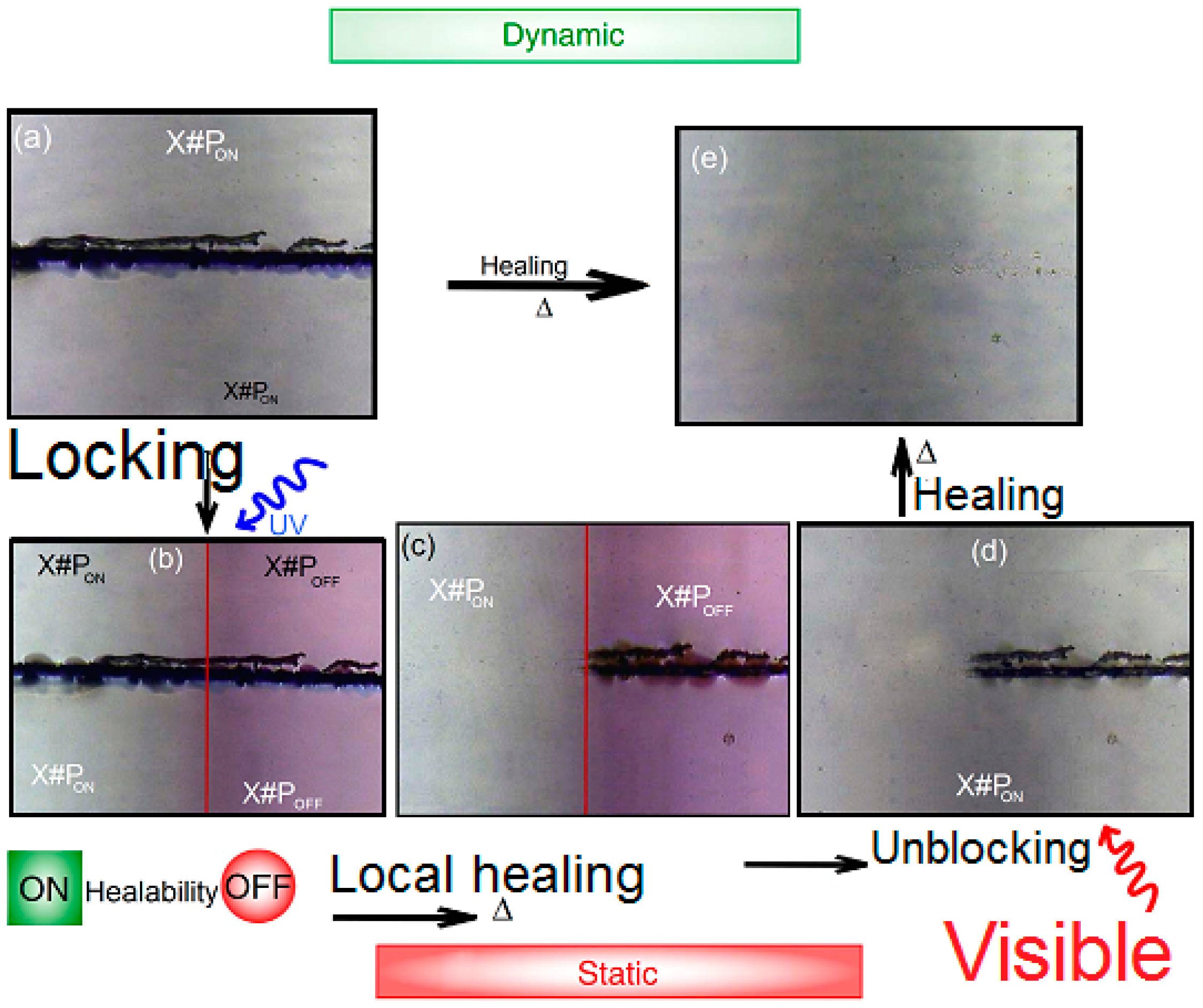
- We irradiate about 50% of the material using a LED XSL-365 nm-5E (Roithner Laser TecchnikGmbH, Vienna, Austria,) with electric polarization 4.2 V for LED under electric current 20 mA. The distance is about 3 cm normal to the sample. We kept the locked polymer network sample X#POFF for more analysis.
- For about 2 h, we used LED Engin-460 nm-Blue Emitter LZ4/00B208 (Roithner Laser TecchnikGmbH, Vienna, Austria) with electric polarization 12 V for LED under electric current 2–3 mA to irradiate the locked and heated polymer films. The relatively long period of time (2 h) allows more examining of the reestablishment of the healability.
2.2. Scratching Techniques for Blocking Cross-Linker and Un-Blocking Cross-Linker (X#PON and X#POFF)
2.3. Optical Micrographs
2.4. Fourier-Transform Infrared (FTIR) Spectroscopy
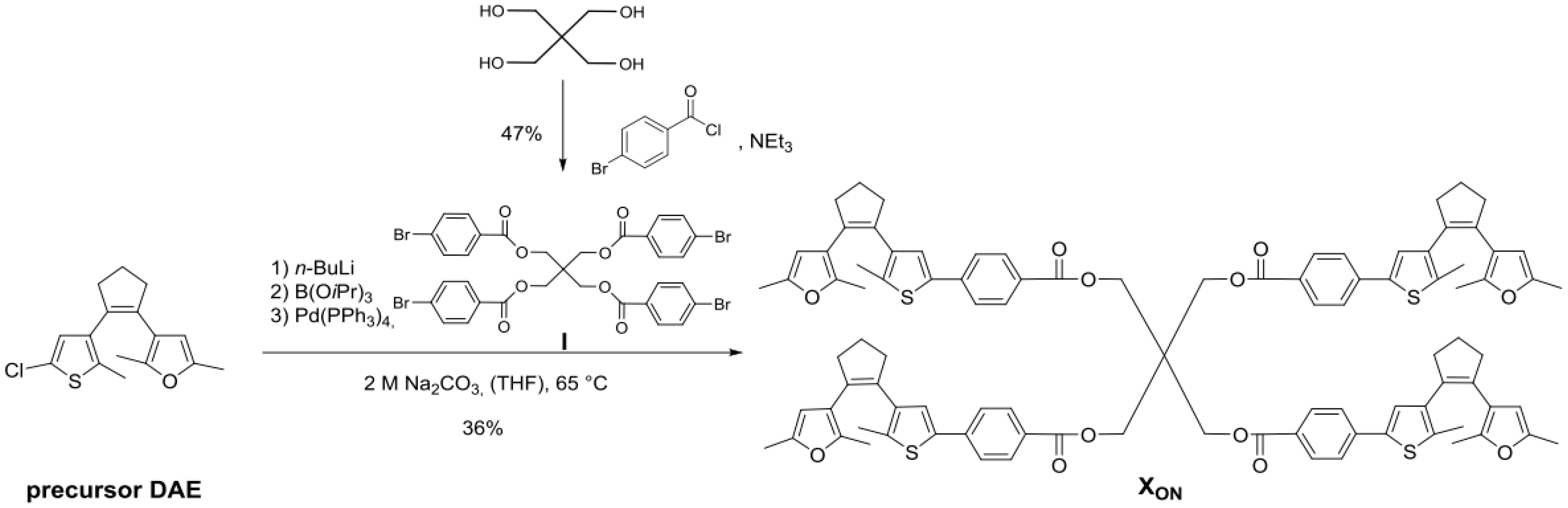
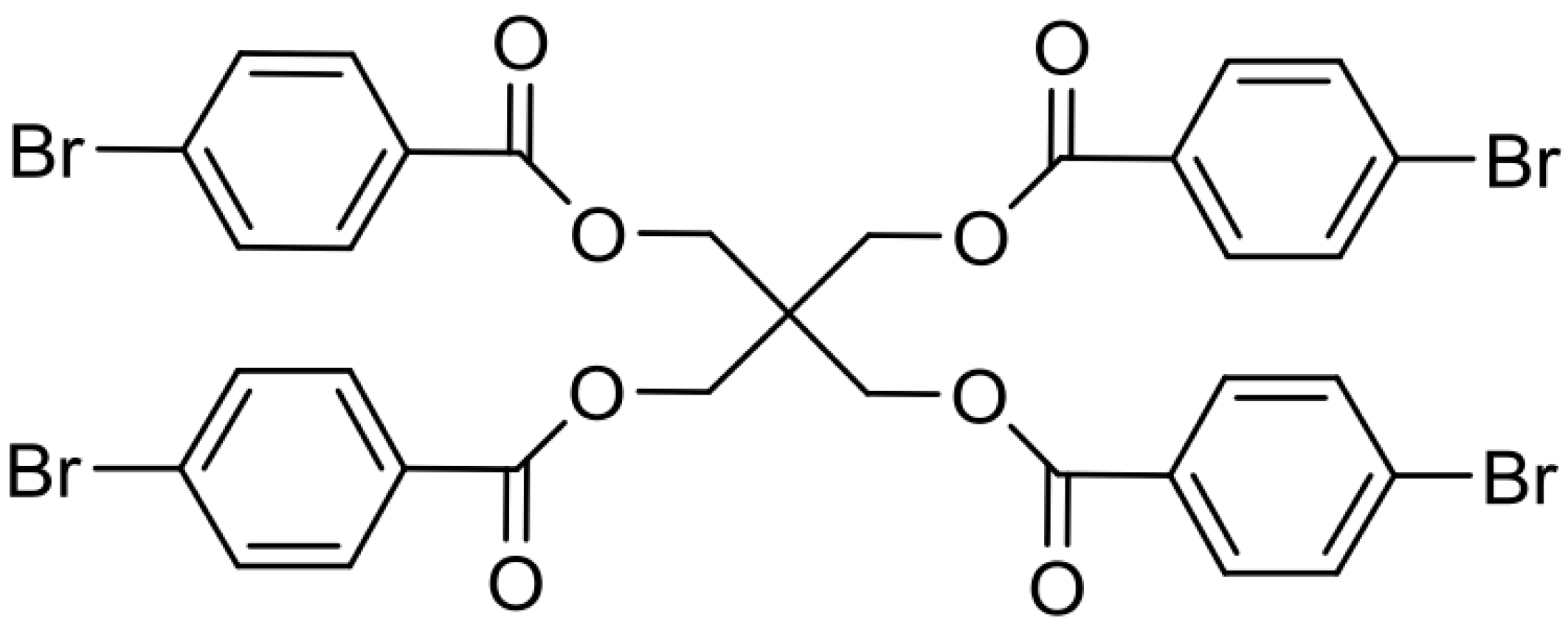
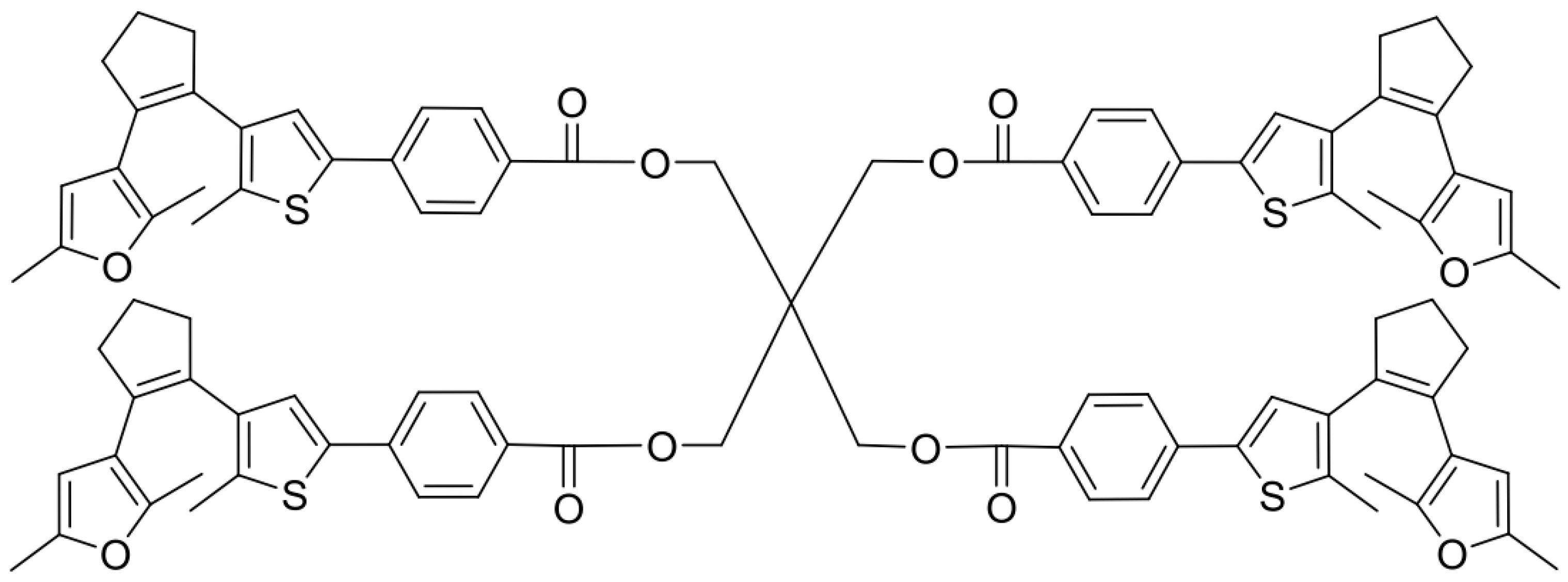
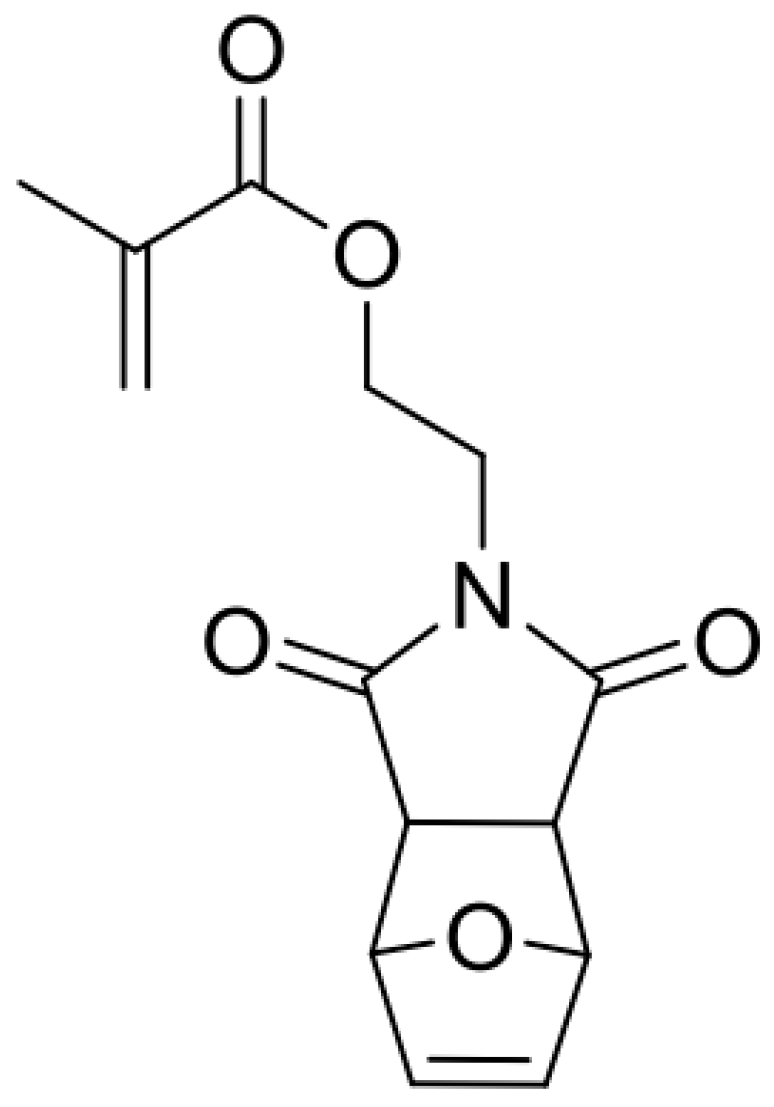
2.5. Synthesis of Cross-Linker XON
2.6. Synthesis of Monomer MIMA and Blank, Linear Polymer P
3. Results
3.1. Preparation of Linear Polymer and Cross-Linker
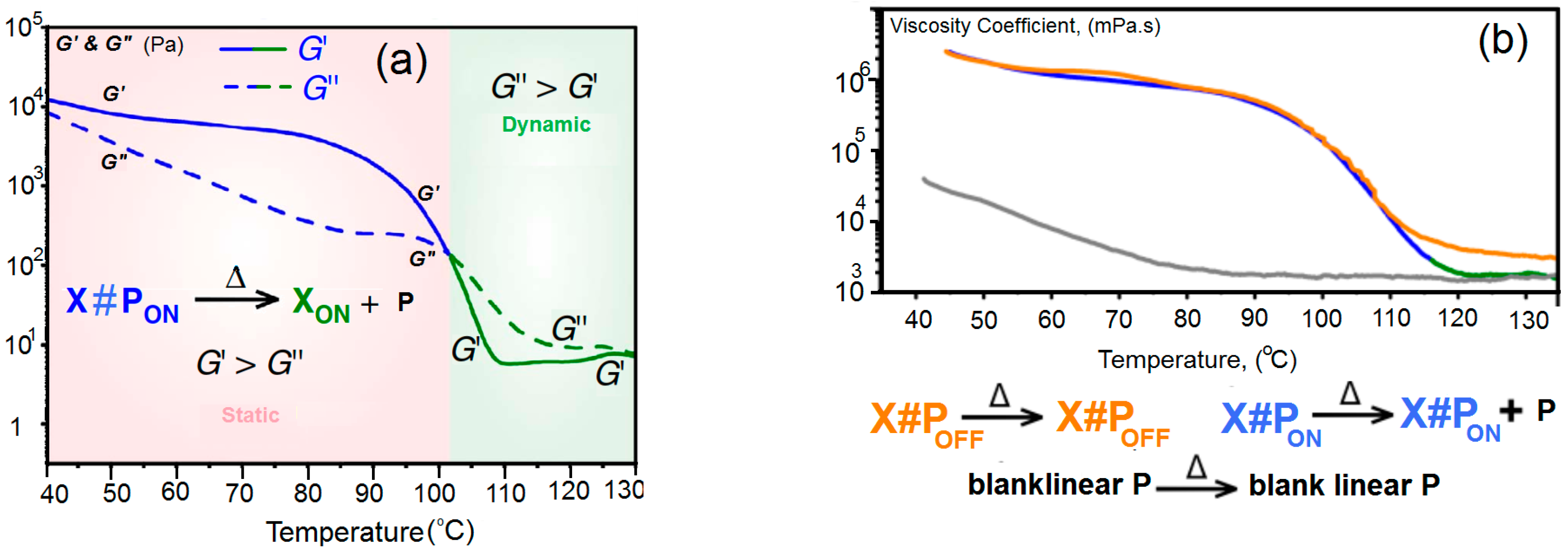
3.2. Mechanical Properties of Linear Polymer P and Polymer Networks
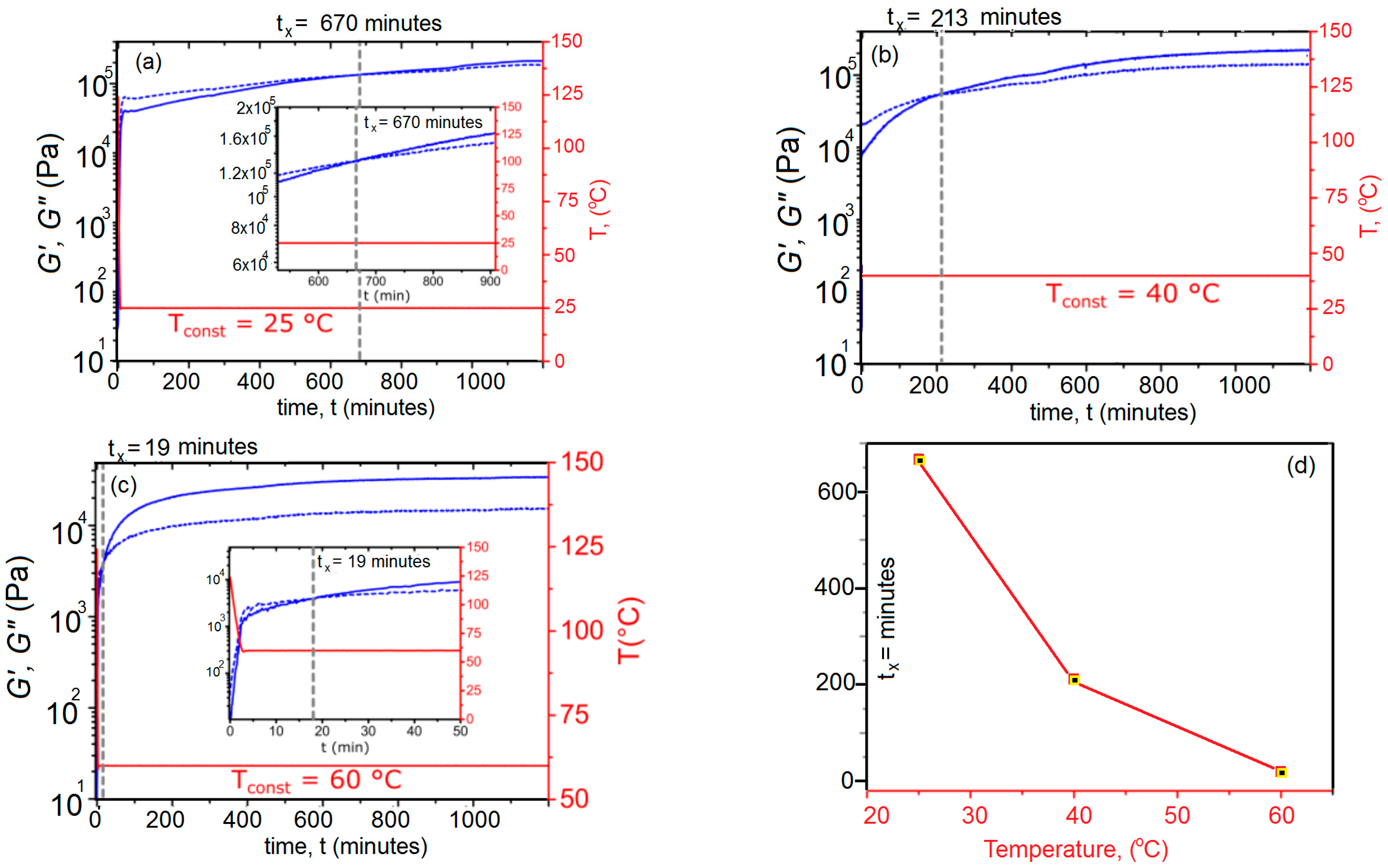
3.3. Temperature-Controlled Sol–Gel Transition in X#PON
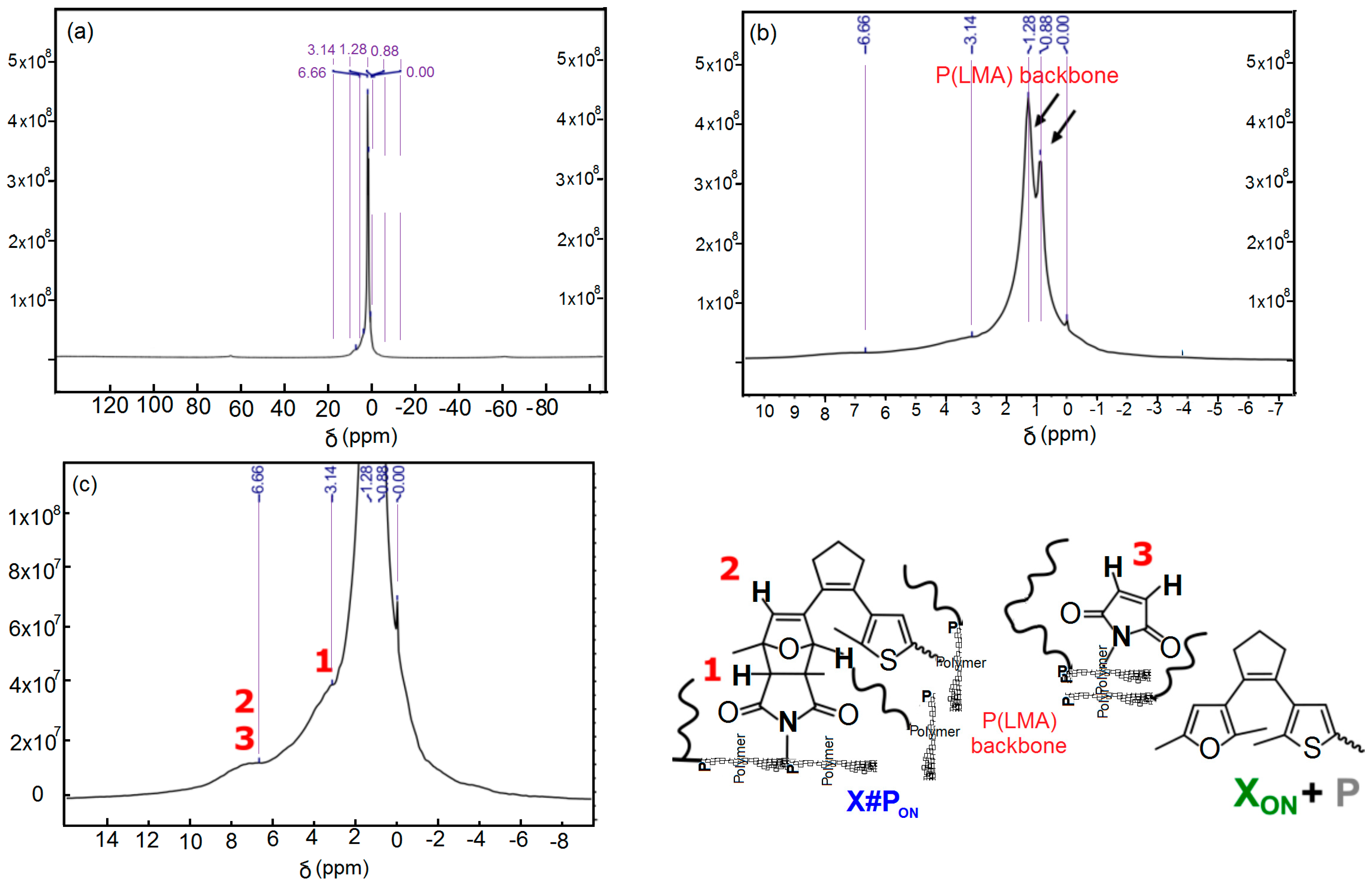
3.4. FT-IR Spectroscopic Analysis of the De-Protection of Furyl-Protected, Inactive Linear Polymer fpP to Form Non-Protected, Active Linear Polymer P via Cleavage of the Furan Protection Group
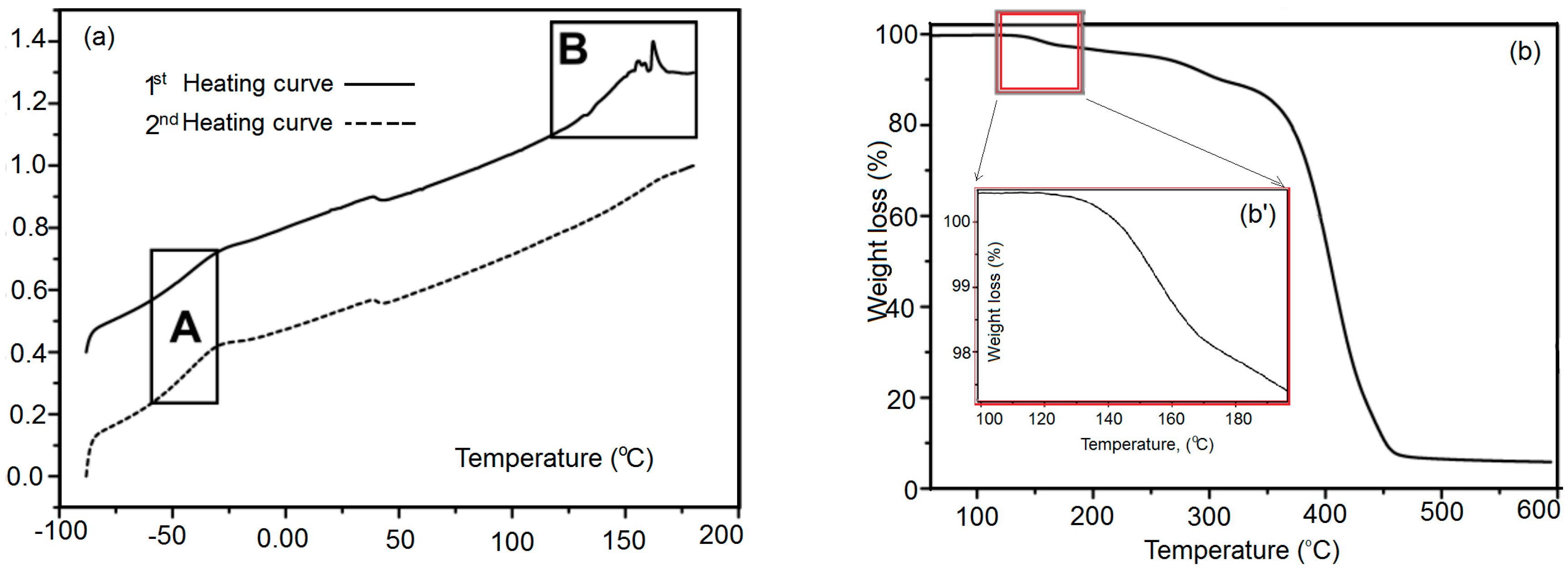
3.5. Differential Scanning Calorimetry Measurements of Polymer Networks X#PON and X#POFF
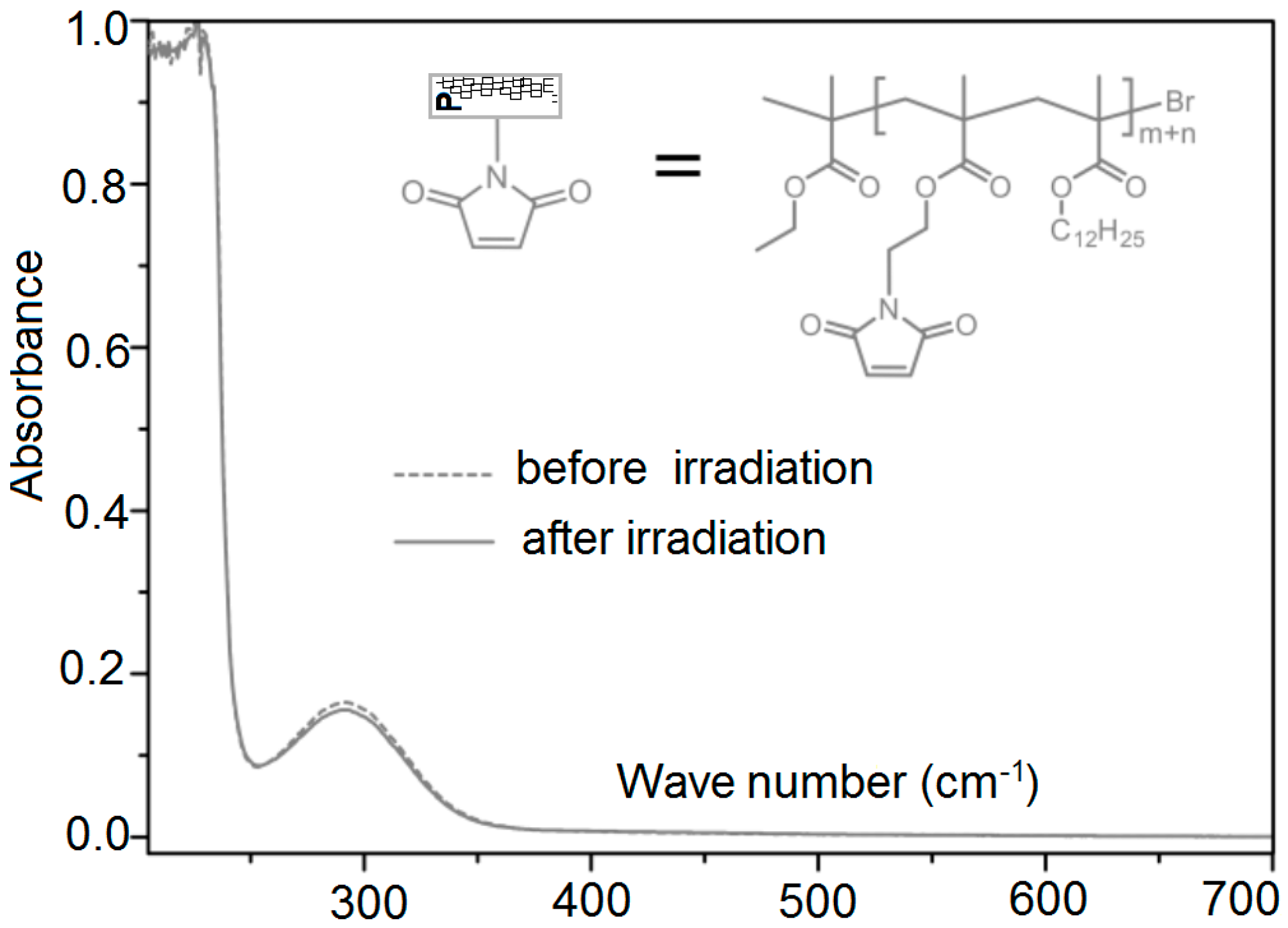
3.6. UV Irradiation of Polymer Chains
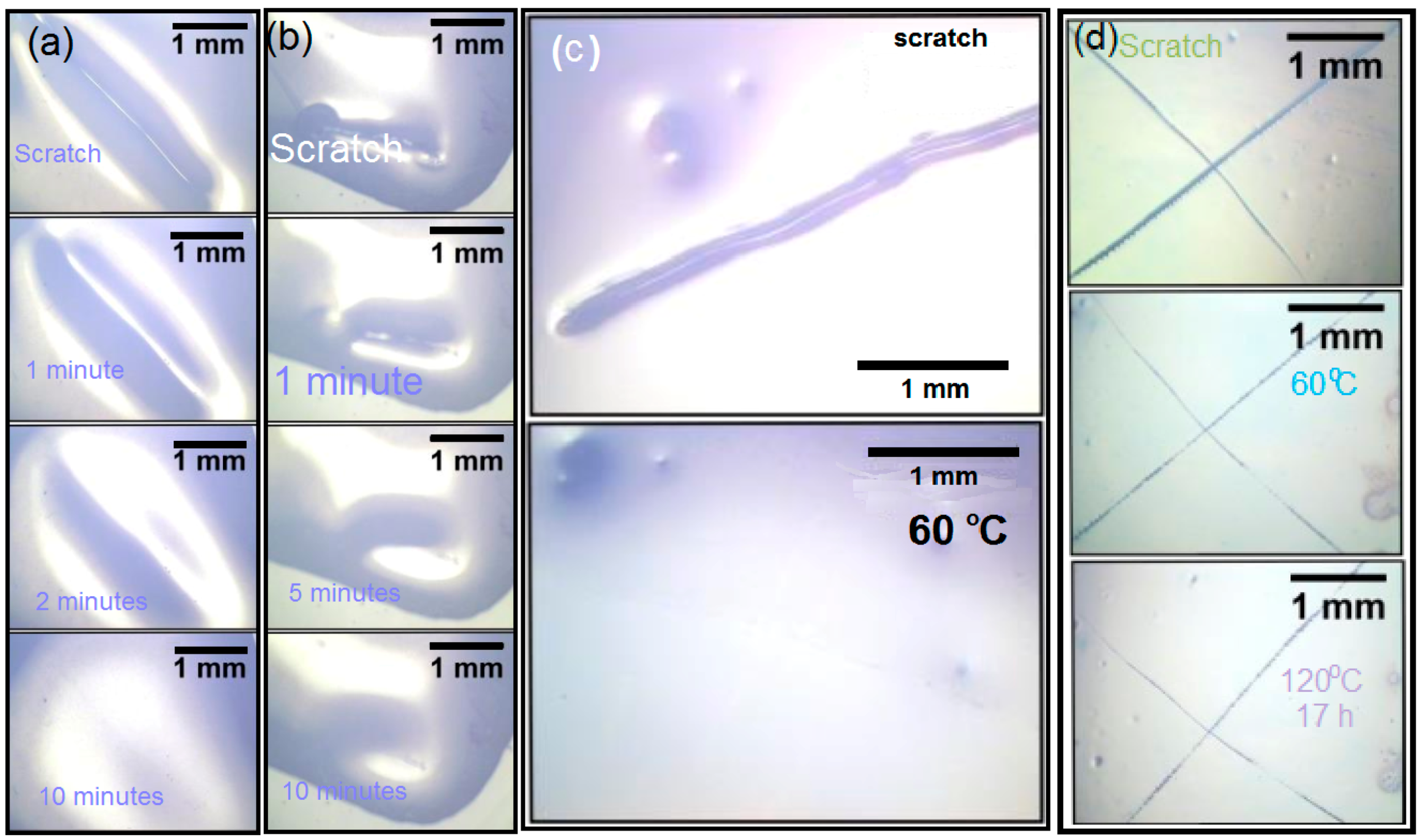
3.7. Scratching Tests of X#P (without Light Control) Upon Variation of Cross-Linking Density
Scratching Tests of the Bulk Material of Blank Sample P without Cross-Linker
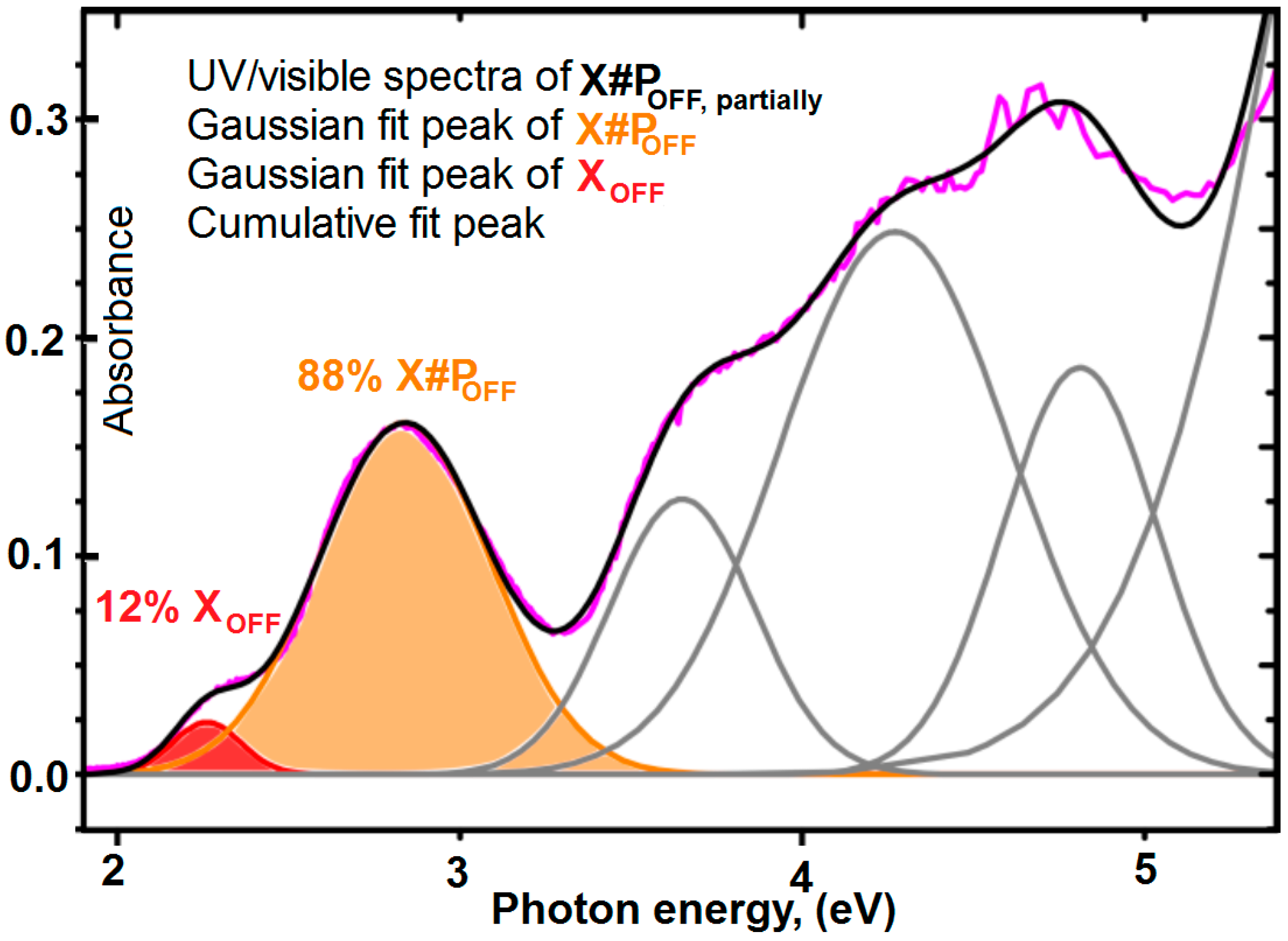
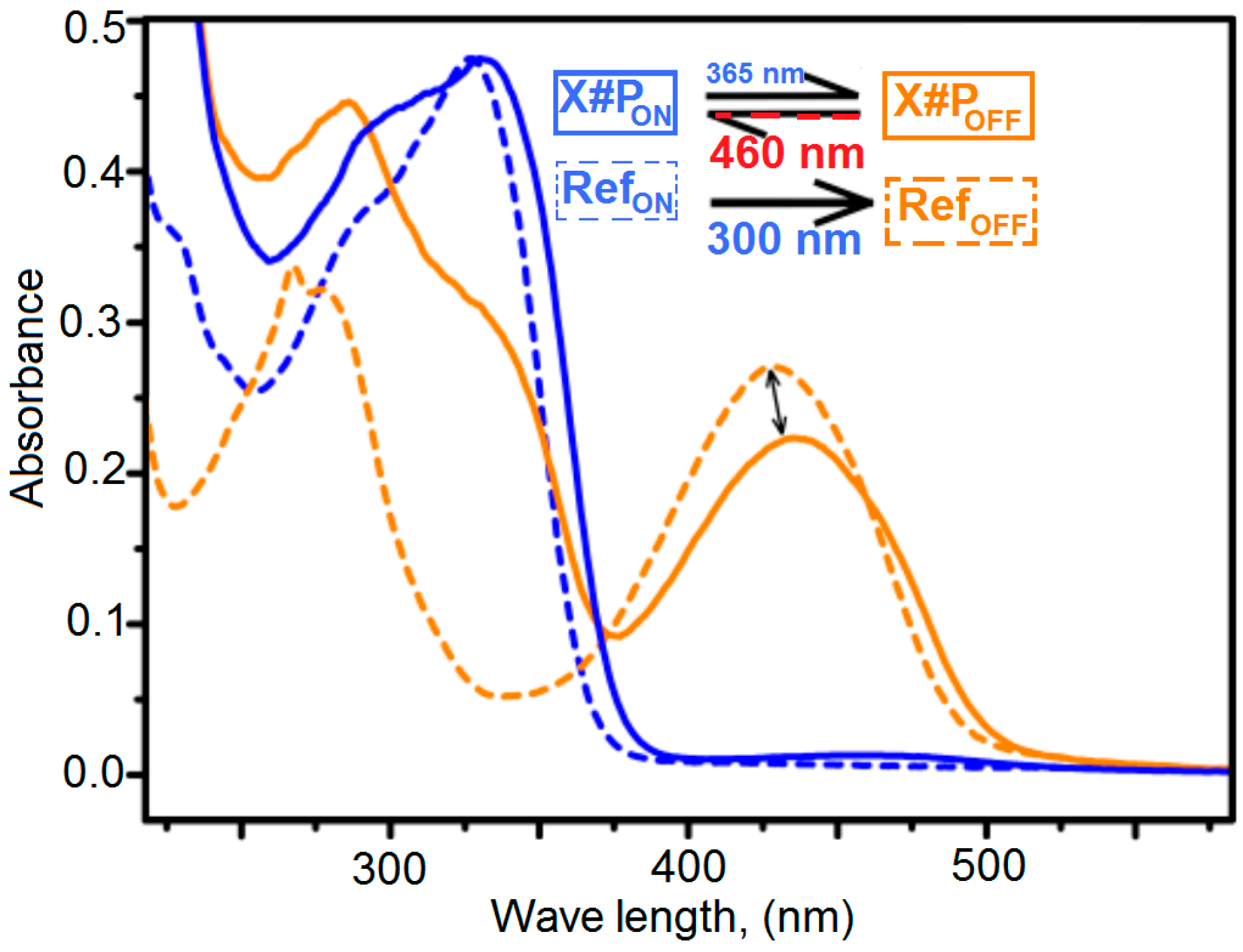
3.8. Determination of the Conversion of the Diels–Alder Cross-Linking Reaction of X#POFF in the Photo Stationary State via UV/Visible Spectroscopic Analysis
3.9. UV-Vis Spectroscopy of Unlocked Polymer Network X#PON
3.10. UV/Visible Spectra for Determination of the Photo-Conversion of X#POFF in the Photo-Stationary State
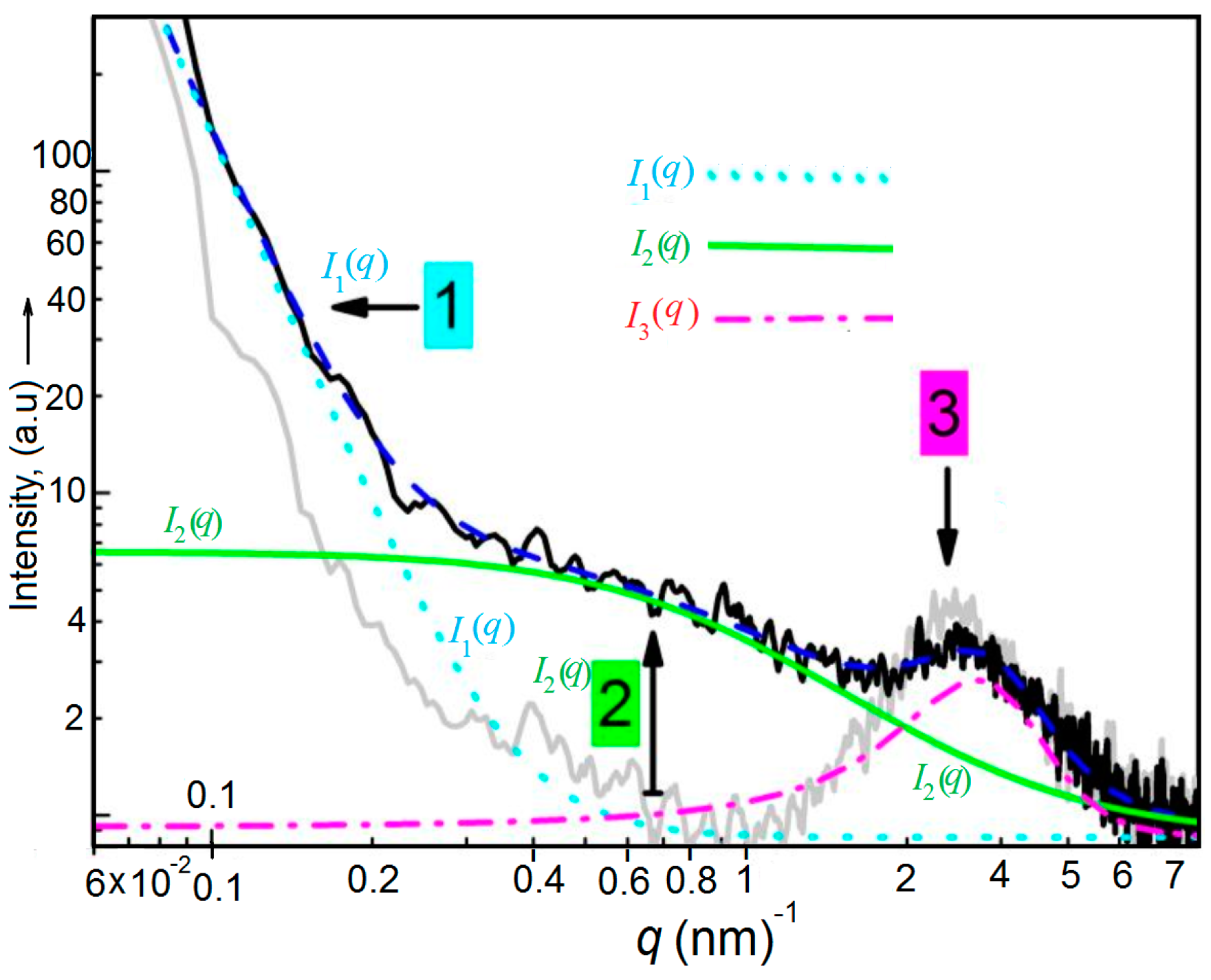
3.11. Small-Angle X-ray Scattering Curves of the Copolymer P without Cross-Linker XON
4. Discussion
4.1. Scattering Contribution 1
4.2. Scattering Contribution 2
4.3. Scattering Contribution 3
4.4. Physical Background and Discussion of Results for X#PON and P
4.5. X#PON at Room Temperature and at 120 °C
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeong, W.-J.; Nishikawa, H.; Gotoh, H.; Takemoto, T. Effect of Solvent Evaporation and Shrink on Conductivity of Conductive Adhesive. Mater. Trans. 2005, 46, 704–708. [Google Scholar] [CrossRef]
- Leung, S.N. Mechanisms of Cell Nucleation, Growth, and Co arsening in Plastic Foaming: Theory, Simulation, and Experiment. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2009. [Google Scholar]
- Pleşa, I.; Noţingher, P.; Schlögl, S.; Sumereder, C.; Muhr, M. Properties of Polymer Composites Used in High-Voltage Applications. Polymers 2016, 8, 173. [Google Scholar] [CrossRef]
- Okamoto, T.; Patil, A.J.; Nissinen, T.; Mann, S. Self-Assembly of Colloidal Nanocomposite Hydrogels Using 1D Cellulose Nanocrystals and 2D Exfoliated Organoclay Layers. Gels 2017, 3, 11. [Google Scholar] [CrossRef]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Chen, J.S. Self-Healing Polymeric Materials. Key Eng. Mater. 2017, 727, 482–489. [Google Scholar] [CrossRef]
- Wu, D.Y.; Meure, S.; Solomon, D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2011, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.P.; Lee, S.; Harmon, J. Ethanol-induced crack healing in poly(methyl methacrylate). J. Polym. Sci. B 1994, 32, 1217. [Google Scholar] [CrossRef]
- Lin, C.B.; Lee, S.; Liu, K.S. Methanol-induced crack healing in poly(methyl methacrylate). Polym. Eng. Sci. 1990, 30, 1399. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Thompson, H.; Temple, R. The infra-red spectra of furan and thiophen. Trans. Faraday Soc. 1945, 41, 27–34. [Google Scholar] [CrossRef]
- Imato, K.; Natterodt, J.C.; Sapkota, J.; Goseki, R.; Weder, C.; Takahara, A.; Otsuka, H. Dynamic covalent diarylbibenzofuranone-modified nanocellulose: Mechano- chromic behaviour and application in self-healing polymer composites. Polym. Chem. 2017, 8, 2115–2122. [Google Scholar] [CrossRef]
- Roth, P.J.; Lowe, A.B. Stimulus-responsive polymers. Polym. Chem. 2017, 8, 10–11. [Google Scholar] [CrossRef]
- Ramaduraia, S.; Wernerb, M.; Slaterc, N.K.H.; Martina, A.; Baulin, V.A.; Keyes, T.E. Dynamic studies of the interaction of a pH responsive, amphiphilic polymer with a DOPC lipid membrane. Soft Matter 2017, 13, 3690–3700. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Basit, H.; Forster, R.J.; Keyes, T.E. Micron dimensioned cavity array supported lipid bilayers for the electrochemical investigation of ionophore activity. Bioelectrochemistry 2016, 112, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive, self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [PubMed]
- Göstl, R.; Senf, A.; Hecht, S. Remote-controlling chemical reactions by light: Towards chemistry with high spatio-temporal resolution. Chem. Soc. Rev. 2014, 43, 1982–1996. [Google Scholar] [CrossRef] [PubMed]
- Asadirad, A.M.; Boutault, S.; Erno, Z.; Branda, N.R. Controlling a polymer adhesive using light and a molecular switch. J. Am. Chem. Soc. 2014, 136, 3024–3027. [Google Scholar] [CrossRef] [PubMed]
- Herder, M.; Schmidt, B.M.; Grubert, L.; Pätzel, M.; Schwarz, J.; Hecht, S. Improving the fatigue resistance of diarylethene switches. J. Am. Chem. Soc. 2015, 137, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.F. Vibrational spectroscopy of N-phenylmaleimide. Spectrochim. Acta A 2006, 63, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Grigg, R.; Knight, J.A.; Sargent, M.V. Studies in furan chemistry. Part I. The infrared spectra of 2, 5-disubstituted furans. J. Chem. Soc. 1965, 6057–6060. [Google Scholar] [CrossRef]
- Breßler, I.; Kohlbrecher, J.; Thünemann, A.F. SASfit: A tool for small-angle scattering data analysis using a library of analytical expressions. J. Appl. Crystallogr. 2015, 48, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P.-G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Panyukov, S.; Rabin, Y. Statistical physics of polymer gels. Phys. Rep. 1996, 269, 1–131. [Google Scholar] [CrossRef]
- Leibler, L. Theory of microphase separation in block copolymers. Macromolecules 1980, 13, 1602–1617. [Google Scholar] [CrossRef]
- Sweat, D.P.; Kim, M.; Schmitt, A.K.; Perroni, D.V.; Fry, C.G.; Mahanthappa, M.K.; Gopalan, P. Phase Behavior of Poly(4-hydroxystyrene-block-styrene) Synthesized by Living Anionic Polymerization of an Acetal Protected Monomer. Macromolecules 2014, 47, 6302–6310. [Google Scholar]
- Oliver, R.C.; Lipfert, J.; Fox, D.O.; Lo, R.H.; Doniach, S.; Columbus, L. Dependence of Micelle Size and Shape on Detergent Alkyl Chain Length and Head Group. PLoS ONE 2013, 8, e62488. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Sakai, T.; Akagi, Y.; Chung, U.I.L.; Shibayama, M. SANS and SLS studies on tetra-arm PEG gels in as-prepared and swollen states. Macromolecules 2009, 42, 6245–6252. [Google Scholar] [CrossRef]
- Hammouda, B.; Ho, D.; Kline, S. SANS from Poly (ethylene oxide)/Water Systems. Macromolecules 2002, 35, 8578–8585. [Google Scholar] [CrossRef]
- Zhang, F.; Ilvasky, J. Ultra-Small-Angle X-ray Scattering of Polymers. Polym. Rev. 2010, 50, 59–90. [Google Scholar] [CrossRef]
- Seiffert, S. Effect and evolution of nanostructural complexity in sensitive polymer gels. Macromol. Chem. Phys. 2015, 216, 9–22. [Google Scholar] [CrossRef]





| Correlation Length | X#PON at 21 °C | X#POFF at 21 °C | ∆ δ2 (nm) |
|---|---|---|---|
| δ2 (nm) | 1.21 ± 0.07 | 1.61 ± 0.10 | → 0.40 + 0.10 |
| Correlation Length | X#PON at 21 °C | X#POFF at 21 °C | ∆ δ2 (nm) |
|---|---|---|---|
| δ2 (nm) | 1.21 ± 0.07 | 1.97 ± 0.20 | → 0.67 + 0.21 |
| Correlation Length | X#POFF at 21°C | X#POFF at 120 °C | ∆ δ (nm) |
|---|---|---|---|
| δ2 (nm) | 1.61 ± 0.10 | 1.91 ± 0.10 | → 0.30 + 0.10 |
| Correlation Length | X#POFF at 21°C | X#POFF at 120 °C | ∆ δ2 (nm) |
|---|---|---|---|
| δ2 (nm) | 1.61 ± 0.10 | 1.91 ± 0.10 | → 0.30 + 0.10 |
| Correlation Length | X#POFF at 21°C | X#POFF at 120 °C | ∆ δ2 (nm) |
|---|---|---|---|
| δ2 (nm) | 1.27 ± 0.11 | 1.93 ± 0.12 | → 0.66 + 0.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, S.; Al-Marzouki, F.; Obaid, A.; Bahabri, F. RETRACTED: Controlled Light Cross-Linking Technique to Prepare Healable Materials. Polymers 2017, 9, 241. https://doi.org/10.3390/polym9060241
Abdalla S, Al-Marzouki F, Obaid A, Bahabri F. RETRACTED: Controlled Light Cross-Linking Technique to Prepare Healable Materials. Polymers. 2017; 9(6):241. https://doi.org/10.3390/polym9060241
Chicago/Turabian StyleAbdalla, Soliman, Fahad Al-Marzouki, Abdullah Obaid, and Fatma Bahabri. 2017. "RETRACTED: Controlled Light Cross-Linking Technique to Prepare Healable Materials" Polymers 9, no. 6: 241. https://doi.org/10.3390/polym9060241
APA StyleAbdalla, S., Al-Marzouki, F., Obaid, A., & Bahabri, F. (2017). RETRACTED: Controlled Light Cross-Linking Technique to Prepare Healable Materials. Polymers, 9(6), 241. https://doi.org/10.3390/polym9060241





